Back to Journals » Drug Design, Development and Therapy » Volume 12
Annatto-derived tocotrienol stimulates osteogenic activity in preosteoblastic MC3T3-E1 cells: a temporal sequential study
Authors Wan Hasan WN, Abd Ghafar N, Chin KY , Ima-Nirwana S
Received 22 March 2018
Accepted for publication 18 April 2018
Published 13 June 2018 Volume 2018:12 Pages 1715—1726
DOI https://doi.org/10.2147/DDDT.S168935
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Wan Nuraini Wan Hasan,1 Norzana Abd Ghafar,2 Kok-Yong Chin,1 Soelaiman Ima-Nirwana1
1Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre, Jalan Yaacob Latif, Bandar Tun Razak, Cheras, Kuala Lumpur, Malaysia; 2Department of Anatomy, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre, Jalan Yaacob Latif, Bandar Tun Razak, Cheras, Kuala Lumpur, Malaysia
Purpose: Annatto-derived tocotrienol (AnTT) has been shown to improve bone formation in animal models of osteoporosis. However, detailed studies of the effects of AnTT on preosteoblastic cells were limited. This study was conducted to investigate the osteogenic effect of AnTT on preosteoblast MC3T3-E1 cells in a time-dependent manner.
Materials and methods: Murine MC3T3-E1 preosteoblastic cells were cultured in the different concentrations of AnTT (0.001–1 µg/mL) up to 24 days. Expression of osteoblastic differentiation markers was measured by qPCR (osterix [OSX], collagen 1 alpha 1 [COL1α1], alkaline phosphatase [ALP], and osteocalcin [OCN]) and by fluorometric assay for ALP activity. Detection of collagen and mineralized nodules was done via Direct Red staining and Alizarin Red staining, respectively.
Results: The results showed that osteoblastic differentiation-related genes, such as OSX, COL1α1, ALP, and OCN, were significantly increased in the AnTT-treated groups compared to the vehicle group in a time-dependent manner (P<0.05). Type 1 collagen level was increased from day 3 to day 15 in the AnTT-treated groups, while ALP activity was increased from day 9 to day 21 in the AnTT-treated groups (P<0.05). Enhanced mineralization was observed in the AnTT-treated groups via increasing Alizarin Red staining from day 3 to day 21 (P<0.05).
Conclusion: Our results suggest that AnTT enhances the osteogenic activity by promoting the bone formation-related genes and proteins in a temporal and sequential manner.
Keywords: bone, differentiation, osteoporosis, tocotrienol, vitamin E
Introduction
Bone formation or osteogenesis is a process well orchestrated by osteoblasts. Osteogenesis is characterized by preosteoblast proliferation, osteoblast differentiation, and collagenous extracellular matrix (ECM) formation. It begins with the active proliferation of undifferentiated cells, and after that, the cells undergo growth arrest and formation of collagenous ECM.1,2 Upon the initiation of matrix synthesis, early osteoblast differentiation marker genes, such as collagen 1 alpha 1 (COL1α1) and alkaline phosphatase (ALP), will be activated. This is followed by the expression of bone sialoprotein (BSP) and osteocalcin (OCN).3 Once these marker genes are activated, mineralization of collagenous ECM will commence with the deposition of calcium and phosphate.3,4
The imbalance between bone formation by osteoblasts and bone resorption by osteoclasts in favor of the latter can lead to degenerative bone diseases, such as osteoporosis. Osteoporosis is characterized by a low bone mass and skeletal microarchitectural deterioration leading to bone fragility and increased fracture risk.5 This silent disease mainly affects postmenopausal women, but it can also occur in men. According to the International Osteoporosis Foundation, one in three women and one in five men >50 years experienced osteoporotic fractures. Osteoporotic fractures contribute to the increased morbidity and mortality of the patients, thus representing a large economic burden.6
Most of the pharmacological agents against osteoporosis aim to prevent excessive resorption (antiresorptive) rather than increased bone formation (anabolic).7 The existing bone anabolic agents, such as teriparatide, are not free from side effects.8,9 Some well-tolerated compounds extracted from natural products have been found to promote bone formation. Natural compounds from grapes (resveratrol), seeds of fenugreek (diosgenin), and hop plant (xanthohumol) have been reported to stimulate osteogenesis in experimental studies.10–12 Of note, vitamin E mixtures derived from natural sources have demonstrated bone anabolic effects in various animal models.13,14
Vitamin E can be found in various natural sources including wheat, barley, rice bran, and palm oil.15 It consists of the following two major families: tocotrienols and tocopherols. Both families contain the following four isomers: alpha (α), beta (β), delta (δ), and gamma (γ). Tocotrienols differ from tocopherols by the presence of an unsaturated side chain, which give rise to the differences in various biological processes between these two families, such as antioxidative, neuroprotective, hypocholesterolemic, anticancer, and bone anabolic actions.16 Ima-Nirwana and Suhaniza17 showed that palm-derived γ-tocotrienol preserved normal body composition and calcium content more effectively compared to α-tocopherol in rats on dexamethasone treatment. Deng et al18 showed that γ-tocotrienol increased the circulating bone formation marker, OCN, bone matrix deposition, and bone formation rate in ovariectomized mice via the mevalonate pathway. Tocotrienols have been studied in osteoporotic rats induced by various stressors and have been confirmed to have positive effects on bone.14 Tocotrienol from the seeds of annatto tree (Bixa orellana) (annatto-derived tocotrienol [AnTT]) contains 100% tocotrienol (~90% δ-tocotrienol and 10% γ-tocotrienol).19 Abdul-Majeed et al20,21 found that combination of a statin with AnTT increased bone formation, reduced bone resorption, and improved bone structure and bone strength in ovariectomized-rats. AnTT also increased osteoblast surface, osteoid surface, and osteoid volume, and reduced osteoclast surface in orchidectomized rats.22 However, there are limited studies on the effects of AnTT on preosteoblastic cells.
