Back to Journals » Drug Design, Development and Therapy » Volume 13
A novel hybrid compound LLP2A-alendronate accelerates open fracture healing in a rabbit model
Authors Wang Z, Zhao Y, Zhang D, Qi B, Xiao W, Hu X, Yu A
Received 25 November 2018
Accepted for publication 30 January 2019
Published 5 April 2019 Volume 2019:13 Pages 1077—1086
DOI https://doi.org/10.2147/DDDT.S195937
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Zheng Wang, Yong Zhao, Dong Zhang, Baiwen Qi, Weidong Xiao, Xiang Hu, Aixi Yu
Department of Orthopaedic Trauma and Microsurgery, Zhongnan Hospital of Wuhan University, Wuhan, Hubei 430071, People’s Republic of China
Purpose: LLP2A-alendronate (LLP2A-Ale) is a novel bone-seeking compound that recruits mesenchymal stem cells to the bone surface and stimulates bone formation. The purpose of this study was to investigate the efficacy of LLP2A-Ale in the treatment of rabbit open fracture.
Methods: Thirty New Zealand White rabbits underwent radius mid-diaphyseal osteotomy and were randomly divided into control and treatment groups with fifteen rabbits in each group. The treatment group received only one injection of LLP2A-Ale (dosage 125 µg/kg), whereas the control group received one injection of PBS. X-ray images were taken to observe the course of fracture healing at 2, 4 and 6 weeks after treatment. Rabbits were sacrificed at 4 and 6 weeks post treatment. Calluses were then harvested and were subjected to histology, immunohistochemistry, molecular biology techniques and biomechanical test.
Results: X-ray images showed that the LLP2A-Ale group exhibited abundant callus formation, stronger bony callus remodeling and earlier marrow cavity recanalization compared to the control group in a time-dependent manner. Histomorphological analysis revealed an advance in woven formation at 4 weeks and lamellar bone formation at 6 weeks in the LLP2A-Ale group. Moreover, gene and protein levels suggested that LLP2A-Ale promoted osteogenesis and angiogenesis probably via upregulating the expression of osteogenesis factors (including bone morphogenetic protein 2 and Runt-related transcription factor 2) and angiogenesis factors (vascular endothelial growth factor). Besides, the radius callus biomechanical properties were significantly enhanced in the LLP2A-Ale group compared with the control group at 6 weeks.
Conclusion: LLP2A-Ale can significantly promote open fracture healing in the rabbit model, probably through enhancing osteogenesis and angiogenesis.
Keywords: osteogenesis drug, fracture healing, angiogenesis, osteogenesis, rabbit fracture model
Corrigendum for this paper has been published
Introduction
Despite the development of modern orthopedics, complications during the course of fracture healing remain a common condition and additional surgical interventions are required to figure out these problems. Open fractures are extremely vulnerable to delayed union and even nonunion compared to closed fractures, with nonunion reported to occur in 10.9% of all fractures.1 Although open fractures have such a high incidence and severe clinical consequences, their available drug treatment is very limited, with only local infusions of recombinant human bone morphogenetic protein (rhBMP) 2 and 7 being used to treat open tibial shaft fractures.2 However, these treatments remain controversial because several side effects have been associated with rhBMP including ectopic bone formation, tumorigenesis and the development of antibodies against rhBMP.3–6 Hence, it is still imperative to discover novel available drug therapies to accelerate open fracture healing so that complications in the course of healing could be avoided as much as possible.
Regeneration of bone is believed to be mediated via osteoprogenitors and their ancestors, mesenchymal stem cells (MSCs).7 Previous experiments with animals and humans have demonstrated that treatment with autologous MSC transplantation could induce the regeneration of bone fracture.8,9 At the same time, there is also sufficient evidence indicating that systemic transplantation of MSCs in vivo has failed to improve osteogenesis in bone fracture due to the inability of MSCs to home to the fracture gap unless they are genetically modified.10–12 In order to solve this dispute, we tried to synthetize a novel chemical compound which could directly enhance autologous MSCs migrating to the fracture gap instead of any cell purification or gene modification.
