Back to Journals » Drug Design, Development and Therapy » Volume 13
Amine derivatives of furocoumarin induce melanogenesis by activating Akt/GSK-3β/β-catenin signal pathway
Authors Zang D, Niu C, Niu C, Aisa HA
Received 20 July 2018
Accepted for publication 28 November 2018
Published 12 February 2019 Volume 2019:13 Pages 623—632
DOI https://doi.org/10.2147/DDDT.S180960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sukesh Voruganti
Deng Zang,1,2 Chao Niu,1 Haji Akber Aisa1
1Key Laboratory of Plant Resources and Chemistry in Arid Regions, State Key Laboratory Basis of Xinjiang Indigenous Medicinal Plants Resource Utilization, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Urumqi 830011, China; 2University of Chinese Academy of Sciences, Beijing 100049, China
Background: Melanogenesis, or the biosynthesis of melanin, plays a critical role in the pigmentation of skin, hair, and eyes. Reduced melanogenesis may lead to depigmentation conditions such as vitiligo. Psoralen, a furocoumarin derivative, is closely associated with melanogenesis, and its derivative 8-methoxypsoralen is used in psoralen and ultraviolet A therapy for pigmentation disorders. In a previous study, we synthesized a new series of amine derivatives of furocoumarin, of which 5-(morpholinomethyl)-3-phenyl-7H-furo[3,2-g]chromen-7-one (encoded as D206008) showed a remarkable melanogenic effect in B16 murine cells.
Methods: In this study, we examined the effects of D206008 on the melanogenesis-related pathways in B16 cells. D206008 increased melanin production and tyrosinase (TYR) activity, as well as the mRNA and protein expression levels of the melanogenic enzymes TYR, TRP-1 and TRP-2, and the melanogenesis-related transcription factor microphthalmia-associated transcription factor (MITF) in a dose-dependent (0–100 µM) and time-dependent (0–48 hours) manner.
Results: Mechanistically, D206008 inhibited β-catenin degradation by enhancing the phosphorylation of Akt and glycogen synthase kinase-3β (GSK-3β), which increased the accumulation of β-catenin in the cytoplasm. Nuclear translocation of β-catenin also increased in response to D206008 treatment.
Conclusion: Taken together, these data indicate that D206008 promotes melanin synthesis by stimulating the nuclear translocation of β-catenin, which activates MITF transcription and eventually melanogenesis.
Keywords: amine derivatives of furocoumarin, melanogenesis, Akt/GSK-3β/β-catenin
Introduction
Pigmentation of skin, hair, and eyes is regulated by melanin synthesis.1,2 Abnormal pigmentation, either due to dysregulated melanin synthesis or melanocyte development, results in esthetic imperfections. Any impairment that decreases melanin biosynthesis either targets the melanocyte number or function and may lead to depigmentation conditions such as vitiligo.3,4 Melanogenesis occurs in specialized vesicles called the melanosomes within the melanocytes.5,6 Three structurally related enzymes, tyrosinase (TYR) and the tyrosinase-related proteins 1 and 2 (TRP-1 and TRP-2), are involved in melanin synthesis. These proteins are transcriptionally regulated by the microphthalmia-associated transcription factor (MITF), which harbors a basic helix–loop–helix leucine zipper structure7 and is a key regulator of melanocyte development and melanogenesis.8 Several signal transduction pathways are involved in melanogenesis via the regulation of MITF and TYR expression, such as the key Akt/glycogen synthase kinase-3β (GSK-3β)/β-catenin pathway.9 Activation by the Frizzled receptor represses GSK-3β, which leads to the accumulation of β-catenin in cytoplasm10 and eventual nuclear translocation, finally resulting in the transcriptional activation of MITF.11,12 Activation of the protein kinase Akt mediates phosphorylation of GSK-3β, which induces melanin synthesis via upregulation of MITF.
