Back to Journals » Drug Design, Development and Therapy » Volume 12
AMPK/AS160 mediates tiliroside derivatives-stimulated GLUT4 translocation in muscle cells
Authors Zhang C, Jiang Y , Liu J, Jin M, Qin N, Chen Y, Niu W, Duan H
Received 2 February 2018
Accepted for publication 10 April 2018
Published 1 June 2018 Volume 2018:12 Pages 1581—1587
DOI https://doi.org/10.2147/DDDT.S164441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Chang Zhang,1 Yue Jiang,1 Jia Liu,1 Meina Jin,1 Nan Qin,1 Ying Chen,1 Wenyan Niu,2 Hongquan Duan1
1School of Pharmacy, Research Center of Basic Medical Science, Tianjin Medical University, Tianjin 300070, People’s Republic of China; 2Department of Immunology, Key Laboratory of Immune Microenvironment and Disease (Ministry of Education), Key Laboratory of Hormones and Development (Ministry of Health), Tianjin Metabolic Diseases Hospital, Tianjin Medical University, Tianjin 300070, People’s Republic of China
Introduction: The Chinese herb Potentilla chinensis can reduce blood glucose level of diabetic mice. Tiliroside is the main effective component, but the detailed mechanism is not clear. Skeletal muscles play an important role in whole body glucose homeostasis. Insulin and exercise/contraction stimulate glucose uptake by muscle cells via redistribution of glucose transporter GLUT4 to the cell surface.
Materials and methods: We explored the effects of tiliroside derivatives on cell surface GLUT4 level (GLUT4 translocation) and the underlying mechanism in L6-GLUT4myc muscle cells.
Results: We showed that tiliroside derivatives D1–22 stimulated GLUT4myc translocation in L6-GLUT4myc skeletal muscle cells. Derivatives D1, D8 and D18 regulated GLUT4myc translocation in a time- and dose-dependent manner. Their effects on GLUT4 were additive with that of acute insulin stimulation. Moreover, they increased phosphorylated adenosine monophosphate-activated protein kinase (AMPK), but not protein kinase B (PKB, also called Akt). Their effects on GLUT4 were inhibited by Compound C. In addition, derivative D8 significantly stimulated AMPK and Akt substrate of 160 kDa (AS160) phosphorylation and GLUT4myc translocation in L6-GLUT4myc cells, but not in L6-AS160 4A-GLUT4myc cells.
Conclusion: Tiliroside derivatives D1, D8 and D18 stimulated GLUT4myc translocation by a mechanism different to that of insulin in skeletal muscle cells. The effect of derivative D8 on GLUT4myc translocation is mediated by AMPK/AS160 signaling pathway.
Keywords: type 2 diabetes, skeletal muscle cells, insulin resistance, AMPK
Introduction
Diabetes is a chronic disease due to impaired glucose homeostasis, that manifests as hyperglycemia. According to the IDF diabetes atlas released by the International Diabetes Federation (IDF) in 2017, there are 425 million individuals with diabetes worldwide and this is estimated to grow to 629 million by 2045.1 The onset of type 2 diabetes is due to the decrease of insulin secretion, the defect of the effect of insulin as well as insulin resistance of the target tissue (fat, muscle and liver).2 Skeletal muscles play an important role in whole body glucose homeostasis. Glucose uptake is mainly mediated by transmembrane protein glucose transporter 4 (GLUT4) in skeletal muscle cells. The amount of glucose uptake is determined by the number of GLUT4 on muscle cell membrane.3,4 The Chinese herb, Potentilla chinensis, has been shown to reduce blood glucose levels of diabetic mice, and tiliroside is the main effective component.5,6 We previously synthesized a series of derivatives, which significantly enhanced glucose uptake in skeletal muscle cells and glucose consumption in insulin resistant (IR) HepG2 liver cells.7–9 However, it is important to determine a compound’s mechanism of action to evaluate its potential usefulness in drug discovery. This study aims to find effective flavonoid derivatives that regulate glucose uptake and illustrate the underlying mechanism in muscle cells. To focus on the role of GLUT4, we used muscle cell lines stably expressing GLUT4myc with an exofacial myc epitope. The effect of these compounds on GLUT4 was investigated by detection of cell surface GLUT4myc levels, via its myc epitope, by the enzyme-linked immunosorbent assay (ELISA).