The main objective of this study was to evaluate the effects of AnTT on cell morphology, proliferation, and differentiation in preosteoblastic MC3T3-E1 cells. It is hypothesized that AnTT would enhance the osteogenic activity in these cells. Through this study, we hope to develop AnTT as a potential anabolic agent in enhancing bone formation for the treatment of bone degenerative diseases including osteoporosis.
Materials and methods
Cell culture
Murine calvariae preosteoblast cell line (MC3T3-E1) was purchased from American Type Culture Collection (ATCC) (no CRL-2594) (Manassas, VA, USA). The cells were grown in α-modified essential medium (α-MEM) (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 10% antibiotic–antifungal agent (Thermo Fisher Scientific) in the humidified condition at 37°C and 5% carbon dioxide. In all experiments, the cells were subcultured at a density of 1×104 cells/mL growth media. On the following day, the media were changed into osteoblast differentiation medium (DM) with α-MEM supplemented with 3 mM sodium phosphate (Sigma-Aldrich Co., St Louis, MO, USA) and 50 μg/mL ascorbic acid (Sigma-Aldrich Co.).
Annatto derived-tocotrienol (AnTT) was a gift from American River Nutrition (Hadley, MA, USA). AnTT preparation was prepared based on a previous study with some modifications.23 Briefly, 5 mg of AnTT was dissolved in 1 mL of ethanol (HmbG Chemicals, Hamburg, Germany) and kept as the stock solution. From the stock solution, 25 μL was added to 60 μL of FBS and incubated overnight. On the next day, 90 μL of DM and 105 μL of ethanol were added and mixed well. AnTT was diluted into 0.001, 0.01, 0.1, and 1 μg/mL. These dilutions were freshly prepared every 2–3 days until the termination of experiments. Treatment for vehicle group contained the same amount of ethanol as the AnTT groups.
Cell viability (MTS assay)
MTS assay was carried out to measure the cell viability of MC3T3-E1 cells after AnTT treatment for 1, 3, and 6 days. The cells were seeded in a 96-well plate in growth media and incubated overnight. On the next day, various concentrations of AnTT treatment in differentiation media were added to the cells. At the end of each time point, 20 μL of MTS solution (Promega Corporation, Fitchburg, WI, USA) was added to each well and incubated for 4 h. The absorbance was measured at 490 nm using a microplate reader (Tecan, Mannedorf, Switzerland).
Cell proliferation (BrdU assay)
BrdU assay was performed to measure the proliferation rate of MC3T3-E1 cells after AnTT treatment for 1, 3, and 6 days. Cells were plated in a 96-well plate in growth media and further incubated with AnTT treatment. At the end of each time point, cell proliferation was determined using the Cell Proliferation ELISA, BrdU (colorimetric) (product code: 11 647 229 001; Hoffman-La Roche Ltd., Basel, Switzerland).
Cell morphology
Morphological changes in MC3T3-E1 cells were observed during differentiation and mineralization with or without AnTT treatment. MC3T3-E1 preosteoblast cells were plated in a six-well plate. After incubating overnight, the cells were treated with AnTT for 3, 6, 9, 15, and 21 days. After each time point, the cells were washed with phosphate-buffered saline (PBS), pH 7.4, and morphology of the cells was visualized using the inverted microscope EVOS Cell Imaging System (Thermo Fisher Scientific).