Natural products or plant extracts have been used for centuries for the treatment of pigmentation disorders. Psoralen corylifolia L. is used for repigmentation in traditional Uygur medicine,13–15 and several psoralen compounds that have been isolated from this plant, for instance, 8-methoxypsoralen (8-MOP) and 5-methoxypsoralen (5-MOP),16 are used with ultraviolet radiation in repigmentation therapies.17 Although these compounds have good melanogenesis potential, they have several side effects such as increased risk of skin cancer and hepatic steatosis.18,19 It is important therefore to isolate/synthesize novel plant-derived compounds with better melanogenesis activity and lower toxicity. Recently, we synthesized a new series of amine derivatives of furocoumarin and evaluated their melanogenic effects in B16 murine cells, relative to 8-MOP. One of derivatives 5-(morpholinomethyl)-3-phenyl-7H-furo[3,2-g]chromen-7-one (D206008) (Figure 1) resulted in higher melanogenesis and TYR activity than 8-MOP in the B16 cells without being cytotoxic. The aim of the present study was to investigate the molecular mechanisms underlying the melanogenic activity of D206008.
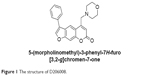  | Figure 1 The structure of D206008. |
Materials and methods
Materials
D206008 was synthesized by The Key Laboratory of Plant Resource Sand Chemistry of Arid Zone, Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences:20 Yield 42%, light yellow solid, m.p. 166°C–167°C; purity for HPLC =98.3%; 1H NMR (400 MHz, CDCl3) δ 8.39 (s, 1H), 7.85 (s, 1H), 7.66 (d, J=7.1 Hz, 2H), 7.57–7.50 (m, 3H), 7.45 (t, J=7.3 Hz, 1H), 6.53 (s, 1H), 3.80–3.73 (m, 4H), 3.71 (s, 2H), 2.63–2.56 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 161.24, 157.18, 152.21, 142.99, 131.22, 129.83, 129.34, 128.23, 127.64, 123.95, 122.42, 116.68, 115.53, 113.81, 100.31, 67.14, 60.15, 53.87; IR (KBr) v: 2,926, 1,725, 1,633, 1,575, 1,455, 1,384, 1,320, 1,263, 1,112, 986, 866 cm−1; HRMS (ESI) calcd for C22H20NO4 [M+H]+ 362.1387, found 362.1390. D206008 was dissolved in DMSO and stored at −20°C as a stock solution (50 mM). L-3-(3,4-dihydroxyphenyl) alanine (L-DOPA) (CAS No 59-92-7) was obtained from Generay Biotech (Shanghai, China), 8-MOP (CAS:298-81-7) was obtained from Sigma Aldrich (Milan, Italy), and Akt inhibitor IV was obtained from EMD Biosciences (La Jolla, CA, USA). 6-Bromoindirubin-3′-oxime (BIO) was purchased from AMQUAR Biology (Shanghai, China). Phosphor-Akt (Thr308, #5106), Akt (#5373), phosphor-GSK-3β (Ser9, #9323), GSK-3β (#9832), phosphor β-catenin (Ser 33,37,41#9561), phospho-β-catenin (Ser675, #4176), β-catenin (#8480), and NUP98 (#2598) were obtained from Cell Signaling Technology (Beverly, MA, USA), and the antibodies against β-actin and tyrosinase (C-19), TRP-1 (H-90), and TRP-2 (H-150) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-MITF was obtained from Chemicon (Temecula, CA, USA). Goat anti-rabbit (BA1054), goat anti-mouse (BA1050), rabbit anti-goat (BA1060), and anti-GADPH were obtained from BOSTER Biological Technology Co Ltd. (Wuhan, China).
B16 cell culture
The B16 murine melanoma cells (Xiangf Bio, Shanghai, China) were maintained in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific), penicillin G (100 U/mL), and streptomycin (100 μg/mL) (Gibco-BRL, Grand Island, NY, USA) and incubated in 5% CO2 at 37°C.
Cell viability assay
Viability of B16 cells was measured by CCK-8 solution (Promega, Madison, MA, USA) assay. After D206008 treatments (0–100 μM), the B16 cells were incubated for 48 hours at 37°C and the cell viability of each treated sample was measured in triplicate using CCK-8 assay kit according to the manufacturer’s instructions. Then culture medium was removed, and 10 μL of CCK solution was added into each well of the 96-well plates. After incubation for 2 hours at 37°C, the absorbance was read at a wavelength of 450 nm using a Spectra Max M5 (Molecular Devices Company, San Diego, CA, USA). The same volume of cells without treatment was used as a blank control. All treatments were performed in triplicate, and all experiments were repeated three times.