Materials and methods
Reagents
Horseradish peroxidase (HRP)-bound goat anti-rabbit IgG antibody and donkey anti-mouse IgM antibody were from Jackson Immuno Research Laboratories (West Grove, PA, USA). Minimum Essential Medium Eagle alpha (α-MEM) from HyClone (Beijing, China). Fetal bovine serum (FBS) and trypsin-EDTA were purchased from BioInd (Beit HaEmek, Israel). Immobilon Western Chemiluminescent HRP Substrate was purchased from Millipore (Billerica, MA, USA). o-Phenylenediamine dihydrochloride (OPD), DMSO, orthovanadate, protease inhibitor cocktail, and other chemicals were from Sigma-Aldrich Co. (St Louis, MO, USA). Polyclonal antibodies to p-AS160 (Thr642) antibody were from Cell Signaling Technology (Danvers, MA, USA). Monoclonal IgM to-actinin-1 (clone BM-75.2) was from Sigma-Aldrich Co. β-actin was obtained from Abmart (Shanghai, China). The antibodies to GLUT4, L6-GLUT4myc and L6-AS160 4A-GLUT4myc cell lines were kindly provided by Dr Amira Klip at The Hospital for Sick Children (Toronto, Canada). This research had approval from The Medical Ethics Committee of Tianjin Medical University.
Synthesis of tiliroside derivatives
The tiliroside derivatives were synthesized via three steps including Classin–Schmitt reaction, α-bromination of the unsaturated ketone and etherification reaction, as previously described.8 Briefly, compounds D1–D22 were synthesized through three steps. The first step was a Classin–Schimitt reaction from the substituted benzaldehyde to the α, β-unsaturated keto. Then the hydrogen atom in this keto was substituted by a bromine atom. Finally, the compounds D1–D22 were prepared by the etherification reaction with kaempferol.8 The structures of compounds D1–D22 were determined by 1H, 13C NMR and 2D NMR spectral data analysis, including correlation spectroscopy (COSY), heteronuclear single quantum coherence (HSQC), heteronuclear multiple bond correlation (HMBC), and rotating-frame nuclear Overhauser effect spectroscopy (ROESY).
Cell culture and treatment
Rat L6-GLUT4myc and L6-AS160 4A-GLUT4myc cell lines were as described.10 Briefly, Akt substrate of 160 kDa (AS160) with 4-point mutations (S318A, S588A, T642A, S751A) which could not be phosphorylated was overexpressed into L6-GLUT4myc cells. Myoblasts were cultured and differentiated into myotubes as described.8 Cells were depleted of serum for 4 h prior to all assays. Pre-treatment with Compound C and stimuli were timed so their completion would coincide with the end of the serum deletion period of 4 h. Compound C was administered 30 min prior to treatment with the derivatives. Insulin (100 nM) and 1 mM metformin were used to treat the cells for 30 min.
Measurement of cell surface GLUT4myc density
GLUT4myc levels at the intact cell surface were measured by ELISA as described.11 Briefly, cells were treated as required and fixed with paraformaldehyde (PFA) prior to quenching with 0.1 M glycine in PBS. Cells were incubated with polyclonal anti-myc antibody in 5% v/v goat serum in PBS, then were incubated with HRP-bound secondary antibody. Cells were incubated with OPD for several minutes and the reaction was stopped with 3M HCl. Supernatant absorbance was measured at 492 nm to obtain readings in the linear range. Background absorbance obtained from cells not incubated with anti-myc antibody was subtracted from all values.
