Back to Journals » Drug Design, Development and Therapy » Volume 9
Antibacterial activity and therapeutic efficacy of Fl-PRPRPL-5, a cationic amphiphilic polyproline helix, in a mouse model of staphylococcal skin infection
Authors Thangamani S, Nepal M, Chmielewski J, Seleem M
Received 15 August 2015
Accepted for publication 16 September 2015
Published 22 October 2015 Volume 2015:9 Pages 5749—5754
DOI https://doi.org/10.2147/DDDT.S94505
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Shankar Thangamani,1 Manish Nepal,2 Jean Chmielewski,2 Mohamed N Seleem1
1Department of Comparative Pathobiology, Purdue University College of Veterinary Medicine, West Lafayette, IN, USA; 2Department of Chemistry, Purdue University, West Lafayette, IN, USA
Abstract: The antibacterial activities and therapeutic efficacy of the cationic, unnatural proline-rich peptide Fl-PRPRPL-5 were evaluated against multidrug-resistant Staphylococcus aureus in a mouse model of skin infection. Fl-PRPRPL-5 showed potent activity against all clinical isolates of S. aureus tested, including methicillin- and vancomycin-resistant S. aureus (MRSA and VRSA, respectively). Fl-PRPRPL-5 was also superior in clearing established in vitro biofilms of S. aureus and Staphylococcus epidermidis, compared with the established antimicrobials mupirocin and vancomycin. Additionally, topical treatment of an MRSA-infected wound with Fl-PRPRPL-5 enhanced wound closure and significantly reduced bacterial load. Finally, 0.5% Fl-PRPRPL-5 significantly reduced the levels of the inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1 beta (IL-1β) in wounds induced by MRSA skin infection. In conclusion, the results of this study suggest the potential application of Fl-PRPRPL-5 in the treatment of staphylococcal skin infections.
Keywords: antimicrobial peptides, Staphylococcus aureus, biofilms, anti-inflammatory, skin infection
Introduction
Staphylococcus aureus, a common and virulent pathogen, is the leading cause of nosocomial wound infections.1–3 Virulence factors, toxins, and exoproteins secreted by multidrug-resistant strains of S. aureus play a major role in evading host immune response, leading to persistent infection, prolonged inflammation, and delayed wound healing.4–6 Furthermore, staphylococcal skin infections often lead to invasive infections that ultimately result in septicemia.7,8 Recently, superficial cutaneous bacterial infections caused by biofilm-producing Staphylococci multidrug-resistant strains have become an emerging clinical problem often associated with treatment failure, even with topical drugs of choice, such as mupirocin and fusidic acid.9–11 Hence, there is an urgent need for novel antimicrobials that can be used to treat cutaneous staphylococcal infections successfully and overcome the antibiotic resistance associated with biofilm-producing Staphylococci spp. One potential alternative approach is the development of antimicrobial peptides as novel therapeutic agents.12 Antimicrobial peptides with anti-inflammatory properties in addition to potent, broad-spectrum antimicrobial activity might form a promising approach to the treatment of staphylococcal skin infections.12,13
In a previous study, we designed a cationic, unnatural proline-rich antimicrobial peptide (Fl-PRPRPL-4) with intrinsic cell penetrating and antibacterial activities (Figure 1). Fl-PRPRPL-4 adopts an amphiphilic polyproline type II helix with a net positive charge and exhibits broad-spectrum activity against various Gram-positive and Gram-negative pathogens, including multidrug-resistant strains of S. aureus.14 However, we recently synthesized a polyproline type II helix with an additional repeat of cationic and hydrophobic moieties, which we call Fl-PRPRPL-5 (Figure 1).15 We previously demonstrated that Fl-PRPRPL-5 shows enhanced cell penetration and higher antimicrobial activity than Fl-PRPRPL-4.15 In the current study, we tested the efficacy of Fl-PRPRPL-5 against clinical isolates of multidrug-resistant S. aureus and evaluated its antibiofilm activity. We also tested its in vivo antimicrobial and anti-inflammatory properties in a mouse model of methicillin-resistant S. aureus (MRSA) skin infection.
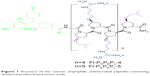  | Figure 1 Structure of the cationic amphiphilic antimicrobial peptides containing unnatural proline-based amino acids. |
Materials and methods
Bacterial strains and reagents
Staphylococcus strains used in this study are presented in Table 1. Mupirocin was purchased from Applichem GmbH (Darmstadt, Germany), and vancomycin hydrochloride from Gold Biotechnology, Inc (Olivette, MO, USA). Mueller–Hinton broth was purchased from Sigma-Aldrich Co (St Louis, MO, USA). Tryptic soy broth, tryptic soy agar, and mannitol salt agar were purchased from BD (Franklin Lakes, NJ, USA).