TYR activity
The cellular TYR activity proceeded as mentioned before method with slight modifications.9 B16 cells were incubated with various concentrations (0–100 μM) of D206008 in DMEM containing 10% FBS for 24 hours; the cells were washed twice with cold PBS, and then lysed in PBS buffer containing 1% sodium deoxycholate + 1% TritonX-100 solution. Next, 3 μL supernatants of cell extracts were used to measure the total protein content by BCA kit assay; the remaining part was used to measure TYR activity 10 μL, 10 mM l-DOPA and 90 μL test supernatants plated in 96-wellplates, then incubated at 37°C for 30 minutes. The OD of supernatants was measured at 490 nm using a microplate reader.
Melanin content measurement
The melanin release of B16 cells was measured using a previously described method with slight modifications.21 Briefly, the cells were treated with various concentrations of D206008 in DMEM containing 10% FBS for 48 hours, washed with ice-cold PBS twice, and then lysed with RIPA lysis buffer containing 1 M PNPP, 1 M sodium NaF, 10 mM PMSF, 100 mM benzamidine, 100 mM DTT, and 200 mM sodium orthovanadate. Three microliters of supernatants of cell extracts were used to measure the total protein content by BCA kit assay and then dissolved in 1 mM NaOH at 80°C for 1 hour, which supernatants was measured at 405 nm.
Western blot analysis
B16 cells were treated with or without D206008. The protein was prepared from B16 cells according to the method mentioned above. The concentration of protein was measured by BCA assay kit. Proteins per lane were separated by 10% SDS-PAGE. Proteins were transferred to a polyvinylidene fluoride membrane. Membranes were blocked with 5% milk or 5% BSA and incubated overnight at 4°C with appropriate antibodies. And then, the membranes were further incubated for 2 hours with the corresponding secondary antibody. Following incubation, the proteins of membranes were detected using enhanced chemiluminescence (ECL) Western blotting detection reagents and photographed with the ChemiDoc MP Imaging System (Bio-Rad Laboratories Inc., Hercules, CA, USA). All determinations were performed three times.
Primer sequence in quantitative real-time PCR
Total cellular RNA was prepared from B16 cells treated with D206008 and isolated with TRIzol reagent in accordance with the manufacturer’s instructions. Quantitative PCR was performed to determine the expression of target genes. The primers were as follow: forward 5′-GTCGTCACCCTGAAAATCCTAACT-3′ and reverse 5′-CATCGCATAAAACCTGATGGC-3′ for Tyr (111 bp); forward 5′-ACCCATTTGTCTCCCAATGA-3′ and reverse 5′-GTCCAATAGGTGCGTTTTCC-3′ for TRP-1 (130 bp); forward 5′-TACCATCTGTTGTGGCTGGA-3′ and reverse 5′-TGGGTCATCTTGCTG-3′ for TRP-2 (147 bp); forward 5′-AGTACAGGAGCTGGAGATG-3′ and reverse 5′-GTGAGATCCAGAGTTGTCGT-3′ for MITF (181 bp). β-Actin was used as an internal control in all cases, and its primer sequence was as follow: forward 5′-TCAAGATCATTGCTCCTCCTG-3′ and reverse 5′-CTGCTTGCTGATCCA-CATCTG-3′ (59 bp). All determinations were performed three times. The reaction parameters were 95°C for 10 minutes, followed by 40 cycles of 15 seconds at 95°C for melting and 1 minute at 60°C for annealing. The real-time PCR was performed using Applied Biosystems 7300 PCR machine (Applied Bioscience, Foster City, CA, USA). The results were normalized to the controls.
Statistics
All results were expressed as mean ± SD and standard error. Statistical analysis was performed with one-way ANOVA followed by Tukey’s multiple comparison test. Statistical analysis was performed using GraphPad Prism 6 (La Jolla, CA, USA). P-values <0.05 were considered statistically significant.
Results
Effect of D206008 on B16 cell viability
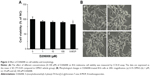
To determine the cytotoxic effects of D206008 on cell viability, B16 melanoma cells were incubated with different concentrations of D206008 (0–100 μM) for 48 hours, and their viability was measured using the CCK-8 assay. As shown in Figure 2, D206008 had no obvious cytotoxic effects on B16 cells.