Cell lysates and immunoblotting
Myotubes were lysed on ice with 100 μL of radioimmunoprecipitation assay (RIPA) buffer and processed as described.12 Samples were resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Protein phosphorylation and expression were detected with specific antibodies. The protein bands were densitometric quantification by using National Institutes of Health (NIH) ImageJ software.
Statistical analysis
Results were presented as mean±standard error (SE). Statistical analyses were carried out using Prism 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA). Student’s t-test was used to compare pairs of data. Data sets with three or more groups were compared using one-way or two-way analysis of variance with Tukey’s post hoc analysis. Results were considered to be statistically significant if p<0.05.
Results
Tiliroside derivatives synthesis and effects on GLUT4 translocation
Tiliroside (Figure 1A) was previously isolated from Chinese herb P. chinensis. Twenty-two tiliroside derivatives were synthesized by modifying the structure of tiliroside as shown in Figure 1B.
  | Figure 1 Chemical structures of tiliroside (A) and 22 tiliroside derivatives (B). |
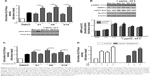
Treatment with 10 μg/mL of tiliroside derivatives for 24 h significantly increased surface GLUT4myc levels in L6-GLUT4myc myotubes. The fold increases above basal levels of D1, D8, D9, D11, D14, D16 and D17–D19 were 3.6±0.3, 3.6±0.2, 2.8±0.4, 3.1±0.3, 3.9±0.3, 4.0±0.5, 2.8±0.4, 3.3±0.6 and 4.4±0.6, respectively (Table 1). Among these derivatives, D1, D8 and D18 had the strongest effects compared to other derivatives. In addition, GLUT4myc translocation in L6-GLUT4myc myotubes responded to derivatives D1, D8 and D18 in a dose- and time-dependent manner (Figure 2A and B). The maximal GLUT4myc translocation stimulated by 10 μg/mL derivatives D1, D8 and D18 for 24 h were 3.3±0.3, 3.7±0.2 and 3.2±0.2, respectively (***p<0.001; Figure 2).
The role of AMPK in the effects of tiliroside derivatives on GLUT4
In order to illustrate the mechanism of the derivatives-regulated GLUT4myc translocation, we detected two well-known signaling pathways which mediated insulin- or contraction-stimulated GLUT4myc translocation, respectively. The results showed that insulin and derivatives D1, D8 and D18 significantly stimulated GLUT4myc translocation 2-fold more than basal untreated cells (Figure 3A). Moreover, the effects of derivatives D1, D8 and D18 on GLUT4myc translocation were additive with insulin (p<0.01, Figure 3A). Importantly, they did not lead to phosphorylation of protein kinase B (Akt) (Figure 3A). These data suggested that the regulatory mechanism of these derivatives on GLUT4 was different with insulin. We next detected the phosphorylation of adenosine monophosphate-activated protein kinase (AMPK). The results showed that derivatives D1, D8 and D18 increased the phosphorylation of AMPK (pAMPK) and its downstream substrate, acetyl-CoA carboxylase (pACC) to a similar degree as metformin (Figure 3B). Derivatives D1, D8 and D18 significantly increased pAMPK by 2.2±0.2, 1.9±0.2 and 1.8±0.3-fold, respectively. The fold increases of pACC by derivatives D1, D8 and D18 were 1.7±0.3, 1.7±0.1 and 1.7±0.3-fold, respectively (p<0.05, p<0.01 vs basal untreated, respectively). These results suggested that AMPK was activated by these derivatives. We next used the AMPK inhibitor, Compound C, to test the role of AMPK in the derivatives-regulated GLUT4myc translocation. Figure 3C showed that Compound C completely reduced the ability of these derivatives to increase cell surface GLUT4myc, implying that AMPK mediated the effect of these derivatives on GLUT4 translocation. As expected, the derivatives reversed insulin resistance induced by high glucose and high insulin (Figure 3D).