The peptidomimetic LLP2A, discovered by one-bead–one compound library screening, shows extraordinarily high affinity and selectivity to the α4β1 integrin receptor.13 For example, previous research has shown that LLP2A binds specifically to α4β1-expressing leukemia and lymphoma cells and can be used as a targeted therapeutic carrier.14 Integrin α4β1 is also highly expressed on the MSC surface and inducing α4β1 signal transduction could enhance MSC proliferation and osteogenic differentiation.15–17 However, single LLP2A ligands have no affinity to bone and could not target MSCs to the surface of bone.13 Thus, we conjugated LLP2A to alendronate (Ale), a kind of bisphosphonate with high affinity for bone, which served as a bone-seeking component to direct both the cells and the compound to bone. Ale has been reported as a widely used drug to treat osteoporosis by inhibiting osteoclastic activity.18 In our combination, we used roughly one-tenth of the therapeutic dose of Ale to avoid the anti-resorptive impacts in the course of treatment. We hypothesized coupling LLP2A and Ale so that LLP2A-Ale could target both bone and MSCs, with Ale functioning as the bone-seeking component to direct LLP2A to bone, and LLP2A directing MSCs to the bone surface to stimulate bone formation without affecting bone resorption.
Our previous experiments in mice have demonstrated that LLP2A-Ale was able to increase homing of the transplanted MSCs to the fracture site, which consequently accelerated closed fracture healing. In this study, rabbit (which undergoes Haversian bone remodeling similar to humans)19 fracture models were chosen to test the efficacy of LLP2A-Ale in accelerating bone healing at histological, molecular and biomechanical levels.
Materials and methods
Ethics statement
Treatment of the animals was carried out in strict accordance with the recommendations described in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The experimental protocol was approved by the Committee on the Ethics of Animal Experiments of Wuhan University, Wuhan, People’s Republic of China. All surgical procedures were performed under pentobarbital sodium anesthesia, and efforts were made to minimize animal suffering.
Establishment of an open rabbit fracture model and grouping
Thirty New Zealand White rabbits (age 5–8 months, weighing 2.0–2.5 kg, half males and half females) were obtained from the Experimental Animal Central Research Laboratory at Wuhan University. After adaptive feeding for 1 week in a room with good light, a temperature between 23 and 30°C and relative humidity of 45%, an open fracture with periosteal stripping was created in the radius of the rabbits. Some modifications have been made based on previous reports.20 Sodium pentobarbital (1%, 3 mL/kg) was used for intravenous anesthesia. After cutting the skin hair on the left forearm, a 2.0-cm longitudinal incision was cut in the lower end of the right radius and the periosteum was stripped circumferentially 15 mm proximal and distal to the planned osteotomy. The periosteum and radius were sawed with a sterilized wire saw to make a 3-mm bone defect. The cut was sutured but not fixed. Success of the fractures was confirmed by X-ray imaging. For the next 3 days after model establishment, 0.5 million U/day penicillin was injected daily. Each rabbit was given normal feed in a single cage and no rabbits died during the modeling process. All rabbits were randomly divided into two groups: the LLP2A-Ale group (125 μg/kg at day 1) and the control group (PBS) (each n=15).
X-ray imaging examination
At 2, 4, and 6 weeks after treatment, an X-ray examination was performed to observe the fracture healing process in both groups. A digital medical X-ray photography system (Digital Diagnose Pro; Philips Inc., Eindhoven, the Netherlands) was used for scanning. Rabbits were anesthetized before the procedure. The bone healing progress and periosteal callus formation were analyzed by three individual blinded observers, to radiologically determine fracture union.