Effects of D206008 on TYR activity and melanin content in B16 cells
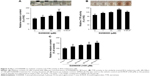
To determine the effects of D206008 on pigmentation, we examined the melanin content of B16 cells treated with different concentrations (0–100 μM) of D206008 for 48 hours, and with 100 μM for varying durations (0–48 hours). As shown in Figure 3, D206008 increased melanin content in B16 cells in a dose-dependent (Figure 3A) and time-dependent manner (Figure 3C). Similarly, the activity of cellular TYR also increased significantly in a concentration-dependent manner following incubation with D206008 (Figure 3B).
Effects of D206008 on the expression level of MITF and TYR family
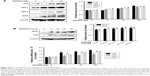
The effect of D206008 on the expression of melanogenic genes was evaluated by real-time PCR and Western blotting. As shown in Figure 4C, the mRNA levels of MITF and its downstream TYR family genes such as TYR, TRP-1, and TRP-2 were increased upon D206008 treatment. The levels of MITF, TYR, TRP-1, and TRP-2 proteins were also significantly increased by D206008 treatment in a dose-dependent (Figure 4A) and time-dependent (Figure 4B) manner in B16 cells. These results indicated that D206008-induced pigmentation occurred via the upregulation of MITF and TYR family genes.
D206008 induces melanogenesis by activating the Akt/GSK-3β/β-catenin pathway
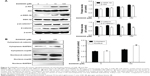
To determine the underlying mechanism of D206008-induced melanogenesis, we investigated the possible role of the Akt/GSK-3β/β-catenin pathway. B16 cells treated with different concentrations of D206008 showed higher levels of p-Akt and p-GSK-3β in a concentration-dependent manner. In contrast, p-β-catenin-ser33 levels decreased and the intracellular β-catenin content increased in a dose-dependent manner (Figure 5A). Furthermore, the nuclear β-catenin content was significantly increased in the D206008-treated cells relative to the untreated cells, while the cytoplasmic protein expression did not change (Figure 5B).
The effect of GSK-3β and Akt inhibitor on D206008-induced melanogenesis
To further confirm the involvement of the Akt/GSK-3β/β-catenin signaling pathway in D206008-induced melanogenesis, the B16 cells treated with D206008 were exposed to either the specific p-GSK-3β inhibitor BIO or Akt inhibitor IV. D206008-induced increase in melanin content and TYR activity were further augmented by BIO and inhibited by Akt inhibitor IV (Figure 6). GSK-3β, which is a negative regulator of Wnt signaling, when GSK-3β was inhibited, could increase β-catenin accumulation in the cytoplasm and promotes β-catenin nuclear translocation, ultimately transcriptionally upregulates MITF and thus activates the melanogenic genes expression. These results clearly indicated that the Akt/GSK-3β/β-catenin signal pathway is involved in D206008-induced melanogenesis (Figure 7).
Discussion
An aberrant decrease in skin melanin biosynthesis may result in depigmentation disorders such as vitiligo,22 which in addition to seriously affecting an individual’s appearance also cause psychological distress and affect quality of life.23 Although a number of melanogenic agents have been developed,24–26 most have serious side effects such as allergies, contact dermatitis, eczema, and cytotoxicity. Therefore, novel, safe, and effective melanogenic drugs have to be developed.
Some plant-derived compounds can promote melanocyte regeneration and have been used in various medicinal systems. Psoralens are furocoumarins isolated from P. corylifolia L, and several natural and synthetic photosensitive derivatives of psoralen have been tested in depigmentation disorders.27 P. corylifolia grows in the high-altitude regions of southern Xin Jiang, and its dried ripe fruits are used to treat vitiligo in Uygur medicine.28 Psoralen compounds isolated from this plant have been used with ultraviolet radiation for the treatment of irregular pigmentation problems such as vitiligo as they activate TYR and promote the synthesis of melanin.29,30 The main psoralen used in psoralen and ultraviolet A is 8-MOP, which unfortunately has secondary gastrointestinal effects and increases the risk of severe complications.31,32 Therefore, it is vital to isolate or synthesize compounds with better activity and low toxicity to treat pigmentation disorders. Pang et al synthetized coumarin derivatives bearing isoxazole moieties as melanogenic stimulator from 5-(bromomethyl) I soxazoles and 4-methylumbelliferone.33 Niu et al synthetized ester coumarin derivatives that were potent on melanin synthesis and TYR stimulations.34 Recently, we synthesized a new series of amine derivatives of furocoumarin compounds and evaluated their melanogenic effects in B16 murine cells. One of derivatives D206008 exhibited better melanogenesis compared to the positive control (8-MOP), in terms of both melanin synthesis and TYR activity, without any cytotoxicity.