Gene expressions
Cells were seeded in a six-well plate and incubated overnight. The cells were treated with AnTT for 3, 6, 9, 15, 18, 21, and 24 days. After each time point, the cells were washed with PBS, and RNA extraction was carried out using the TRI reagent (Molecular Research Centre, Inc., Cincinnati, OH, USA). PCR amplification was performed with a thermal cycler (Techne, Staffordshire, UK). Levels of mRNA were quantified by iQ5 (Bio-Rad Laboratories Inc., Hercules, CA, USA) under the amplification condition of 40 cycles, 10 s at 95°C (denaturation) and 30 s at 56°C (annealing). The primers were synthesized by First Base (Singapore Science Park II, Singapore), and primer sequences for the genes are listed in Table 1.
  | Table 1 Oligonucleotide sequences for bone formation-related genes for qPCR |
Collagen staining
To determine the formation of collagenous matrix, collagen content in the preosteoblastic culture was stained with Direct Red (Sigma-Aldrich Co.) for 3, 9, 15, and 21 days. MC3T3-E1 cells were plated in a 48-well plate, and after overnight incubation, the cells were treated with AnTT. At the end of each time point, the cells were washed with PBS, and 0.1% Direct Red 80 in picric acid (Sigma-Aldrich Co.) was added into the wells. The plates were then incubated for 1 h at 37°C. The cells were washed with 10 mM hydrochloric acid (Fisher, Hampton, VA, USA), and the staining was visualized under the microscope EVOS Cell Imaging System. The bound collagen was dissolved with 0.1 M sodium hydroxide, and the absorbance was measured at 450 mm using a microplate reader.
ALP activity
ALP activity was measured to determine the early marker of bone formation. The cells were plated in a six-well plate and incubated overnight. The cells were then treated with AnTT for 3, 9, 15, and 21 days. At the end of each time point, the cells were harvested and assayed for ALP activity using the ALP assay kit (fluorometric) (product code: ab83371; Abcam, Cambridge, UK).
Alizarin Red staining
To determine the effects of AnTT on mineralization, the matrix was stained with Alizarin Red dye (Sigma-Aldrich Co.) that binds to calcium in the ECM from day 3 to day 21. The cells were plated in a 48-well plate and incubated overnight. The cells were treated with AnTT for 3, 9, 15, and 21 days. At the end of each time point, the cells were washed with PBS and fixed with 10% buffered formalin for 10 min. Then, 40 mM Alizarin Red, pH 4.4, was added to the wells and incubated for 1 h at room temperature. The cells were washed with PBS and observed under the inverted microscope EVOS Cell Imaging System. Then, the cells were dissolved in 10% cetylpyridinium chloride (Sigma-Aldrich Co.) and shaken overnight at room temperature. The absorbance was measured at 562 nm using an EnSpire microplate reader (PerkinElmer Inc., Waltham, MA, USA).
Statistical analysis
Statistical analysis was done using the SPSS software for Windows, version 20 (IBM Corporation, Armonk, NY, USA). Differences between mean values of multiple groups were analyzed by the mixed design ANOVA with post hoc pairwise analysis. A P-value of <0.05 was considered as statistically significant.
Results
Effects of AnTT on the viability and proliferation of MC3T3-E1 cells
Cell viability (Figure 1A) and cell proliferation (Figure 1B) were measured after preosteoblastic cells were treated with increasing doses of AnTT for days 1, 3, and 6. There were significant “time” (P<0.05) and “time × treatment” (P<0.05) effects on cell viability and cell proliferation. At day 1, AnTT treatments showed no toxicity to the cells. After 3 and 6 days of AnTT treatments, cell viability at 20 μg/mL AnTT was significantly decreased compared to vehicle. Cell viability at day 3 and day 6 for all the groups was significantly increased compared to day 1 and day 3, respectively (P<0.05). In contrast, cell proliferation showed a significant decrease at 5, 10, and 20 μg/mL of AnTT compared to vehicle at day 1 (P<0.05). After 3 and 6 days of treatment, AnTT at 5, 10, and 20 μg/mL significantly decreased cell proliferation compared to vehicle (P<0.05). At 10 μg/mL, AnTT significantly increased cell proliferation at day 3 but not at day 6 compared to day 1 (P<0.05). These results indicate that AnTT at high doses reduced cell viability and cell proliferation.
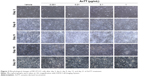
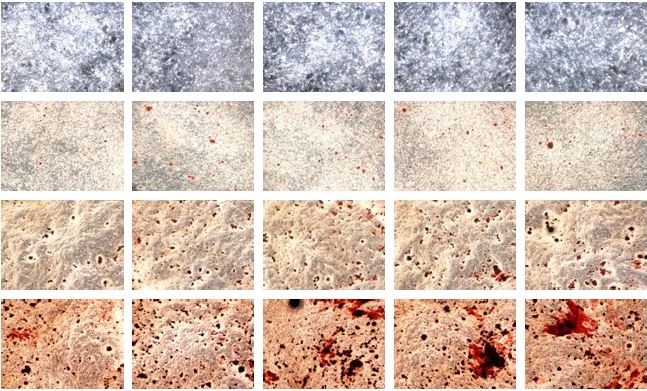
Effects of AnTT on the morphology of preosteoblast MC3T3-E1 cells
Preosteoblasts were characterized by their spindle-shaped appearance. At day 3, the cells reached a subconfluent monolayer appearance (Figure 2). The shape of the cells changed, and their confluency increased from day 9 to day 21. From the observation, a semiopaque layer on top of the cells appeared from day 6 to day 21, and it was presumed as the collagenous matrix as well as calcified nodules.