Serum biochemical analysis
Blood samples were collected from the central ear artery 4 weeks after the injection of LLP2A-Ale or PBS in each group (n=3). The serum was promptly separated and stored at −80°C prior to the assay. The serum concentrations of plasma osteocalcin (OCN, ng/mL, a crucial bone formation marker), bone alkaline phosphatase (BALP, pg/mL, another crucial bone formation marker) and tartrate-resistant acid phosphatase (TRACP, ng/mL, a significant bone resorption marker) were measured using commercial ELISA kits (Bio-Swamp Inc., Wuhan, People’s Republic of China) for the rabbits. All samples were run in the same assay unless an individual value required repeating.
Histology
Three 0.5-cm specimens were obtained from two sides of the fracture line in each group at 4 and 6 weeks respectively post treatment, fixed in PLP fixative (2% paraformaldehyde containing 0.075 mol/L lysine and 0.01 mol/L sodium periodate) overnight at 4°C and decalcified in EDTA–glycerol solution for 3–5 weeks at 4°C. Then, decalcified specimens were dehydrated, paraffin-embedded and sectioned (5 μm) by a rotary microtome. The sections were stained with H&E or Safranin O/light green, or immunohistochemically, as described below. (H&E staining was used for basic tissue morphological analysis, while Safranin O/light green stained cartilaginous callus.)
Immunohistochemistry
To assess blood vessel formation of the callus, vascular endothelial growth factor (VEGF) immunohistochemistry was performed 4 weeks after injection. Additional slices were firstly deparaffinized, dehydrated, rinsed and incubated with 3% hydrogen peroxide for 20 min. Trypsin-induced epitope retrieval was performed for 20 min at room temperature. All sections were then blocked with 0.1% BSA in PBS for 1 h. Thereafter, the callus sections described above were incubated with primary antibodies (anti-VEGF; Abcam, Cambridge, UK) overnight at 4°C and then incubated with horseradish peroxidase-coupled secondary antibodies (Aspen, Guangzhou, People’s Republic of China).
Real-time quantitative PCR
Total RNA was prepared at 4 weeks per group after treatment and quantitative reverse transcription (qRT)-PCR was carried out as previously described.21 Before RNA extraction, samples (n=3 for each group) were immediately crushed into powder in a mortar containing liquid nitrogen using the pestle and then mixed with the monophasic solution of phenol and guanidine thiocyanate. Total RNA was extracted using Trizol (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. Then, the PrimeScript II First Strand cDNA Synthesis Kit was used to synthesize cDNA from RNA (Takara Bio, Japan). Real-time fluorescence quantitative PCR was performed on an ABI 7300 Real-Time PCR system using the SYBR Premix Ex Taq (Takara Bio, Shiga, Japan). The primers were synthesized by Genecreate Biotechnology Inc. (Wuhan, People’s Republic of China). All mRNA levels were normalized by the housekeeping gene β-actin. The relative quantity of mRNA was calculated using the 2−ΔΔCt method. The primers used for qRT-PCR are listed in Table 1.
Western blot analysis
Total protein was extracted from three callus tissues harvested at 4 weeks per group and quantified using a commercially available kit (Aspen). Immunoblotting was carried out as described previously.22 Equal amounts of proteins from specimen lysates were loaded onto a 5% SDS polyacrylamide gel (Aspen) and transferred to a polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5% BSA in TBS and then incubated with primary antibodies against VEGF (1:2,000), Runt-related transcription factor 2 (Runx2, 1:1,000), BMP-2 (1:1,000), TRACP (1:1,000) and β-actin (loading control, 1:10,000) purchased from Abcam. Bands were visualized using an enhanced chemiluminescence kit (Aspen). Quantification of the protein bands was performed with AlphaEaseFC software (Alpha Inc., San Leandro, CA, USA).
