Back to Journals » Drug Design, Development and Therapy » Volume 9
A meta-analysis for C-X-C chemokine receptor type 4 as a prognostic marker and potential drug target in hepatocellular carcinoma
Authors Hu F, Miao L, Zhao Y, Xiao Y, Xu Q
Received 3 April 2015
Accepted for publication 8 May 2015
Published 15 July 2015 Volume 2015:9 Pages 3625—3633
DOI https://doi.org/10.2147/DDDT.S86032
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Fei Hu, Lin Miao, Yu Zhao, Yuan-Yuan Xiao, Qing Xu
Department of Medical Oncology, Shanghai Tenth People’s Hospital, Tongji University, School of Medicine, Shanghai, People’s Republic of China
Abstract: Chemokines (CKs), small proinflammatory chemoattractant cytokines that bind to specific G-protein coupled seven-span transmembrane receptors, are major regulators of cell trafficking and adhesion. C-X-C chemokine receptor type 4 (CXCR4) has gained tremendous attention over the last decade, since it was found to be upregulated in a wide variety of cancer types, including hepatocellular carcinoma (HCC). The clinical relevance of expression of CXCR4 in HCC remains controversial; our aim was to identify the precise relationship of CXCR4 to prognosis and clinicopathological features. We searched the database from MEDLINE, PubMed, Web of Science, Scopus and Embase and then conducted a meta-analysis from publications met the inclusion criteria for the qualitative study. Our data showed that 1) CXCR4 is overexpressed in HCC tissues but not in normal hepatic tissue, OR =84.26, 95% confidence interval (CI) =11.86–598.98, P<0.0001. CXCR4 expression is higher in HCC than those in cirrhosis as well, OR =20.71, 95% CI =7.61–56.34, P<0.00001. 2) The expression levels of CXCR4 does not increase during local progression, however, CXCR4 expression increases the risk of distant metastases in HCC, OR =5.84, 95% CI =2.84–12.00, P<0.00001. 3) High levels of CXCR4 gene expression are associated with worse survival in HCC, HR =0.18, 95% CI =0.10–0.32, Z=5.77, P<0.00001. These data indicate that CXCR4 expression correlates with an increased risk and worse survival in HCC patients. The aberrant CXCR4 expression plays an important role in the carcinogenesis and metastasis of HCC. Our conclusion also supports that the promise of CXCR4 signaling pathway blockade as a potential strategy for HCC patients.
Keywords: CXCR4, CXCL12, prognosis, marker, hepatocellular carcinoma, cirrhosis, meta-analysis
Introduction
Primary liver cancer is the fifth leading cause of cancer-related mortality in males and ninth in females. It is estimated that 28,720 people in US have suffered from liver cancer, and deaths from the year of 2012 are 20,550 people.1 The majority of primary liver cancers belong to hepatocellular carcinoma (HCC). In developing countries, hepatitis B virus is the major cause of HCC, while in developed countries, hepatitis C virus and alcohol consumption are the primary causative factors.2 In most of HCC, cirrhosis is present prior to the malignant transformation. C-X-C chemokine receptor type 4 (CXCR4) is one of the receptors to the stromal cell-derived factor-1α (SDF-1α or CXCL12). However, some other ligands3,4 such as MIF5 can bind and activate CXCR4, while CXCL12 activates not only CXCR4 but also CXCR7.6 CXCL12–CXCR4 signaling has been found to be involved in migration of tumor cells to sites of metastasis in various malignancies, such as breast, ovarian, prostate, lung, pancreatic, colorectal, thyroid, renal cancers, neuroblastoma, rhabdomyosarcoma, as well as leukemia and lymphoma.7–9 The CXCR4 has also aroused interest in HCC carcinogenesis. CXCL12–CXCR4 is involved in cell growth, migration, and invasion of HCC cells. The majority of studies demonstrated CXCR4 expression in HCC tissue but not in normal hepatic tissue10,11 and correlations between high CXCR4 expression and aggressive tumor behavior, metastasis development, and poor prognosis.11–13 However, the clinical relevance of expression of CXCR4 in HCC remains controversial, and the association between CXCR4 expression and clinicopathological features is inconclusive due to the relatively small number of tested samples in each study. In the current study, we will perform a meta-analysis from the qualified publications for the qualitative study.