The role of AS160 in the effects of tiliroside derivatives on GLUT4
We previously showed that AS160 lies downstream of AMPK to mediate contraction-stimulated GLUT4 translocation in skeletal muscle cells.10–12 AS160 is known as a Rab-GTPase activating protein (GAP). In basal conditions, AS160 has Rab-GAP activity that maintains the Rab substrates in the inactive GDP-bound form, leading to intracellular GLUT4 retention.13 When phosphorylated by AMPK, its Rab-GAP activity becomes inactivated and the inhibition on its Rab substrates is removed; in turn, the activated Rabs regulate GLUT4 storage vesicle mobilization to the cell surface.14,15 Here, we found that AS160 responded to derivative D8 and became phosphorylated in L6-GLUT4myc cells (Figure 4A). To further explore the requirement for AS160 in D8-promoted GLUT4myc translocation, we used L6-GLUT4myc cells overexpressing non-phosphorylatable AS160 4A (L6-AS160 4A-GLUT4myc). D8 could not phosphorylate AS160 in L6-AS160 4A-GLUT4myc myotubes (Figure 4A). Accordingly, D8-stimulated GLUT4myc translocation was reduced in L6-AS160 4A-GLUT4myc cells (2.7±0.3-fold in L6-GLUT4myc myotubes vs 1.7±0.1-fold in L6-AS160 4A-GLUT4myc myotubes, p<0.01, Figure 4B). These data indicated that D8-enhanced GLUT4myc translocation is partly mediated by AS160.
Discussion
Skeletal muscles take up approximately 80% of dietary glucose following a meal. This plays an important role in insulin resistance and type 2 diabetes, which are conditions in which insulin-regulated signal and glucose uptake are impaired. However, other than insulin there are no clinically used treatments that target muscle cell glucose utilization. Exercise/contraction can also enhance glucose uptake via GLUT4 by signaling pathways different to that of insulin. A drug which activates insulin or contraction signaling pathways may potentially relieve insulin resistance. Tiliroside in the Chinese herb P. chinensis can reduce blood glucose level of diabetic mice.5,6 We previously synthesized a small series of tiliroside derivatives and found they promoted GLUT4 translocation to the cell surface.9 We revealed 3-O-[(E)-4-(4-cyanophenyl)-2-oxobut-3-en-1-yl] kaempferol (D3) was the most active compound to increase surface GLUT4myc levels in L6-GLUT4myc and C2C12-GLUT4myc myotubes.9 In order to find more effective compounds to regulate glucose metabolism, we synthesized another set of 3-substituted derivatives of kaempferol with different modifications. In the current study, we investigated their effects on GLUT4 translocation and related signaling pathways.
Upon challenge with insulin or contraction, GLUT4 translocates to and inserts into the cell surface of skeletal muscle myofibers from intracellular storage vesicles to begin transporting glucose into the cells. In this study, overexpressing GLUT4 with a myc epitope in the L6 muscle cell line allows us to score cell surface GLUT4 content which correlates with glucose uptake. Specifically, when GLUT4myc translocates to the cell surface, the myc epitope is exposed to outside of the cell and this can be detected by ELISA, in the intact cells. By measuring GLUT4myc translocation to the cell surface, we focus on GLUT4 without the involvement of other GLUTs, like GLUT1 and GLUT3.
We found that the newly synthesized derivatives more effectively enhance GLUT4myc translocation than our previous reported tiliroside derivatives. We further found that derivatives D1, D8 and D18 stimulated GLUT4myc translocation in a time- and dose-dependent manner. Insulin and contraction are two main stimuli to enhance glucose metabolism in muscle, but they utilize different proximal signaling mechanisms and together, they can have additive effects on the stimulation of glucose uptake. We found the effects of D1, D8 and D18 derivatives tiliroside on GLUT4myc translocation were additive with acute insulin stimulation and had no effect on the key insulin signal molecule, Akt.16 AMPK is the key signal molecule stimulated by muscle contraction. GLUT4 translocation is stimulated by AMPK activition.10 To examine the contribution of AMPK to the gain in surface GLUT4 in derivatives-stimulated myotubes, we inhibited AMPK with its inhibitor, Compound C. We found Compound C completely reduced GLUT4myc translocation stimulated by tiliroside derivatives. This demonstrated that AMPK mediates the effect of tiliroside derivatives on GLUT4 translocation.