Biomechanical bone strength measurements
A three-point-bending test was performed to assess the biomechanical strength of the fracture calluses using a mechanical testing machine. Three right fractured radii were collected after sacrifice at 6 weeks per group, cleaned for soft tissue, wrapped in saline-moistened gaze and stored at −20°C until testing. The specimens were placed with the anterior surface facing upward and the proximal and distal ends were fixed to two in-house support pins (span =9 mm) to ensure sample stability during the procedure. The anterior surface of the middle part of the callus was subjected to vertical compression at a constant velocity (1 mm/min) until the callus ruptured. Sample loads and displacements were continuously recorded throughout each test. Maximum load, flexure strength, load displacement and elasticity modulus were determined from the load-displacement curve.23
Statistical analysis
The data were presented as the mean±SD and analyzed with SPSS 20.0 (IBM Corporation, Armonk, NY, USA). For comparison of two groups, a two-tailed t-test was used. P<0.05 was considered statistically significant.
Results
Radiographic union grading
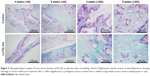
Representative X-ray images of the course of bone healing 2, 4 and 6 weeks after treatment are presented in Figure 1. Radiographs showed that the fracture line was still visible in all groups at 2 weeks. However, the LLP2A-Ale group had evident external bone callus and apparent connection of fracture lines. By contrast, the control group revealed slight periosteal reaction and the fracture line was still clear. At 4 weeks after treatment, there is bigger external bone callus and blurry fracture lines in the LLP2A-Ale group, while the control group exhibited a smaller callus and still clear fracture lines. At 6 weeks, the bone calluses in the LLP2A-Ale group started to remold and almost achieved bony union. The performances on the X-ray film were the callus resorption, marrow cavity recanalization and invisible fracture line. But, in comparison, the bone defect area in the control group grew slowly with a more obvious external bone callus than before and an obstructed marrow cavity. In addition, no case showed union.
H&E staining
H&E-stained sections of the fracture callus illustrate the entire process of endochondrostosis around the fracture callus (Figure 2). At 4 weeks post treatment, the calluses of the treatment group contained large amounts of newly formed woven bone around the fractured radius as an external callus. Meanwhile, in the control group, a large amount of fibrocartilage and little woven bone were predominant in the calluses, which was in the conversion stage of a primary callus between fibrocartilage formation (soft callus) and bone formation (hard callus).
At 6 weeks after treatment, the calluses in the LLP2A-Ale group showed that the majority of woven bones had calcified and been remodeled into lamellar bones. The remaining collagen fibers in the lamellar bone were disorganized, suggesting that the calluses in the LLP2A-Ale group had undergoing bone remodeling. However, there was a typical appearance of lamellar bone accompanying a small amount of woven bones in the calluses of the control group, which implied that the phase of callus remodeling in this group was delayed.
Safranin O/light green staining
At 4 weeks, the quantity of woven bone in the LLP2A-Ale group was greater than that in the control group. In the LLP2A-Ale group, fibrous callus had become almost completely calcified into woven bone, leaving only a few red-stained cells remaining within the trabeculae. At 6 weeks, primary lamellar bone was observed in the calluses of the LLP2A-Ale group, and trabeculae and collagen fibers had both become oriented parallel to the longitudinal direction of the radius. At that time point, little lamellar bone was observed in the control group, suggesting that callus remodeling seemed to be delayed (Figure 3).
Immunohistochemistry
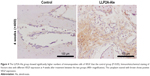
To determine the effect of LLP2A-Ale on angiogenesis during fracture healing, VEGF (a marker for angiogenesis) was examined in callus tissues at 4 weeks by immunohistochemistry. The cytoplasm stained brown was VEGF expression positive, while the cytoplasm without brown color meant that VEGF expression was negative (Figure 4). At 4 weeks after treatment, the LLP2A-Ale group had higher expression of VEGF than the control group (P<0.05).