Synthesis of Fl-PRPRPL-5
The unnatural amino acids needed for the synthesis of Fl-PRPRPL-5 were synthesized, as previously described.16 The peptide was synthesized on the Rink amide resin using a fluorenylmethyloxycarbonyl- (Fmoc)-based strategy with 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate (HATU) as the coupling agent. At the conclusion of the synthesis of the peptide, the resin was treated with N-hydroxysuccinimide fluorescein. The resin-bound fluorescein-modified peptide was cleaved from the resin with a trifluoroacetic acid cocktail; the resulting material was purified to homogeneity by reverse-phase high-performance liquid chromatography, and was then characterized by mass spectrometry.
Antibacterial assays
The broth micro-dilution method was used to determine the minimum inhibitory concentration (MIC) of Fl-PRPRPL-5, as per the guidelines outlined by the Clinical and Laboratory Standards Institute (CLSI).17 Fl-PRPRPL-5 was incubated with bacteria for 16 hours at 37°C, and the lowest concentration of peptide capable of inhibiting visible growth of bacteria by visual inspection was recorded as MIC. The minimum bactericidal concentration of the peptide was determined by sub-culturing the wells where no growth was observed onto tryptic soy agar plates. The plates were incubated for 24 hours, and the concentration where ≥99.9% reduction in bacterial colony-forming units was observed was recorded as the minimum bactericidal concentration.18
Biofilm assay
The ability of Fl-PRPRPL-5 and control antibiotics (mupirocin and vancomycin) to disrupt established biofilms of S. aureus (American Type Culture Collection [ATCC] 6538) and S. epidermidis (ATCC 35984) was evaluated by using the microtiter dish biofilm formation assay.9,19 Briefly, bacteria were grown in 96-well tissue-culture-treated plates in tryptic soy broth supplemented with 1% glucose for 24 hours at 37°C. The media were removed, and plates were washed four times with phosphate-buffered saline, and the adherent biofilms attached to the wells were treated with the concentration mentioned in the figure of peptide and control antibiotics. After 24 hours, plates were again washed with water, and biofilms were stained with 0.1% (weight/volume) crystal violet for 30 minutes at room temperature. The plates were then air dried, and biofilms were solubilized using 95% ethanol. Intensity of crystal violet was measured using a microplate reader (Bio-Tek Instruments Inc, Winooski, VT, USA) at the optical density of 595 nm.
Mice infection
Murine MRSA skin infection study was performed, as described before.20 Briefly, groups of mice (five mice per group) were infected intradermally with 1.65×108 colony-forming units of MRSA USA300 and were left for 48 hours before an open wound formed at the injection site. Mice were divided into three groups, and each group of mice was treated with either 0.5% Fl-PRPRPL-5, 2% mupirocin, or vehicle alone (petroleum jelly). All groups of mice were treated once a day for 4 days. Exactly 24 hours after the last treatment, mice were euthanized, and the area around the wound was slightly swabbed with 70% ethanol. Wound area (1 cm2) was precisely excised, homogenized, and plated onto mannitol salt agar plates to count viable bacterial colony-forming units. The animal care and all experiments were performed in accordance with the guidelines approved by Purdue University Animal Care and Use Committee (PACUC).
Quantifying inflammatory cytokines
Skin homogenates obtained from the MRSA-infected wounds were centrifuged, and the supernatants were used to quantify the levels of inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), interleukin-1 beta (IL-1β), and monocyte chemoattractant protein-1. Duo-set enzyme-linked immunosorbent assay kits (R&D Systems, Inc, Minneapolis, MN, USA) were employed to detect cytokine levels, and the experiment was carried out using the manufacturer’s protocol.21
Statistical analyses
Statistical analyses were assessed by Graph Pad Prism 6.0 (Graph Pad Software, La Jolla, CA, USA). P-values were calculated by the two-tailed Student’s t-test. P-values of <0.05 were considered as significant.
Results and discussion
Fl-PRPRPL-5 has potent activity against clinical isolates of S. aureus
The antimicrobial activity of Fl-PRPRPL-5 was evaluated against a panel of clinical S. aureus isolates (Table 1). Fl-PRPRPL-5 showed bactericidal activity against this panel of multidrug-resistant S. aureus strains that are resistant to methicillin, gentamicin, mupirocin, vancomycin, clindamycin, and erythromycin. Fl-PRPRPL-5 was also active against MRSA USA300, which has been linked to the majority of skin and soft tissue infections present in the US.22 Fl-PRPRPL-5 showed bactericidal activity against all tested strains of S. aureus, regardless of their antibiotic resistance, with MICs ranging from 8–16 μM (Table 1).