Effects of AnTT on the expression of bone markers’ gene
In this study, the transcription factor involved in osteoblast differentiation and bone formation, osterix (OSX) (Figure 3A), was measured after 3, 6, 9, and 15 days of AnTT treatment. Besides, three genes related to bone formation markers namely COL1α1 (Figure 3B), ALP (Figure 3C), and OCN (Figure 3D) were measured after MC3T3-E1 cells treated with AnTT from day 3 to day 24.
There were significant time (P<0.05) and time × treatment (P<0.05) effects of OSX gene expression. At day 3, 0.001 μg/mL of AnTT significantly increased OSX gene expression compared to its vehicle (P<0.05). Besides, OSX gene expression in all AnTT-treated groups was significantly increased compared to the vehicle at day 6 (P<0.05). Meanwhile, at day 9, OSX gene expression in cells treated with 0.01, 0.1, and 1 μg/mL of AnTT was significantly increased compared to the vehicle (P<0.05). At day 15, 0.001 and 0.1 μg/mL of AnTT significantly increased OSX gene expression compared to the vehicle (P<0.05). In the vehicle group, OSX gene was significantly increased at day 9 compared to day 6 (P<0.05). Treatment with 0.001 μg/mL of AnTT significantly increased OSX gene expression at day 6 and day 15 compared to day 3 and day 9, respectively (P<0.05). Treatment with 0.01 μg/mL of AnTT significantly increased OSX gene expression at day 6 and day 9 compared to day 3 and day 6, respectively (P<0.05). The OSX gene expression of the 0.1 μg/mL AnTT group was significantly increased at day 9 compared to day 3 (P<0.05). The 1 μg/mL of AnTT significantly increased OSX gene expression at day 6 and day 9 compared to day 3 and day 6, respectively (P<0.05). The results showed that AnTT treatment hastened OSX gene expression as compared to vehicle. The expression of OSX gene was significantly increased in all AnTT groups at all time points compared to day 3 (P<0.05).
There were significant time (P<0.05) and time × treatment (P<0.05) effects for COL1α1 gene expression. At day 3, COL1α1 gene expression was significantly increased by 0.01, 0.1, and 1 μg/mL of AnTT compared to the vehicle (P<0.05). COL1α1 gene expression was significantly increased by 0.001, 0.01, and 0.1 μg/mL AnTT compared to the vehicle at day 6 (P<0.05). While at day 9, COL1α1 gene expression was significantly increased by 0.001, 0.01, 0.1, and 1 μg/mL of AnTT compared to its vehicle (P<0.05). COL1α1 gene was significantly increased by 0.01 μg/mL of AnTT compared to the vehicle at day 6 (P<0.05). In the vehicle group, COL1α1 gene expression was significantly increased at day 6, day 9, and day 15 compared to day 3, day 6, and day 9, respectively (P<0.05). Meanwhile, COL1α1 gene expression was significantly increased in all AnTT groups at day 6 and day 9 compared to day 3 and day 9, respectively (P<0.05). These data indicated that with AnTT treatment, COL1α1 gene expression reached its maximum level earlier at day 9 compared to vehicle at day 15. The expression of COL1α1 gene was significantly increased in all AnTT groups at all time points compared to day 3 (P<0.05).
There were significant time (P<0.05) and time × treatment (P<0.05) effects for ALP gene expression. At day 3, ALP gene expression was significantly increased by 0.001, 0.01, and 0.1 μg/mL of AnTT compared to its vehicle (P<0.05). ALP gene expression was significantly increased by 0.001, 0.01, and 0.1 μg/mL of AnTT compared to the vehicle at day 6 and day 9 (P<0.05). While at day 15, COL1α1 gene expression was significantly increased by 0.01 and 0.1 μg/mL of AnTT compared to its vehicle (P<0.05). COL1α1 gene expression was significantly increased by 0.01 μg/mL of AnTT compared to the vehicle at day 18 (P<0.05). At day 21, ALP gene expression was significantly increased by 0.001, 0.01, and 0.1 μg/mL of AnTT compared to the vehicle (P<0.05). In the vehicle group, ALP gene expression was significantly increased at day 9, day 15, day 18, day 21, and day 24 compared to its previous time points (day 6, day 9, day 15, day 18, and day 21, respectively) (P<0.05). Meanwhile, ALP gene expression was significantly increased in all AnTT groups at day 6 and day 9 compared to day 3 and day 9, respectively (P<0.05). The 0.001 and 1 μg/mL of AnTT increased ALP gene expression significantly at day 18 compared to day 15 (P<0.05). The 0.01 μg/mL of AnTT increased ALP gene expression significantly at day 15 and day 21 compared to day 9 and day 18, respectively (P<0.05). The 0.1 μg/mL of AnTT increased ALP gene expression significantly at day 18 compared to day 15. These data indicated that with AnTT treatment, ALP gene expression reached its maximum level earlier at day 21 compared to the vehicle, which still increased at day 24 (P<0.05). The expression of ALP gene expression was significantly increased in all AnTT groups at all time points compared to day 3 (P<0.05).