Serum biochemical parameters
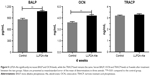
The concentrations of serum markers reflecting bone turnover are shown in Figure 5. After 4 weeks of treatment, rabbits in the LLP2A-Ale group exhibited significantly higher serum OCN(+) and BALP(+) levels than those in the control group (P<0.05), indicating the induction of high bone turnover following the injection of LLP2A-Ale. However, no significant difference in serum TRACP concentrations was observed among the control and LLP2A-Ale groups (P>0.05). This result implies that LLP2A-Ale does not alter bone resorption during fracture healing.
Real-time PCR and western blot examination
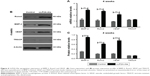
These assay results are shown in Figure 6. Rabbits in the LLP2A-Ale group exhibited significant increases in osteoblastic bone formation-related gene expression and proteins compared to the control group, including BMP2 and Runx2 (P<0.05). Apart from the result observed above, we also detected that the gene expression and proteins of VEGF, an important angiogenesis regulator, were significantly increased (P<0.05). This revealed that LLP2A-Ale could also effectively improve the impaired vascular invasion as well as prominently promote osteogenesis in the callus during fracture healing. Besides, in order to confirm whether LLP2A-Ale has an influence in bone resorption during fracture healing, we examined TRACP at genetic and protein levels and found there was no difference between the two groups (P>0.05).
Biomechanical testing
Biomechanical testing is the final method to judge a treatment’s effect on bone healing. Maximum load, flexure strength, load displacement and elastic modulus, which represent the intrinsic quality of bone structure, were increased in the LLP2A-Ale groups at 6 weeks, with increases of 59.31%, 58.24%, 32.66% and 24.79% separately compared to the control group fracture calluses (P<0.05) (Table 2). These results demonstrate that LLP2A-Ale treatment enhances the strength of the callus compared to PBS controls.
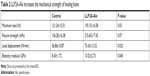  | Table 2 LLP2A-Ale increases the mechanical strength of healing bone |
Discussion
In this study, we have examined the efficacy of LLP2A-Ale in a fracture model with poor bone healing. Our research group has previously reported that engraftment efficacy can be increased via the “bone-targeting” agent LLP2A-Ale, which improves the homing of transplanted MSCs to the fracture callus, using a mouse model with closed fracture.24 However, that study was performed in mice, which have limited intracortical Haversian remodeling during the course of fracture healing.19 Therefore, it was of interest to investigate the effects of LLP2A-Ale on improving bone healing using a larger animal model with a cortical bone remodeling pattern more similar to human cortical bone.
Additionally, the present study showed for the first time that administration of LLP2A-Ale could accelerate bone healing through enhancing osteogenesis and angiogenesis in the rabbit open fracture model. Compared to the closed fracture, an open fracture often suffers from higher energy damage and is particularly vulnerable to delayed union and nonunion. The periosteal stripping leads to impaired bone healing, and aptly recapitulates the conditions associated with a major orthopedic injury. Although the Einhorn closed fracture model is often used to screen interventions, the osteotomy model used in this study was selected to better represent a challenging clinical scenario. Some therapeutic reagents that improve healing in closed fractures have been subsequently found to be ineffective in this open fracture model, for example parathyroid hormone (1–34).20
Through the X-ray quantitative analysis of the fracture line and external callus variation, our study found that LLP2A-Ale could lead to disappearance of the fracture line and the healing of bony callus. These results indicated that the formation of external callus was accelerated via LLP2A-Ale, so it could shorten the healing process.
Our study also manifests that single administration of LLP2A-Ale more potently enhanced woven bone formation and callus remodeling in histology. The large amount of newly formed woven bone observed during the early stage may indicate that LLP2A-Ale augmented more MSCs to the bone surface and stimulated bone formation.
Furthermore, we observed significant motivation of osteoblast activity and bone formation in the course of fracture healing upon the administration of LLP2A-Ale, as evidenced by increased serum OCN and BALP, osteoblastic bone formation-related gene expression and proteins (including Runx2, BMP-2) in callus tissues.