Downstream of AMPK, contraction induces phosphorylation of the Rab-GAPs AS160 and TBC1 domain family member 1 (TBC1D1), to regulate insulin- or contraction-stimulated GLUT4 vesicle traffic.10,17 When the key regulatory serine or threonine residues of AS160 and TBC1D1 are phosphorylated by insulin or contraction, their Rab-GAP activity is inhibited and their target Rabs become GTP-loaded to promote GLUT4 translocation.17 We found Compound C inhibited the D8-mediated phosphorylation of AS160 Thr642 (data not shown). This suggests that AS160 may lie downstream of AMPK to mediate derivative D8-regulated GLUT4 traffic. In adipocytes and muscle cells, overexpression of the AS160 mutant AS160 4A, which cannot become phosphorylated, acts in a constitutively active, dominant manner, preventing the phosphorylation of the endogenous AS160 and hence signal transmission in response to insulin.18 A key finding of the present study is that stable expression of the constitutively active AS160 4A mutant in L6-GLUT4myc myotubes effectively prevented the induction of GLUT4myc translocation by the tiliroside derivative, D8. This suggested that AS160 is required for the effect of derivative D8 on GLUT4 translocation. However, the AS160 4A mutant only partly inhibited derivative D8-induced GLUT4myc translocation. It is likely that other signaling molecules such as TBC1D1 may be also involved in this mechanism. We also measured the effect of tiliroside derivatives on the activation of Akt, the kinase utilized by insulin to regulate AS160 phosphorylation and GLUT4 translocation. In fact, D1, D8 or D18 tended to reduce the basal levels of phospho-Akt (Figure 3A). This mechanism requires further investigation, but it could involve down-regulation of mTOR activity through lowering of phosphatidic acid levels by activated AMPK, as recently illustrated for AICAR.19,20 Together, our result demonstrates that phosphorylation/inactivation of the Rab-GAPs is required for signal transmission toward GLUT4 translocation upon derivatives D8 treatment.
In the present study, we also report that derivative D8 could reverse muscle cell insulin resistance-induced by chronic exposure to high glucose and high insulin. This further suggests that derivative D8 stimulated GLUT4 translocation by a different mechanism compared to insulin.
Conclusion
Tiliroside derivatives promote GLUT4 translocation in muscle cells by a mechanism different from insulin. AMPK and AS160 are required for this effect and tiliroside-derivative D8 could reverse insulin resistance. Importantly, contraction-stimulated glucose uptake is not impaired in insulin-resistant states such as obesity and type 2 diabetes.21 Thus, our study may contribute to the drug development for treatments of type 2 diabetes.
Acknowledgments
This work was supported by grant from National Natural Science Foundation of China (number 81373297). H Duan and W Niu were supported by grants from National Natural Science Foundation of China (#81670731, #81170740 and #81161120545), the Tianjin Municipal Science and Technology Commission (#15JCZDJC35500), the Tianjin Health and Family Planning Commission (#15KG102) and the Science & Technology Development Fund of Tianjin Education Commission for Higher Education (#2017KJ212).
Disclosure
The authors report no conflicts of interest in this work.