There were significant time (P<0.05) and time × treatment (P<0.05) effects for OCN gene expression. From day 3 to day 21, OCN gene expression was significantly increased by 0.001, 0.01, and 0.1 μg/mL of AnTT compared to its vehicle (P<0.05). ALP gene expression was significantly increased by 1 μg/mL of AnTT compared to the vehicle at day 15 and day 18 (P<0.05). In the vehicle group, OCN gene expression was significantly increased at day 15 compared to day 6 (P<0.05). Meanwhile, OCN gene expression was significantly increased by 0.001 and 0.01 μg/mL of AnTT at day 6, day 9, day 15, and day 18 compared to its previous time points (day 3, day 6, day 9, and day 15, respectively) (P<0.05). The 0.1 μg/mL of AnTT increased ALP gene expression significantly at day 6, day 15, and day 21 compared to its previous time points (day 3, day 9, and day 18, respectively) (P<0.05). The 1 μg/mL of AnTT increased OCN gene expression significantly at day 15 compared to day 9 (P<0.05). These data indicated that with AnTT treatment, OCN reached its maximum level earlier at day 21 compared to vehicle, which still increases at day 24. The expression of OCN gene was significantly increased in all the AnTT groups at all time points compared to day 3 (P<0.05).
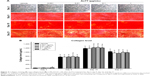
Effects of AnTT on collagen formation
The intensity of the red color increased in a time-dependent manner in all the groups from day 3 to day 15 (Figure 4A). At day 21, the intensity of the red color appeared to decrease. Quantitative measurement of collagen level was done after the positive staining was dissolved (Figure 4B). There were significant time (P<0.05) and time × treatment (P<0.05) effects for collagen level. Collagen level was significantly increased at day 3 in the 1 μg/mL AnTT-treated group compared to the vehicle (P<0.05). At day 9, collagen level was significantly increased in the 0.1 μg/mL AnTT-treated group compared to the vehicle (P<0.05). In addition, collagen level was significantly increased at day 15 in the 0.01, 0.1, and 1 μg/mL AnTT-treated groups compared to the vehicle (P<0.05). At day 21, collagen level was significantly decreased in the 0.01 μg/mL AnTT-treated group compared to vehicle (P<0.05). Collagen level was significantly increased in all the groups at day 9 and day 15 compared to its previous time points (P<0.05) (day 6 and day 9, respectively). At day 21, however, collagen levels were significantly decreased compared to day 15 in all the groups (P<0.05). Collagen levels were significantly increased in all the groups compared to day 3 (P<0.05).
Effects of AnTT on ALP activity
ALP activity was measured to determine the effects of AnTT on osteoblast differentiation (Figure 5). There were significant time (P<0.05) and time × treatment (P<0.05) effects for ALP activity. At day 9, ALP activity was significantly increased in the 0.1 and 1 μg/mL AnTT-treated groups compared to the vehicle (P<0.05). ALP activity was significantly increased in the 0.01, 0.1, and 1 μg/mL AnTT-treated groups compared to the vehicle at day 15 (P<0.05). In contrast, ALP activity was significantly increased in all the groups compared to its previous time points (P<0.05). The ALP activity was significantly increased in a time-dependent manner in all the groups compared to day 3 (P<0.05).
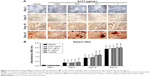
Effects of AnTT on mineralization
The intensity of the red color for Alizarin Red-positive staining increased in a time-dependent manner in all the groups from day 3 to day 21 (Figure 6A). There were significant time (P<0.05) and time × treatment (P<0.05) effects for calcium deposition. Quantitative data revealed that calcium deposition was significantly increased in the 1 μg/mL AnTT group compared to the vehicle at day 9 (P<0.05) (Figure 6B). At day 15 and day 21, calcium deposition was significantly increased in the 0.1 and 1 μg/mL AnTT groups compared to the vehicle (P<0.05). Calcium deposition was significantly increased in all the groups at all time points compared to their previous time points (P<0.05). Calcium deposition was significantly increased in all the groups at all time points compared to day 3 (P<0.05).