Melanin is produced in melanocytes and involves three structurally related enzymes – TYR, TRP-1, and TRP-2 – of which TYR is the main rate-limiting enzyme. It hydroxylates L-tyrosine to L-DOPA and subsequently oxidizes L-DOPA to dopaquinone. TRP-1 and TRP-2 catalyze further oxidation steps in melanin synthesis.35 Therefore, studies have largely focused on enhancing TYR activity to restore melanin synthesis. D206008 promoted melanin synthesis by increasing TYR activity and therefore total melanin content in a dose- and time-dependent manner. Interestingly, even low doses of D206008 (1 μM) had a positive effect on melanogenesis. Furthermore, D206008 treatment significantly increased the expression levels of TYR, TRP-1, and TRP-2. The melanogenic enzymes are regulated by the MITF, a key regulator of melanocyte development and melanogenesis.36,37 To determine the underlying molecular mechanism of the pro-melanogenic effect of D206008, we analyzed the expression of MITF and TYR genes, both of which were significantly upregulated by D206008 in a time- and dose-dependent manner, as was the MITF protein. Taken together, D206008 promotes melanogenesis in B16 melanoma cells by upregulating MITF and its downstream TYR family genes.
The Akt/GSK-3β/β-catenin signaling pathway is closely related to melanocyte development and melanogenesis. Akt phosphorylation and activation lead to the phosphorylation of GSK-3β,38 which prevents degradation of β-catenin and increases its stability. Accumulation of β-catenin in the cytoplasm promotes its nuclear translocation, where it transcriptionally upregulates MITF and thus activates the melanogenic genes.39,40 The key regulator of this pathway is the level of intracellular β-catenin.41 A previous study found that β-catenin regulates melanogenesis, which upregulated MITF trancription by activation of β-catenin signal pathway, then MITF enhance tyrosinase gene expression and promote melanogenesis.42,43 D206008 also enhanced Akt and GSK-3β phosphorylation and decreased that of β-catenin, which increased its accumulation in the cytoplasm of B16 cells. D206008 promoted phosphorylation of Akt and led to increased intracellular p-GSK-3β expression, resulting in the accumulation of β-catenin in the cytoplasm by inhibiting the phosphorylation of β-catenin, which translocated into the nucleus and activated the transcription of melanogenic genes TYR, TRP-1, and TRP-2 via MITF. These results show that D206008 induced melanogenesis via phosphorylation of GSK-3β and Akt, which increased the nuclear translocation of β-catenin and activated MITF. To further confirm the possible involvement of GSK-3β and Akt in melanogenesis, we pretreated the cells with the specific GSK-3β inhibitor BIO and Akt inhibitor IV. Previous research had revealed that BIO makes β-catenin accumulate in cells, which results in an increase in TYR activity and melanin content. The Akt inhibitor reversed the TYR activity and melanin content.44 In our study, BIO increased TYR activity and melanin content in B16 cells treated with D206008; opposite effects were seen with the Akt inhibitor IV. In conclusion, D206008 promotes melanogenesis via the Akt/GSK-3β/β-catenin pathway. Although ester coumarin derivatives synthetized by Niu et al were potent on melanin synthesis and TYR stimulations by upregulation of MITF and TYR family via Akt/GSK-3β/β-catenin signaling pathways, it was low water solubility, which may reduce its effect in vivo. Thus D206008 was designed and synthesized to improve this deficiency since the nitrogen atoms could easily form hydrogen bond in water. Further, our data indicated that D206008 increased melanin production and TYR activity by Akt/GSK-3β/β-catenin signaling pathway, a finding that needs to be supported in animal models of hypopigmentation to comprehensively understand the repigmentation effect of this drug.
Acknowledgments
This work was supported by the funds for the Xinjiang Key Research and Development Program (No 2016B03038-3; No 2016B03038-1) and Foundation of Director of Xinjiang Technical Institute of Physics and Chemistry, Chinese Academy of Sciences (No 2016TP001).