References
International Diabetes Federation. Q&A: Key points for IDF Diabetes Atlas 2017. Diabetes Res Clin Pract. 2018;135:235–236. | ||
Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: possible roles in Type 2 diabetes. Am J Physiol. 1999;277(1 Pt 1):E1–E10. | ||
Klip A. The many ways to regulate glucose transporter 4. Appl Physiol Nutr Metab. 2009;34(3):481–487. | ||
Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J Appl Physiol. 2005;99(1):330–337. | ||
Zhao C, Qiao W, Zhang YW, Lu B, Duan HQ. 委陵菜抗糖尿病有效部位及有效成分的研究 [Study on anti-diabetes active fraction and constituents from Potentilla chinesis]. Zhongguo Zhong Yao Za Zhi. 2008;33(6):680–682. Chinese. | ||
Ninomiya K, Matsuda H, Kubo M, Morikawa T, Nishida N, Yoshikawa M. Potent anti-obese principle from Rosa canina: structural requirements and mode of action of trans-tiliroside. Bioorg Med Chem Lett. 2007;17(11):3059–3064. | ||
Zhu Y, Zhang Y, Liu Y, Chu H, Duan H. Synthesis and biological activity of trans-tilioside derivatives as potent anti-diabetic agents. Molecules. 2010;15(12):9174–9183. | ||
Qin N, Li CB, Jin MN, Shi LH, Duan HQ, Niu WY. Synthesis and biological activity of novel tiliroside derivants. Eur J Med Chem. 2011;46:5189–5195. | ||
Shi L, Qin N, Hu L, Liu L, Duan H, Niu W. Tiliroside-derivatives enhance GLUT4 translocation via AMPK in muscle cells. Diabetes Res Clin Pract. 2011;92(2):e41–e46. | ||
Li Z, Yue Y, Hu F, et al. Electrical pulse stimulation induces GLUT4 glucose transporter translocation in C2C12 myotubes that depends on Rab8A, Rab13 and Rab14. Am J Physiol Endocrinol Metab. Epub 2017 Oct 31. | ||
Zhao Y, Li N, Li Z, et al. Conditioned medium from contracting skeletal muscle cells reverses insulin resistance and dysfunction of endothelial cells. Metabolism. 2018;82:36–46. | ||
Li Z, Yue Y, Hu F, et al. Electrical pulse stimulation induces GLUT4 glucose transporter translocation in C2C12 myotubes that depends on Rab8A, Rab13 and Rab14. Am J Physiol Endocrinol Metab. Epub 2017 Oct 31. | ||
Niu W, Bilan PJ, Ishikura S, et al. Contraction-related stimuli regulate GLUT4 traffic in C2C12-GLUT4myc skeletal muscle cells. Am J Physiol Endocrinol Metab. 2010;298(5):E1058–E1071. | ||
Mîinea CP, Sano H, Kane S, et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem J. 2005;391(Pt 1):87–93. | ||
Hargett SR, Walker NN, Keller SR. Rab GAPs AS160 and Tbc1d1 play nonredundant roles in the regulation of glucose and energy homeostasis in mice. Am J Physiol Endocrinol Metab. 2016;310(4):E276–E288. | ||
Niu W, Bilan PJ, Hayashi M, Da Y, Yao Z. Insulin sensitivity and inhibition by forskolin, dipyrodamole and pentobarbital of glucose transport in three L6 muscle cell lines. Sci China C-Life Sci. 2007;50(6):739–747. | ||
Taylor EB, An D, Kramer HF, et al. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem. 2008;283(15):9787–9796. | ||
Thong FS, Bilan PJ, Klip A. The Rab GTPase-activating protein AS160 integrates Akt, protein kinase C, and AMP-activated protein kinase signals regulating GLUT4 traffic. Diabetes. 2007;56(2):414–423. | ||
Mukhopadhyay S, Chatterjee A, Kogan D, Patel D, Foster DA. 5-Aminoimidazole-4-carboxamide-1-β-4-ribofuranoside (AICAR) enhances the efficacy of rapamycin in human cancer cells. Cell Cycle. 2015;14(20):3331–3339. | ||
Mukhopadhyay S, Saqcena M, Chatterjee A, Garcia A, Frias MA, Foster DA. Reciprocal regulation of AMP-activated protein kinase and phospholipase D. J Biol Chem. 2015;290(11):6986–6993. | ||
Kawano Y, Rincon J, Soler A, et al. Changes in glucose transport and protein kinase Cbeta(2) in rat skeletal muscle induced by hyperglycaemia. Diabetologia. 1999;42(9):1071–1079. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.