Discussion
Bone formation is a complex process and involves various signaling cascades that activate a series of markers. All the markers appear in a sequential manner starting from the expression of the transcription factor, OSX, to the formation of collagen fibrils in the ECM, followed by the expression of ALP and OCN, and finally the mineralization of ECM.24 The present study showed that AnTT promoted preosteoblasts differentiation to the mature and functional osteoblasts, indicated by the expression of osteoblast differentiation markers. It also encouraged the synthesis and mineralization of the ECM, indicated by collagen and calcium staining. Therefore, AnTT is a potent skeletal anabolic agent.
First, to determine the cytotoxicity and effective doses of AnTT on preosteoblastic cell lines, cell viability, and cell proliferation were investigated. We found that cells treated with AnTT at lower concentration (0.001–1 μg/mL) showed active DNA synthesis and no toxicity effect. However, AnTT at high concentrations significantly reduced cell viability (20 μg/mL) and cell proliferation (5–20 μg/mL) of MC3T3-E1 cells. In line with our findings, γ-tocotrienol at low doses (1 and 10 μM) was reported to protect osteoblast cells against hydrogen peroxidase (H2O2) toxicity, while at high dose (100 μM), γ-tocotrienol induced further apoptosis caused by H2O2, which was a sign of toxicity.25,26 In addition, γ-tocotrienol at doses >16 μg/mL caused a reduction in cell viability and morphological changes indicating toxicity in MC3T3-E1 cells.27 Vitamin E is capable to act as antioxidant and prooxidant. In the absence of other antioxidants and at high doses, vitamin E tends to act as pro-oxidants.28 In contrast to our data, γ-tocotrienol (2–8 μmol/L) was shown to significantly increase cell viability and cell proliferation in preosteoblastic MC3T3-E1 cells.27 This indicated that γ-tocotrienol was less toxic to the preosteoblasts compared to AnTT.
Next, morphological changes that occured during osteogenesis were evident from day 3 to day 21. The morphology of the cells cultured was in accordance with other studies. A previous study reported that the morphology of the cells changed from fusiform into cuboidal during early days of culture (1–9 days) in murine preosteoblast MC3T3-E1 cells.1 The MC3T3-E1 cells showed a typical fibroblastic morphology that reached a confluent monolayer with a mosaic appearance at day 4. Then, the cells continued to grow and formed multiple cell layers.29 Subsequently, the osteoblasts formed noncalcified nodules and, later, calcified nodules if cultured continuously after confluency.30 This was similar with the observation of the present study, whereby the presence of collagen was detected early in the culture, followed by the calcified nodules at the later stage.
Since OSX was the transcription factor triggering the expression of bone formation markers in osteoblasts, we measured mRNA level of OSX and found that AnTT upregulated OSX gene expression. In agreement with our finding, γ-tocotrienol increased OSX gene expression in ovariectomized mice.18 Subsequently, expression of genes related to osteoblastic differentiation such as COL1α1, ALP, and OCN was increased by AnTT in preosteoblastic cells. In parallel with our findings, AnTT was reported to significantly increase the expression of COL1α1 and ALP genes in the bone of orchidectomized rats.31 In the same study, the gene expression of OCN was not statistically different between the AnTT-treated group and the sham group, which was in contrast with our data. Since in cell culture study, the osteoblasts in monolayer were exposed to the treatment directly; thus, this would explain a greater response compared to the animal studies.32
We also showed that AnTT increased the formation of collagen, ALP activity, and calcium deposition in the culture. This might be a direct response to enhanced expression of genes related to bone formation. Vitamin E members, α- and δ-tocopherol, were found to increase the ALP activity from day 7 to day 14 in rats’ calvariae osteoblasts,30 which was in line with our findings. Another study reported that γ-tocotrienol increased ALP activity and mineralized nodules in MC3T3-E1 cells.27 In the same study, γ-tocotrienol did not alter collagen protein level, which was in contrast with our data.
Taken together, this study demonstrated that AnTT enhanced osteogenesis, as reflected at the transcriptional level (increased expression of genes coding OSX, COL1α1, ALP, and OCN) and the translational level (increased protein expression of collagen, ALP activity, and calcium deposition). AnTT enhanced the bone formation by upregulating OSX gene expression and subsequently promoted osteoblast differentiation markers such as COL1α1, ALP, OCN, and calcified nodules in a sequential manner. Collagen deposition in the ECM serves as an autocrine signal to enhance the synthesis of osteoblast markers, such as ALP and OCN.33,34 This eventually induces the deposition of minerals, such as calcium and phosphate, to form hydroxyapatite crystals within the collagen fibrils.35,36 The expression of ALP appears at the transition of cell proliferation stage to matrix development or maturation, while OCN appears at subsequent mineralization stage.37 Induction of the ECM maturation gene, OCN, is related to the onset of calcium deposition.38 OCN is the most abundant noncollagenous protein found in the bone that plays a role in bone mineralization by binding calcium ions to hydroxyapatite crystals.39 Our data revealed that low concentrations of AnTT such as 0.001, 0.01, and 0.1 μg/mL effectively increased the genes’ level (OSX, COL1α1, ALP, and OCN), while AnTT at 0.1 and 1 μg/mL effectively increased the proteins’ level (collagen, ALP activity, mineralized nodules) for bone formation markers.