Fl-PRPRPL-5 is effective in clearing preformed biofilms
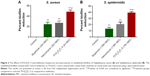
Bacterial biofilms play a major role in bacterial resistance. They often impede antimicrobial penetration, leading to treatment failures that increase the rate of emergence of bacterial resistance.9,23 Therefore, we evaluated the effect of Fl-PRPRPL-5 on preformed biofilms. As shown in Figure 2, treatment with Fl-PRPRPL-5 and control antibiotics (mupirocin and vancomycin) significantly reduced the masses of preformed biofilms generated by biofilm-forming strains of S. aureus and S. epidermidis. Fl-PRPRPL-5 was found to be significantly superior to mupirocin and vancomycin in reducing biofilm mass. Fl-PRPRPL-5, at twice the MIC reduced the mass of the S. aureus biofilm, by more than 50%. Mupirocin and vancomycin reduced the mass of the established biofilm by only 25%, even at 32 times their respective MICs (Figure 2A). Similarly, Fl-PRPRPL-5, at a concentration 16 times its MIC, significantly reduced the mass of a pre-established S. epidermidis biofilm by approximately 40%, whereas mupirocin and vancomycin, at 128 times their respective MICs, reduced the mass of the biofilm by approximately 10% and 20%, respectively (Figure 2B). These results suggest that Fl-PRPRPL-5 exhibits substantially more potent activity against staphylococcal biofilms than the traditional antimicrobials mupirocin and vancomycin.
Fl-PRPRPL-5 enhances wound closure and reduces bacterial load
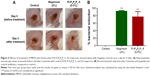
Confident in the broad-spectrum antimicrobial activity and potent antibiofilm properties of Fl-PRPRPL-5, we tested its in vivo anti-staphylococcal activity in a mouse model of MRSA skin infection. Three groups of BALB/C mice were intradermally infected with MRSA USA300. The open wound that formed 2 days after infection was treated topically with vehicle alone (petroleum jelly), 2% mupirocin, or 0.5% Fl-PRPRPL-5 once a day for 4 days. As shown in Figure 3A, topical treatment with 0.5% Fl-PRPRPL-5 for 4 days considerably enhanced the rate of wound closure compared with the control and 2% mupirocin treatment groups. However, the mean bacterial count was significantly reduced in both the mupirocin and Fl-PRPRPL-5 treatment groups, compared with the control group (P≤0.05). As shown in Figure 3B, the groups treated with 2% mupirocin had the highest reduction in bacterial count (95%) followed by 0.5% Fl-PRPRPL-5 (75%). These results suggest that topical application of 0.5% Fl-PRPRPL-5 exerts a positive effect on the wound healing process and reduces the bacterial load in MRSA-infected wounds.
Fl-PRPRPL-5 reduces inflammatory cytokines induced by MRSA infection
Excess inflammation, which is often associated with S. aureus skin infections, increases the clinical severity of the infection.4–6 Therefore, we tested the immunomodulatory properties of Fl-PRPRPL-5 in a mouse model of MRSA skin infection. The effect of Fl-PRPRPL-5 on levels of the inflammatory cytokines TNF-α, IL-6, IL-1β, and monocyte chemoattractant protein-1 was measured in the infected wounds using enzyme-linked immunosorbent assays. As shown in Figure 4, Fl-PRPRPL-5 (0.5%) significantly reduced the levels of TNF-α, IL-6, and IL-1β. In contrast, 2% mupirocin significantly reduced the level of only IL-6. These results indicate that, in addition to having potent antimicrobial activity, Fl-PRPRPL-5 has considerably higher anti-inflammatory properties than the traditional antimicrobial mupirocin.
Conclusion
In summary, we conclude that Fl-PRPRPL-5, a new antimicrobial peptide, possesses anti-staphylococcal and antibiofilm activities against multidrug-resistant clinical isolates of S. aureus. In addition, this peptide enhances wound closure and reduces bacterial load in MRSA-infected wounds. Fl-PRPRPL-5 reduced levels of the inflammatory cytokines TNF-α, IL-6, and IL-1β in wounds induced by MRSA infection. The anti-inflammatory properties of Fl-PRPRPL-5 might also have a beneficial effect on the healing of chronic and diabetic wounds.23–27 However, additional studies are needed to determine the exact mechanisms involved in the wound healing process. In conclusion, our study suggests that Fl-PRPRPL-5 might constitute a novel therapeutic option for the treatment of cutaneous staphylococcal skin infections. The current study also forms a strong platform for the further exploration of the immunomodulatory activities of antimicrobial peptides as part of a novel strategy for the treatment of staphylococcal skin infections.
Acknowledgments
The authors would like to thank the Network of Antimicrobial Resistance in Staphylococcus aureus (NARSA) program supported under the US National Institute of Allergy and Infectious Diseases (NIAID)/ US National Institutes of Health (NIH) contract number HHSN272200700055C for providing MRSA strains used in this study.
Disclosure
The authors report no conflicts of interest in this work.