The present research pointed out that serum BALP and OCN levels in the treatment groups significantly increased at 4 weeks after treatment. The possible explanation for this could be that BALP and OCN were produced by osteoblasts and could be rich in cytoplasm. The secreted BALP and OCN was infiltrated into the blood, contributing to elevated serum BALP and OCN levels, indicating the increase in bone regeneration and osteoblast activity.
As one of the most important regulatory factors for bone formation, BMP-2 has been proved to have strong ability in promoting bone cell differentiation and inducing bone formation in vitro.25,26 Runx2 expression and activation is considered another crucial point incorporating many signals that regulate osteoblast differentiation.27 High expression of BMP-2 and Runx2 in bone marrow stromal cells could promote bone marrow stem cells to differentiate into osteoblasts, thus promoting the formation of bone.27,28
Angiogenesis is an important stage of bone healing as it provides nutrition and various essential osteoblast components to the catagmatic area. Blood vessels are conducive to osteogenesis, both in bone development and bone repair,29 since an adequate bone tissue blood supply is able to provide various essential cells and cytokines required for fracture repair. VEGF is an essential angiogenesis factor for bone formation during bone healing. In the present research, the results showed that, compared to the control group, gene expression and protein levels of VEGF were higher in the treatment group. Besides, immunohistochemistry showed that VEGF in calluses of the treatment group was significantly higher than that in the control group. Taken together, these results revealed that LLP2A-Ale is a positive regulator of angiogenesis and could enhance bone healing via angiogenesis during rabbit fracture healing.
There is a concern that Ale may delay fracture healing.30 In our study, Ale was used as a bone-targeted delivery agent in a conjugated compound (LLP2A-Ale) to increase homing of endogenous or transplanted stem cells to bone as Ale has very high affinity for hydroxyapatite. Our findings revealed no obvious alteration in serum TRACP in the treatment group compared with those in the control group. On the other hand, gene expression and protein levels of TRACP demonstrated that LLP2A-Ale could not influence bone resorption during fracture healing. These observations indicate that no antiresorptive effects were caused by the administration of LLP2A-Ale.
The most essential test of fracture healing is to determine whether one intervention restores bone strength. In the present study, we have analyzed maximum load, flexure strength and load displacement of bone at 6 weeks after treatment. We have indeed observed that the above indicators were much higher in the LLP2A-Ale treatment group compared to the control group. Compared to the treatment group, the elastic modulus of the control group decreased by about 24.79%, which is mainly related to the fact that the fracture site of the control group had more woven bone and fiber connective tissue, which improved the adaptability and ductility of the tissue and reduced the elastic modulus of the callus.
In conclusion, the present study demonstrates that LLP2A-Ale promoted open fracture healing in the rabbit model, which undergoes Haversian bone remodeling similar to that in humans. Single injection of LLP2A-Ale is able to accelerate bone healing through enhancing osteogenesis and angiogenesis without influencing bone resorption in the open rabbit fracture model. Thus, this study not only enriches our basic knowledge for understanding rabbit fracture healing in response to a novel bone-seeking compound of LLP2A conjugated with Ale but also offers new treatment alternatives for osteopenia/osteoporosis and osseous defects in open fracture patients. In our next experiment, LLP2A-Ale will be tested in a rabbit model of both osteoporosis and bone nonunion.
Acknowledgment
We thank Prof Kit S Lam and Prof Yu Aiming (Department of Biochemistry and Molecular Medicine, University of California at Davis Medical Center) for providing LLP2A-Ale. The work was funded by Wuhan Clinical Medical Research Center of Microsurgery.
Disclosure
The authors report no conflicts of interests in this work. Dr Dong Zhang, Dr Baiwen Qi, Dr Weidong Xiao, Dr Xiang Hu, and Dr Aixi Yu report grants from Wuhan Clinical Medical Research Center of Microsurgery during the conduct of the study.