This study highlights the potential effects of AnTT during bone formation. However, some limitations of this study should be acknowledged. Only murine preosteoblasts were used in this study, which may not be the model that best resembles the bone formation in humans. Next, the upstream pathways affecting osteogenesis could be potentially affected by AnTT, but they were not characterized in this study. Previous studies suggested that tocotrienol protected bone loss in ovariectomized rats by inhibiting mevalonate pathway.18 It is noted that dose-dependent effect of AnTT was not evident in this study. The action of tocotrienol may be limited by its cellular uptake, although this speculation was not tested in this study. Similar results were observed in a study by Mohamad et al,40 which showed no dose dependent of annatto tocotrienol (60 and 100 mg/kg/day) on the skeletal parameters in male rats. From the results, the most effective dose for the induction of mRNA expression and protein activity did not match. Since tocotrienols may act at transcriptional, translational, and post-translational stages or directly with the protein, the effects of tocotrienol on protein activity are not merely dependent on its translation level. At high concentration, tocotrienol may influence protein stability and degradation rate.41 Nevertheless, there are limited anabolic antiosteoporotic agents available to treat osteoporosis currently. Based on our findings, AnTT showed promising anabolic effects on preosteoblastic cells, indicating that it might be an alternative anabolic agent to preserve bone health. However, this speculation awaits further verification in a clinical trial.
Conclusion
AnTT enhances osteoblast differentiation by upregulating the expression of OSX, collagen, ALP, and OCN. This translates to an increase in bone matrix synthesis and mineralization. Therefore, the antiosteoporosis action of AnTT demonstrated in previous animal studies can be explained by its bone anabolic actions. It has the potential to be developed as an antiosteoporosis therapy in humans.
Acknowledgments
We express our sincere gratitude to American River Nutrition (USA) for providing the annatto-derived tocotrienol. This research was funded by RGS/1/2013/SKK03/UKM/01/1 grant and FF-2017-270 grant provided by the Ministry of Education, Malaysia, and Universiti Kebangsaan Malaysia.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Quarles LD, Yohay DA, Lever LW, Caton R, Wenstrup RJ. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J Bone Miner Res. 1992;7(6):683–692. | ||
Choi JY, Lee BH, Song KB, et al. Expression patterns of bone-related proteins during osteoblastic differentiation in MC3T3-E1 cells. J Cell Biochem. 1996;61(4):609–618. | ||
Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14(6):893–903. | ||
Lian JB, Stein GS. Concepts of osteoblast growth and differentiation: basis for modulation of bone cell development and tissue formation. Crit Rev Oral Biol Med. 1992;3(3):269–305. | ||
World Health Organization. Assessment of Fracture Risk and Its Application to Screening for Postmenopausal Osteoporosis: Report of a WHO Study Group [Meeting Held in Rome from 22 to 25 June 1992]; 1994. | ||
Hernlund E, Svedbom A, Ivergård M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1–2):136. | ||
Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26(5):688–703. | ||
Cejka D, Benesch T, Krestan C, et al. Effect of teriparatide on early bone loss after kidney transplantation. Am J Transplant. 2008;8(9):1864–1870. | ||
Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1838–1845. | ||
Mizutani K, Ikeda K, Kawai Y, Yamori Y. Resveratrol stimulates the proliferation and differentiation of osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun. 1998;253(3):859–863. | ||
Alcantara EH, Shin M-Y, Sohn H-Y, et al. Diosgenin stimulates osteogenic activity by increasing bone matrix protein synthesis and bone-specific transcription factor Runx2 in osteoblastic MC3T3-E1 cells. J Nutr Biochem. 2011;22(11):1055–1063. | ||
Jeong HM, Han EH, Jin YH, Choi YH, Lee KY, Jeong HG. Xanthohumol from the hop plant stimulates osteoblast differentiation by RUNX2 activation. Biochem Biophys Res Commun. 2011;409(1):82–89. | ||
Shen CL, Klein A, Chin KY, et al. Tocotrienols for bone health: a translational approach. Ann N Y Acad Sci. 2017;1401(1):150–165. | ||
Chin K-Y, Soelaiman IN. The biological effects of tocotrienol on bone: a review on evidence from rodent models. Drug Des Devel Ther. 2015;9:2049–2061. | ||