References
Sen CK, Gordillo GM, Roy S, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. | ||
Mohamed MF, Seleem MN. Efficacy of short novel antimicrobial and anti-inflammatory peptides in a mouse model of methicillin-resistant Staphylococcus aureus (MRSA) skin infection. Drug Des Devel Ther. 2014;8:1979–1983. | ||
Sisirak M, Zvizdic A, Hukic M. Methicillin-resistant Staphylococcus aureus (MRSA) as a cause of nosocomial wound infections. Bosn J Basic Med Sci. 2010;10(1):32–37. | ||
Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. | ||
Montgomery CP, Daniels MD, Zhao F, Spellberg B, Chong AS, Daum RS. Local inflammation exacerbates the severity of Staphylococcus aureus skin infection. PLoS One. 2013;8(7):e69508. | ||
Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev. 2005;18(3):521–540. | ||
del Rio A, Cervera C, Moreno A, Moreillon P, Miró JM. Patients at risk of complications of Staphylococcus aureus bloodstream infection. Clin Infect Dis. 2009;48 Suppl 4:S246–S253. | ||
Keynan Y, Rubinstein E. Staphylococcus aureus bacteremia, risk factors, complications, and management. Crit Care Clin. 2013;29(3):547–562. | ||
Mohamed MF, Hamed MI, Panitch A, Seleem MN. Targeting methicillin-resistant Staphylococcus aureus with short salt-resistant synthetic peptides. Antimicrob Agents Chemother. 2014;58(7):4113–4122. | ||
McNeil JC, Hulten KG, Kaplan SL, Mason EO. Mupirocin resistance in Staphylococcus aureus causing recurrent skin and soft tissue infections in children. Antimicrob Agents Chemother. 2011;55(5):2431–2433. | ||
Farrell DJ, Castanheira M, Chopra I. Characterization of global patterns and the genetics of fusidic acid resistance. Clin Infect Dis. 2011;52 Suppl 7:S487–S492. | ||
Mohammad H, Thangamani S, Seleem MN. Antimicrobial peptides and peptidomimetics – potent therapeutic allies for staphylococcal infections. Curr Pharm Des. 2015;21(16):2073–2088. | ||
Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol. 2006;24(12):1551–1557. | ||
Kuriakose J, Hernandez-Gordillo V, Nepal M, et al. Targeting intracellular pathogenic bacteria with unnatural proline-rich peptides: coupling antibacterial activity with macrophage penetration. Angew Chem Int Ed Engl. 2013;52(37):9664–9667. | ||
Nepal M, Thangamani S, Seleem MN, Chmielewski J. Targeting intracellular bacteria with an extended cationic amphiphilic polyproline helix. Org Biomol Chem. 2015;13(21):5930–5936. | ||
Fillon YA, Anderson JP, Chmielewski J. Cell penetrating agents based on a polyproline helix scaffold. J Am Chem Soc. 2005;127(33):11798–117803. | ||
Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7-A7. CLSI, Wayne, PA. 2007. | ||
Mohammad H, Mayhoub AS, Cushman M, Seleem MN. Anti-biofilm activity and synergism of novel thiazole compounds with glycopeptide antibiotics against multidrug-resistant staphylococci. J Antibiot (Tokyo). 2014;68(4):259–266. | ||
O’Toole GA. Microtiter dish biofilm formation assay. J Vis Exp. 2011;47:2437. | ||
Thangamani S, Younis W, Seleem MN. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci Rep. 2015;5:11596. | ||
Rioja I, Bush KA, Buckton JB, Dickson MC, Life PF. Joint cytokine quantification in two rodent arthritis models: kinetics of expression, correlation of mRNA and protein levels and response to prednisolone treatment. Clin Exp Immunol. 2004;137(1):65–73. | ||
King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144(5):309–317. | ||
de la Fuente-Núñez C, Mansour SC, Wang Z, et al. Anti-biofilm and immunomodulatory activities of peptides that inhibit biofilms formed by pathogens isolated from cystic fibrosis patients. Antibiotics (Basel). 2014;3(4):509–526. | ||
Wallace HJ, Stacey MC. Levels of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in chronic venous leg ulcers – correlations to healing status. J Invest Dermatol. 1998;110(3):292–296. | ||
Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. | ||
Jialal I, Miguelino E, Griffen SC, Devaraj S. Concomitant reduction of low-density lipoprotein-cholesterol and biomarkers of inflammation with low-dose simvastatin therapy in patients with type 1 diabetes. J Clin Endocrinol Metab. 2007;92(8):3136–3140. | ||
Cowin AJ, Hatzirodos N, Rigden J, Fitridge R, Belford DA. Etanercept decreases tumor necrosis factor-alpha activity in chronic wound fluid. Wound Repair Regen. 2006;14(4):421–426. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.