References
Zura R, Xiong Z, Einhorn T, et al. Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. 2016;151(11):e162775. doi:10.1001/jamasurg.2016.2775 | ||
McKay WF, Peckham SM, Badura JM. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop. 2007;31(6):729–734. doi:10.1007/s00264-007-0418-6 | ||
Spiro AS, Beil FT, Baranowsky A, et al. BMP-7-induced ectopic bone formation and fracture healing is impaired by systemic NSAID application in C57BL/6-mice. J Orthop Res. 2010;28(6):785–791. doi:10.1002/jor.21044 | ||
Spiro AS, Beil FT, Schinke T, et al. Short-term application of dexamethasone enhances bone morphogenetic protein-7-induced ectopic bone formation in vivo. J Trauma. 2010;69(6):1473–1480. doi:10.1097/TA.0b013e3181dc59e4 | ||
Mines D, Gu Y, Kou TD, Cooper GS. Recombinant human bone morphogenetic protein-2 and pancreatic cancer: a retrospective cohort study. Pharmacoepidemiol Drug Saf. 2011;20(2):111–118. doi:10.1002/pds.2057 | ||
Katayama Y, Matsuyama Y, Yoshihara H, et al. Clinical and radiographic outcomes of posterolateral lumbar spine fusion in humans using recombinant human bone morphogenetic protein-2: an average five-year follow-up study. Int Orthop. 2009;33(4):1061–1067. doi:10.1007/s00264-008-0600-5 | ||
Jones E, Yang X. Mesenchymal stem cells and bone regeneration: current status. Injury. 2011;42(6):562–568. doi:10.1016/j.injury.2011.03.030 | ||
Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16(2):155–162. doi:10.1002/jor.1100160202 | ||
Livingston TL, Gordon S, Archambault M, et al. Mesenchymal stem cells combined with biphasic calcium phosphate ceramics promote bone regeneration. J Mater Sci Mater Med. 2003;14(3):211–218. | ||
Gutwald R, Haberstroh J, Kuschnierz J, et al. Mesenchymal stem cells and inorganic bovine bone mineral in sinus augmentation: comparison with augmentation by autologous bone in adult sheep. Br J Oral Maxillofac Surg. 2010;48(4):285–290. doi:10.1016/j.bjoms.2009.06.226 | ||
Vertenten G, Lippens E, Girones J, et al. Evaluation of an injectable, photopolymerizable, and three-dimensional scaffold based on methacrylate-endcapped poly (D,L-lactide-co-epsilon-caprolactone) combined with autologous mesenchymal stem cells in a goat tibial unicortical defect model. Tissue Eng Part A. 2009;15(7):1501–1511. doi:10.1089/ten.tea.2008.0367 | ||
Longobardi L, Granero-Molto F, O’Rear L, et al. Subcellular localization of IRS-1 in IGF-I-mediated chondrogenic proliferation, differentiation and hypertrophy of bone marrow mesenchymal stem cells. Growth Factors. 2009;27(5):309–320. doi:10.1080/08977190903138874 | ||
Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against α4β1 integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2(7):381–389. doi:10.1038/nchembio798 | ||
Zwingenberger AL, Kent MS, Shi C, Taylor SL, Chen X, Lam KS. Affinity of the alpha4-beta1 integrin-targeting peptide LLP2A to canine lymphoma. Vet Immunol Immunopathol. 2012;145(1–2):298–304. doi:10.1016/j.vetimm.2011.11.018 | ||
Herberg S, Hill WD. Two birds with one bone? IBMS Bonekey. 2012;9(9):115. doi:10.1038/bonekey.2012.115 | ||