Disclosure
The authors report no conflicts of interest in this work.
References
Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84(4):1155–1228. | ||
Leyden JJ, Shergill B, Micali G, Downie J, Wallo W. Natural options for the management of hyperpigmentation. J Eur Acad Dermatol Venereol. 2011;25(10):1140–1145. | ||
Pillaiyar T, Manickam M, Jung SH. Downregulation of melanogenesis: drug discovery and therapeutic options. Drug Discovery Today. 2017;2(22):282–298. | ||
Fiorito S, Epifano F, Preziuso F, et al. Natural oxyprenylated coumarins are modulators of melanogenesis. Eur J Med Chem. 2018;152(25):274–282. | ||
Tachibana M, Hara Y, Vyas D, et al. Cochlear disorder associated with melanocyte anomaly in mice with a transgenic insertional mutation. Mol Cell Neurosci. 1992;3(5):433–445. | ||
Hodgkinson CA, Moore KJ, Nakayama A, et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74(2):395–404. | ||
Tachibana M. MITF: a stream flowing for pigment cells. Pigment Cell Res. 2000;13(4):230–240. | ||
Levy C, Khaled M, Fisher DE. MITF: master regulator of melanocyte development and melanoma oncogene. Trends Mol Med. 2006;12(9):406–414. | ||
Hwang I, Park JH, Park HS, et al. Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J Dermatol Sci. 2013;72(3):274–283. | ||
Zhu PY, Yin WH, Wang MR, Dang YY, Ye XY. Andrographolide suppresses melanin synthesis through Akt/GSK3β/β-catenin signal pathway. J Dermatol Sci. 2015;79(1):74–83. | ||
Bellei B, Flori E, Izzo E, Maresca V, Picardo M. GSK3beta inhibition promotes melanogenesis in mouse B16 melanoma cells and normal human melanocytes. Cell Signal. 2008;20(10):1750–1761. | ||
Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clin Cancer Res. 2010;16(12):3153–3162. | ||
Wang YF, Liu YN, Xiong W, et al. A UPLC-MS/MS method for in vivo and in vitro pharmacokinetic studies of psoralenoside, isopsoralenoside, psoralen and isopsoralen from Psoralea corylifolia extract. J Ethnopharmacol. 2014;151(1):609–617. | ||
Pei T, Zheng C, Huang C, et al. Systematic understanding the mechanisms of vitiligo pathogenesis and its treatment by Qubaibabuqi formula. J Ethnopharmacol. 2016;190(22):272–287. | ||
Niu C, Pang GX, Li G, et al. Synthesis and biological evaluation of furocoumarin derivatives on melanin synthesis in murine B16 cells for the treatment of vitiligo. Bioorg Med Chem. 2016;24(22):5960–5968. | ||
Jois HS, Manjunath BL, Venkatarao SJ. Chemical examination of the seeds of Psoralea corylifolia. Indian Chem Soc. 1933;10:41. | ||
Späth E, Kainrath P. Über Bergamottin und über die Auffindung von Limettin im Bergamottöl (XXXIV). Mitteil. über natürliche Cumarine. Ber Deutsch Chem Ges. 1937;70(11):2272–2276. | ||
Verma SB, Wollina U. Accidental PUVA burns, vitiligo and atopic diathesis resulting in prurigo nodularis: a logical but undocumented rarity. An Bras Dermatol. 2012;87(6):891–893. | ||
Li H, Min YS, Park KC, Kim DS. Inhibition of melanogenesis by Xanthium strumarium L. Biosci Biotechnol Biochem. 2012;76(4):767–771. | ||
Niu C, Zang D, Aisa HA. Design, synthesis and biological activity of novel furocoumarin derivatives as stimulators of melanogenesis and tyrosinase in B16 cells. Chem Res Chin Univ. 2018;34(3):408–414. | ||
Kim HJ, Kim JS, Woo JT, Lee IS, Cha BY. Hyperpigmentation mechanism of methyl 3,5-di-caffeoylquinate through activation of p38 and MITF induction of tyrosinase. Acta Biochim Biophys Sin. 2015;47(7):548–556. | ||
Patel S, Rauf A, Khan H, Meher BR, Hassan SSU. A holistic review on the autoimmune disease vitiligo with emphasis on the causal factors. Biomed Pharmacother. 2017;92:501–508. | ||