Methods
Search strategy
We searched the databases of MEDLINE, PubMed, Web of Science, Scopus, and Embase in April 2015 using the search terms: “CXCR4”, “expression”, “hepatocellular carcinoma”, “liver cancer”, and “clinical studies”. Investigations identified through the search methods aforementioned were sheltered by titles and abstracts. Nonrelevant and duplicate publications from the different databases were excluded, and the remaining papers were evaluated in the full text version for inclusion and exclusion criteria. All clinical studies except case reports were chosen, for instance, randomized controlled trials, cohort studies, case-controls studies, and case series. Publications in English and Chinese were chosen. We screened references of selected investigations and selected the most complete study to avoid duplicate reports.
Selection criteria
We collected all relevant articles regarding CXCR4 expression and clinicopathological features in HCC patients in this study. The inclusion criteria were as follows: 1) CXCR4 expression that was detected in the circulation and/or hepatic tissues, 2) researches that revealed the relationship between CXCR4 expression and HCC clinicopathological parameters and prognosis, 3) CXCR4 expression that was examined by ELISA, mRNA expression, and protein expression, 4) articles that were written in English and Chinese, 5) articles that include data of hazard ratio (HR) about overall survival (OS) with 95% confidence interval (CI) and probabilities for OS where applicable. For this meta-analysis, we excluded in vitro/ex vivo studies, nonresearch articles, studies missing HR and OS information, and studies written in non-English language.
Data extraction
The eligible publications were chosen by two investigators independently based on inclusion and exclusion criteria. The authors’ name, time of publication, sample source, number of cases, clinicopathological stage, CXCR4 expression, and patient survival were recorded. Data for clinical characteristics were summarized in a table format. We also calculated heterogeneity of studies to determine whether the investigations are eligible for a meta-analysis.
Statistical analysis
Review Manager 5.2 (Cochrane Collaboration, Oxford, UK) was used to analyze the data. Odds ratios (ORs) and their 95% CIs were pooled and compared. P-value <0.05 was considered to be statistically significant. A chi-square examination was used to determine heterogeneity with significance being set at P<0.10. The I2 value is an estimate of the amount of variance due to between-study heterogeneity rather than chance (the Cochran’s Q statistics). Substantial heterogeneity exists when I2 exceeds 50%. A random effect model will be selected to pool the ORs if there heterogeneity exists; otherwise, a fixed effect model will be chosen.
In total, 166 publications from MEDLINE, PubMed, Web of Science, Scopus, and Embase were selected. One hundred and thirty-six of noneligible publications were excluded, while 30 full-text studies were chosen for more detailed assessment. Ten publications were eventually included in this study, which met the inclusion criteria for qualitative study and for meta-analysis. The article search and study selection are depicted in Figure 1.
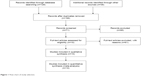  | Figure 1 Flow chart of study selection. |
Results
Identification of eligible studies
One hundred and sixty-six publications were identified by the search method as described earlier. One hundred and fifty-six of those were excluded due to in vitro/in vivo studies, reviews, lacking of matched controls, or studies irrelevant to the current analysis. Eventually, there were ten studies included in final meta-analysis (Figure 1).
Study characteristics
Ten studies published from 2006 to 2014 were eligible for meta-analysis.10–19 A total of 843 HCC and 54 liver cirrhosis patients from People’s Republic of China, Germany, Italy, and USA were enrolled. One hundred and one normal hepatic tissues served as controls. Their basic characteristics are summarized in Table 1.
The difference in CXCR4 expression in patients with HCC and cirrhosis as well as normal individuals
The staining for CXCR4 was predominately a cytoplasmic staining and, in a few cases, an additional weak membranous or nuclear staining.11,12 We found that expression levels of CXCR4 are higher in HCC than those in normal hepatic tissues that serve as controls and cirrhosis patients. The pooled OR from six studies10,12,14,15,18–20 including 249 HCC patients and 101 normal controls is shown in Figure 2 (OR =84.26, 95% CI =11.86–598.98, P<0.0001). The “Events” in the second column means the number of HCC patients (described as “Total” in the third column) with CXCL4 positive expression. The “Events” in the fourth column means the number of normal individuals (described as “Total” in the fifth column) with CXCL4 positive expression. The OR is shown numerically in the seventh column, and the CI of the summary of OR does not include 1.0 (ie, 11.86–598.98) suggesting that the association is statistically significant. These findings that CXCR4 overexpresses in HCC tumor tissues but negatively expresses in normal tissues indicate that CXCR4 could be a diagnostic biomarker for HCC. The pooled OR from three studies14,17,19 including 127 HCC patients and 52 cirrhosis patients is shown in Figure 3A (OR =20.71, 95% CI =7.61–56.34, P<0.00001). The “Events” in the second column means the number of HCC patients (described as “Total” in the third column) with CXCL4 positive expression. The “Events” in the fourth column means the number of patients with cirrhosis (described as “Total” in the fifth column) with CXCL4 positive expression. The OR is shown numerically in the seventh column, and the CI of the summarized OR does not include 1.0 (ie, 7.61–56.34) suggesting that patients with HCC expressed significantly higher levels of CXCR4 than those with cirrhosis. The pooled OR from two studies14,19 including 36 cirrhosis patients and 26 normal controls is shown in Figure 3B (OR =3.82, 95% CI =0.62–23.55, P=0.15). The “Events” in the second column means the number of hepatic cirrhosis patients (described as “Total” in the third column) with CXCL4 positive expression. The “Events” in the fourth column means the number of normal individuals (described as “Total” in the fifth column) with CXCL4 positive expression. The OR is shown numerically in the seventh column, and the CI of the summarized OR includes 1.0 (ie, 0.62–23.55) suggesting that patients with hepatic cirrhosis expressed CXCR4 protein as high as those normal individuals. These findings indicate that CXCR4 expression in cirrhosis tissues is not significantly higher than that in normal tissues.