Brooke G, Tong H, Levesque JP, Atkinson K. Molecular trafficking mechanisms of multipotent mesenchymal stem cells derived from human bone marrow and placenta. Stem Cells Dev. 2008;17(5):929–940. doi:10.1089/scd.2007.0156 | ||
Sanjay K, Selvarangan P. Bone homing of mesenchymal stem cells by ectopic alpha 4 integrin expression. FASEB J. 2007;21(21):3917–3927. doi:10.1096/fj.07-8275com | ||
Pongchaiyakul C, Leerapun T, Wongsiri S, Songpattanasilp T, Taechakraichana N. Value and validation of RCOST and TOPF clinical practice guideline for osteoporosis treatment. J Med Assoc Thai. 2012;95(12):1528–1535. | ||
Hirano T, Burr David B, Turner Charles H, Sato M, Cain Rick L, Hock Janet M. Anabolic effects of human biosynthetic parathyroid hormone fragment (1–34), LY333334, on remodeling and mechanical properties of cortical bone in rabbits. J Bone Miner Res. 2009;14(4):536–545. doi:10.1359/jbmr.1999.14.4.536 | ||
Tagil M, McDonald MM, Morse A, et al. Intermittent PTH(1–34) does not increase union rates in open rat femoral fractures and exhibits attenuated anabolic effects compared to closed fractures. Bone. 2010;46(3):852–859. doi:10.1016/j.bone.2009.11.009 | ||
Liu A, Li Y, Wang Y, Liu L, Shi H, Qiu Y. Exogenous parathyroid hormone-related peptide promotes fracture healing in Lepr(−/−) mice. Calcif Tissue Int. 2015;97(6):581–591. doi:10.1007/s00223-015-0041-2 | ||
Yu J, Xu L, Li K, et al. Zinc-modified calcium silicate coatings promote osteogenic differentiation through TGF-beta/Smad pathway and osseointegration in osteopenic rabbits. Sci Rep. 2017;7(1):3440. doi:10.1038/s41598-017-03661-5 | ||
Jing D, Yan Z, Cai J, et al. Low-level mechanical vibration improves bone microstructure, mechanical properties and porous titanium implant osseointegration by promoting a major skeletal anabolic response in type 1 diabetic rabbits. Bone. 2017;106:11–21. doi:10.1016/j.bone.2017.10.001 | ||
Yao W, Lay YAE, Kot A, et al. Improved mobilization of exogenous mesenchymal stem cells to bone for fracture healing and sex difference. Stem Cells. 2016;34(10):2587–2600. doi:10.1002/stem.2433 | ||
Matsumoto T, Yamada A, Aizawa R, et al. BMP-2 induced expression of Alx3 that is a positive regulator of osteoblast differentiation. PLoS One. 2013;8(6):e68774. doi:10.1371/journal.pone.0068774 | ||
Ramazzotti G, Bavelloni A, Blalock W, Piazzi M, Cocco L, Faenza I. BMP-2 induced expression of PLCβ1 that is a positive regulator of osteoblast differentiation. J Cell Physiol. 2015;231(3):623–629. doi:10.1002/jcp.25107 | ||
Tuzmen C, Campbell PG. Crosstalk between neuropeptides SP and CGRP in regulation of BMP2-induced bone differentiation. Connect Tissue Res. 2018;59(sup1):81–90. doi:10.1080/03008207.2017.1408604 | ||
Xiaoyu S, Panpan D, Yixin M, Xumin L, Shengbin H. A crucial role for upstream regulators of the bone morphogenetic protein (BMP) signaling in osteoblast differentiation. Bone. 2016;93:219. doi:10.1016/j.bone.2016.01.021 | ||
Zhan Q, Gui X, Wang F, et al. Sialoglycoprotein isolated from the eggs of Gadus morhua enhances fracture healing in osteoporotic mice. Food Funct. 2017;8(3):1094–1104. doi:10.1039/c6fo01346e | ||
Einhorn TA. Can an anti-fracture agent heal fractures? Clin Cases Miner Bone Metab. 2010;7(1):11–14. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.