Doppalapudi S, Mahira S, Khan W. Development and in vitro assessment of psoralen and resveratrol co-loaded ultradeformable liposomes for the treatment of vitiligo. J Photochem Photobiol B. 2017;174:44–57. | ||
Goh BK, Pandya AG. Presentations, signs of activity, and differential diagnosis of vitiligo. Dermatol Clin. 2017;35(2):135–144. | ||
Speeckaert R, Speeckaert MM, van Geel N. Why treatments do(n’t) work in vitiligo: an autoinflammatory perspective. Autoimmun Rev. 2015;14(4):332–340. | ||
Frisoli ML, Harris JE, Michael L, Frisoli BS, John E, Harris MD. Vitiligo: mechanistic insights lead to novel treatments. J Allergy Clin Immunol. 2017;140(3):654–662. | ||
Abdel Naser MB, Wollina U, El Okby M, El Shiemy S. Psoralen plus ultraviolet A irradiation-induced lentigines arising in vitiligo: involvement of vitiliginous and normal appearing skin. Clin Exp Dermatol. 2004;29(4):380–382. | ||
Niu C, Yin L, Nie LF, et al. Synthesis and bioactivity of novel isoxazole chalcone derivatives on tyrosinase and melanin synthesis in murine B16 cells for the treatment of vitiligo. Bioorg Med Chem. 2016;24(21):5440–5448. | ||
Yang YF, Zhang YB, Chen ZJ, Zhang YT, Yang XW. Plasma pharmacokinetics and cerebral nuclei distribution of major constituents of Psoraleae fructus in rats after oral administration. Phytomedicine. 2018;38(1):166–174. | ||
Feng L, Wang L, Jiang X. Pharmacokinetics, tissue distribution and excretion of coumarin components from Psoralea corylifolia L. in rats. Arch Pharm Res. 2010;33(2):225–230. | ||
Herr H, Cho HJ, Yu S. Burns caused by accidental overdose of photochemotherapy (PUVA). Burns. 2007;33(3):372–375. | ||
Kassem AA, Abd El-Alim SH, Asfour MH. Enhancement of 8-methoxypsoralen topical delivery via nanosized niosomal vesicles: formulation development, in vitro and in vivo evaluation of skin deposition. Int J Pharm. 2017;517(1–2):256–268. | ||
Pang GX, Niu C, Mamat N, Aisa HA. Synthesis and in vitro biological evaluation of novel coumarin derivatives containing isoxazole moieties on melanin synthesis in B16 cells and inhibition on bacteria. Bioorg Med Chem Lett. 2017;27(12):2674–2677. | ||
Niu C, Yin L, Aisa H. Novel furocoumarin derivatives stimulate melanogenesis in b16 melanoma cells by up-regulation of MITF and TYR family via Akt/GSK3β/β-catenin signaling pathways. Int J Mol Sci. 2018;19(3):746–765. | ||
Hearing VJ. Biochemical control of melanogenesis and melanosomal organization. J Investig Dermatol Symp Proc. 1999;4(1):24–28. | ||
Kim ES, Jeon HB, Lim H, et al. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells inhibits melanogenesis by promoting proteasomal degradation of MITF. PLoS One. 2015;10(5):e0128078. | ||
Hearing VJ, Tsukamoto K. Enzymatic control of pigmentation in mammals. FASEB J. 1991;5(14):2902–2909. | ||
Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29(2):95–102. | ||
Hart MJ, de Los Santos R, Albert IN, Rubinfeld B, Polakis P. Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta. Curr Biol. 1998;8(10):573–581. | ||
Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275(5307):1790–1792. | ||
Amit S, Hatzubai A, Birman Y, et al. Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev. 2002;16(9):1066–1076. | ||
Helene CE, Arturo C. Synthesis and assembly of fungal melanin. Microbiol Biotechnol. 2012;93(3):931–940. | ||
Regazzetti C, Joly F, Marty C, et al. Transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: a promising target for repigmenting vitiligo patients. J Invest Dermatol. 2015;135(12):3105–3114. | ||
Huang YC, Yang CH, Chiou YL. Citrus flavanone naringenin enhances melanogenesis through the activation of Wnt/β-catenin signalling in mouse melanoma cells. Phytomedicine. 2011;18(14):1244–1249. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.