CXCR4 expression in the progression and metastasis of liver cancers
We observed that expression levels of CXCR4 did not increase during local progression as grouped as T3 + T4 vs T1+T2 in HCC. The pooled OR from three studies11,12,17 including 259 HCC patients is shown in Figure 4A (OR =1.9, 95% CI =0.48–7.57, P=0.36). The “Events” in the second column means the number of HCC patients categorized as stages 3 and 4 (described as “Total” in the third column) expressing CXCL4 protein. The “Events” in the fourth column means the number of HCC patients categorized as stages 1 and 2 (described as “Total” in the fifth column) expressing CXCL4 protein. The OR is shown numerically in the seventh column, and the CI of the summarized OR includes 1.0 (ie, 0.48–7.57) suggesting that HCC patients at stages 3 and 4 expressed CXCR4 protein as high as those at stages 1 and 2. This suggests that strong CXCR4 expression did not correlate with local progression and proliferation of the primary tumor as indicated by the T-status. In two studies involved in the distant metastasis of HCC, CXCR4 expressions were increased in the specimens of distant metastasis. The pooled OR from two studies11,12 including 219 HCC patients is shown in Figure 4B (OR =5.84, 95% CI =2.84–12.00, P<0.00001). The “Events” in the second column means the number of HCC patients with distant metastasis (described as “Total” in the third column) expressing CXCL4 protein. The “Events” in the fourth column means the number of HCC patients without distant dissemination (described as “Total” in the fifth column) expressing CXCL4 protein. The OR is shown numerically in the seventh column, and the CI of the summarized OR does not include 1.0 (ie, 2.84–12.0) suggesting that HCC patients having distant metastasis expressed significantly much more CXCR4 protein than those just having limited local lesions. These data suggest that CXCR4 expression increases the risk of distant metastases in HCC.
CXCR4 expression and survival
Four included studies11–13,16 estimated the relationship between OS in HCC and CXCR4 expression; the pooled results (Figure 5) showed the presence of prognostic impact of CXCR4 gene in HCC patients (HR =0.18, 95% CI =0.10–0.32, Z=5.77, P<0.00001). High expressions of CXCR4 are the prognostic factor for reduced OS.
Sensitivity analyses and publication bias
We removed one study at a time to examine the result stability, which was called a sensitivity analysis. The pooled ORs stayed unchanged, suggesting the stability of our analyses. The symmetric funnel plots implied no publication biases in the meta-analysis (Figure 6).
Discussion
Chemokines contribute to cancer progression, which are secreted by different cell types and acting on endothelial, epithelial, leukocytes, and tumor cells.21 They sustain cancer cell growth and proliferation, induce angiogenesis, and assist in cancer cells escaping from immune surveillance mechanisms.22–24 CXCL12–CXCR4 pathway has attracted much attention in HCC pathogenesis and progression. Our data showed that 1) CXCR4 expression is overexpressed in HCC tissues but not in normal hepatic tissues. CXCR4 expression is higher in HCC patients than whom in cirrhosis as well, 2) the expression levels of CXCR4 does not increase during local progression; however, CXCR4 expression increases the risk of distant metastases in HCC, 3) High levels of CXCR4 gene expression are associated with worse survival in HCC.