Aggarwal BB, Sundaram C, Prasad S, Kannappan R. Tocotrienols, the vitamin E of the 21st century: its potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80(11):1613–1631. | ||
Sen CK, Khanna S, Roy S. Tocotrienols: vitamin E beyond tocopherols. Life Sci. 2006;78(18):2088–2098. | ||
Ima-Nirwana S, Suhaniza S. Effects of tocopherols and tocotrienols on body composition and bone calcium content in adrenalectomized rats replaced with dexamethasone. J Med Food. 2004;7(1):45–51. | ||
Deng L, Ding Y, Peng Y, et al. γ-Tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as HMG-CoA reductase inhibitor. Bone. 2014;67:200–207. | ||
Frega N, Mozzon M, Bocci F. Identification and estimation of tocotrienols in the annatto lipid fraction by gas chromatography-mass spectrometry. J Am Oil Chem Soc. 1998;75(12):1723–1727. | ||
Abdul-Majeed S, Mohamed N, Soelaiman I-N. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid Based Complement Alternat Med. 2012;2012:9. | ||
Abdul-Majeed S, Mohamed N, Soelaiman I-N. The use of delta-tocotrienol and lovastatin for anti-osteoporotic therapy. Life Sci. 2015;125:42–48. | ||
Chin K-Y, Abdul-Majeed S, Fozi NFM, Ima-Nirwana S. Annatto tocotrienol improves indices of bone static histomorphometry in osteoporosis due to testosterone deficiency in rats. Nutrients. 2014;6(11):4974–4983. | ||
Makpol S, Durani LW, Chua KH, Mohd Yusof YA, Ngah WZ. Tocotrienol-rich fraction prevents cell cycle arrest and elongates telomere length in senescent human diploid fibroblasts. Biomed Res Int. 2011;2011:11. | ||
Franceschi RT, Iyer BS. Relationship between collagen synthesis and expression of the osteoblast phenotype in MC3T3-E1 cells. J Bone Miner Res. 1992;7(2):235–246. | ||
Nizar A, Nazrun A, Norazlina M, Norliza M, Ima NS. Low dose of tocotrienols protects osteoblasts against oxidative stress. Clin Ter. 2011;162(6):533–538. | ||
Abd Manan N, Mohamed N, Shuid AN. Effects of low-dose versus high-dose γ-tocotrienol on the bone cells exposed to the hydrogen peroxide-induced oxidative stress and apoptosis. Evid Based Complement Alternat Med. 2012;2012:680834. | ||
Xu W, He P, He S, et al. Gamma-tocotrienol stimulates the proliferation, differentiation, and mineralization in osteoblastic MC3T3-E1 cells. J Chem. 2018;2018:9. | ||
Tafazoli S, Wright JS, O’Brien PJ. Prooxidant and antioxidant activity of vitamin E analogues and troglitazone. Chem Res Toxicol. 2005;18(10):1567–1574. | ||
Sudo H, Kodama H-A, Amagai Y, Yamamoto S, Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983;96(1):191–198. | ||
Soeta S, Higuchi M, Yoshimura I, Itoh R, Kimura N, Aamsaki H. Effects of vitamin E on the osteoblast differentiation. J Vet Med Sci. 2010;72(7):951–957. | ||
Chin K-Y, Ima-Nirwana S. Effects of annatto-derived tocotrienol supplementation on osteoporosis induced by testosterone deficiency in rats. Clin Interv Aging. 2014;9:1247–1259. | ||
Edmondson R, Broglie JJ, Adcock AF, Yang L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev Technol. 2014;12(4):207–218. | ||
Shi S, Kirk M, Kahn AJ. The role of type I collagen in the regulation of the osteoblast phenotype. J Bone Miner Res. 1996;11(8):1139–1145. | ||
Beck GR, Sullivan EC, Moran E, Zerler B. Relationship between alkaline phosphatase levels, osteopontin expression, and mineralization in differentiating MC3T3-E1 osteoblasts. J Cell Biochem. 1998;68(2):269–280. | ||
Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 2010;77(1):4–12. | ||
Addison WN, Azari F, Sørensen ES, Kaartinen MT, McKee MD. Pyrophosphate inhibits mineralization of osteoblast cultures by binding to mineral, up-regulating osteopontin, and inhibiting alkaline phosphatase activity. J Biol Chem. 2007;282(21):15872–15883. | ||
Owen TA, Aronow M, Shalhoub V, et al. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143(3):420–430. | ||
Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4(13):3111–3123. | ||
Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003;425(6961):977–980. | ||
Mohamad N-V, Ima-Nirwana S, Chin K-Y. Effect of tocotrienol from Bixa orellana (annatto) on bone microstructure, calcium content, and biomechanical strength in a model of male osteoporosis induced by buserelin. Drug Des Devel Ther. 2018;12:555. | ||
Song BL, DeBose-Boyd RA. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta- and gamma-tocotrienols. J Biol Chem. 2006;281(35):25054–25061. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.