CXCR4 is a marker of hematopoietic cells and has been involved in hematopoiesis and leukemias.25 In solid tumors, CXCR4 has been found dramatically overexpressed compared to normal tissues and is present predominantly on cancer cells.26–32 Our conclusion that CXCR4 expressions are overexpressed in HCC tissues but not in normal hepatic tissue is consistent with the previous reports. Most HCC develops as a result of persistent inflammation and fibrosis, a cirrhosis of liver. The risk of HCC development is strongly associated with the stage and extent of the liver fibrosis.33 Our analysis showed that CXCR4 expressions are significantly higher in HCC than those in cirrhosis that are in agreement with previous reports. These findings indicate that CXCR4 could be an early marker for diagnosis of HCC.
CXCL12–CXCR4 activation can increase cell proliferation, survival, migration, and invasion in HCC cells. CXCR4 exerts its aforementioned functions through various target cells and a variety of signaling pathways. 1) T lymphocytes: CD4+CD25+ T regulatory cells migrated and accumulated in HCC tissues through activation of CXCL12–CXCR4, then increased IL-10/TGF-β and decreased IFN-γ promoting HCC formation.34–40 2) Endothelial cells: CXCL12–CXCR4 axis is involved in tumor neovascularization through VEGF-dependent and independent mechanisms.41 3) HCC cells: upon binding of CXCL12 ligand with CXCR4 receptor expressed on the surface of HCC cells, the CXCL12–CXCR4 pathway is activated and subsequently activated downstream pathways such as JNK/SAPK, ERK2, and MMP2/MMP9, finally cancer cell proliferation, migration, and invasion are increased.2 SDF-1/CXCR4 axis can also induce epithelial-mesenchymal transition in HCC and facilitate cell invasion.42
CXCR4 expression correlates with more aggressive behavior of many cancers9,43,44 predicting worse outcome. However, CXCR4 expression is also reported to predict better outcome45–47 in cancer patients. The discrepancy between different studies could be explained by variations in methods, interpretation of the histoimmuno-staining, heterogeneous patient population,45 limited number of patients of studies but most probably different role of CXCR4 between metastatic and primary sites as well as CXCR4 cellular localization. CXCR4 has been demonstrated to be present in cytoplasm, nucleus and cell membrane in a variety of human cancers. In secondary cancer in liver (for instance, liver metastases of colorectal cancer), expression of nuclear CXCR4 is associated with better survival, whereas the expression of cytoplasmic CXCR4 with worse prognosis. Contrary to the observation from secondary cancer in liver, patients with nuclear CXCR4 expression have a worse OS in primary colon tumor.48,49 Since the relationship between CXCR4/CXCL12 expression and prognosis has not been examined in a large number of HCC patients, we included four studies with large number of patients in this meta-analysis. In these studies, both detectable expression of CXCR4 at cytoplasm and nucleus were taken into consideration as positive staining. The rational for this is increased CXCL12 levels induce both cytoplasmic and nuclear CXCR4 internalization. Our data systemically and quantitatively analyzed the eligible studies and showed that nuclear and cytoplasmic CXCR4 gene expression was associated with worse survival in metastatic HCC, HR =0.18, 95% CI =0.10–0.32, Z=5.77, P<0.00001.
CXCL12 (SDF-1α)-CXCR4 pathway inhibition is an emerging sensitizer for anticancer therapies, and multiple reagents are currently being developed and tested to target the CXC12–CXCR4 pathway in cancer.50 The anti-CXCR4 drug AMD3100, also known as plerixafor (Mozobil), is a US Food and Drug Administration-approved drug with a relatively mild toxicity.51,52 However, some questions about the drug still remain unanswered, for instance, is inhibition/blockade of the CXCL12–CXCR4 pathway alone efficacious in solid tumor? Can blockade the CXCL12–CXCR4 pathway in combination with other therapies synchronize the each monotherapy? Is tumor sensitized to other therapies once blocking the CXCL12–CXCR4 pathway? In addition, we should keep in mind that CXCL12 binds and initiates signaling pathway through its cognate receptors CXCR4 and CXCR7.50,53 In recent years, upregulation of CXCR7 has been discovered in numerous malignancies. Therefore, CXCR4 blockade with ADM3100 may not be sufficient to block the effects of CXCL12, which may also bind to CXCR7 on cancer or stromal cells.50 More basic research and clinical work will be needed to overcome these challenges and to increase the chances of CXCR4 pathway inhibition as a potential adjunctive therapy for solid tumor.
Disclosure
The authors declare that they have no competing interests and have no financial disclosures.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. | ||
Ghanem I, Riveiro ME, Paradis V, Faivre S, de Parga PM, Raymond E. Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. Am J Transl Res. 2014;6:340–352. | ||
Tripathi A, Davis JD, Staren DM, Volkman BF, Majetschak M. CXC chemokine receptor 4 signaling upon co-activation with stromal cell-derived factor-1alpha and ubiquitin. Cytokine. 2014;65:121–125. | ||
Sano K, Masuda R, Hisada H, et al. A radiogallium-DOTA-based bivalent peptidic ligand targeting a chemokine receptor, CXCR4, for tumor imaging. Bioorg Med Chem Lett. 2014;24:1386–1388. | ||
Klasen C, Ohl K, Sternkopf M, et al. MIF promotes B cell chemotaxis through the receptors CXCR4 and CD74 and ZAP-70 signaling. J Immunol. 2014;192:5273–5284. | ||
Hattermann K, Holzenburg E, Hans F, Lucius R, Held-Feindt J, Mentlein R. Effects of the chemokine CXCL12 and combined internalization of its receptors CXCR4 and CXCR7 in human MCF-7 breast cancer cells. Cell Tissue Res. 2014;357:253–266. | ||
Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J Mol Med (Berl). 2014;92:433–439. | ||
Hinton CV, Avraham S, Avraham HK. Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis. 2010;27:97–105. | ||
Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107: 1761–1767. | ||
Liu H, Pan Z, Li A, et al. Roles of chemokine receptor 4 (CXCR4) and chemokine ligand 12 (CXCL12) in metastasis of hepatocellular carcinoma cells. Cell Mol Immunol. 2008;5:373–378. | ||
Xiang ZL, Zeng ZC, Tang ZY, et al. Chemokine receptor CXCR4 expression in hepatocellular carcinoma patients increases the risk of bone metastases and poor survival. BMC Cancer. 2009;9:176. | ||
Schimanski CC, Bahre R, Gockel I, et al. Dissemination of hepatocellular carcinoma is mediated via chemokine receptor CXCR4. Br J Cancer. 2006;95:210–217. | ||
Xiang Z, Zeng Z, Tang Z, et al. Increased expression of vascular endothelial growth factor-C and nuclear CXCR4 in hepatocellular carcinoma is correlated with lymph node metastasis and poor outcome. Cancer J. 2009;15:519–525. | ||
Li W, Gomez E, Zhang Z. Immunohistochemical expression of stromal cell-derived factor-1 (SDF-1) and CXCR4 ligand receptor system in hepatocellular carcinoma. J Exp Clin Cancer Res. 2007;26:527–533. | ||
Liu WZL, Li J, Zhang Y. Expression and clinical significance of chemokine receptor 4 and endothelial growth factor in hepatocellular carcinoma. Clin Focus. 2010;25:409. | ||
Neve Polimeno M, Ierano C, D’Alterio C, et al. CXCR4 expression affects overall survival of HCC patients whereas CXCR7 expression does not. Cell Mol Immunol. 2014;3:102. | ||
Shi GYSD, Lu WB, Chen YK, Tong XM. A study of CXCR4/SDF-1 in hepatocellular carcinoma and liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2007;15:276–278. | ||
Yang QCY, Xu N. Expression and clinical significance of CXCR4 in human hepatocellular cancer. Jinan Med J. 2010;21:7. | ||
Yu YCQ, Qian Y, Li S, Mei M. Expression and significance of chemokine CXCL12 and CXCR4 in hepatocellular carcinoma. Shandong Pharmaceut J. 2010;50:15–16. | ||
Liu HZW, Pan Z, Wu M. Roles of CXCR4 in metastasis of hepatocellular carcinoma cells. Zhonghua Shi Yan Wai Ke Za Zhi. 2008;11:1363–1365. | ||
Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392: 565–568. | ||
Mehrad B, Keane MP, Strieter RM. Chemokines as mediators of angiogenesis. Thromb Haemost. 2007;97:755–762. | ||
Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. | ||
Zhu Q, Han X, Peng J, Qin H, Wang Y. The role of CXC chemokines and their receptors in the progression and treatment of tumors. J Mol Histol. 2012;43:699–713. | ||
Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. | ||
Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. | ||
Huang EH, Singh B, Cristofanilli M, et al. A CXCR4 antagonist CTCE-9908 inhibits primary tumor growth and metastasis of breast cancer. J Surg Res. 2009;155:231–236. | ||
Ma WF, Du J, Fu LP, Fang R, Chen HY, Cai SH. Phenotypic knockout of CXCR4 by a novel recombinant protein TAT/54R/KDEL inhibits tumors metastasis. Mol Cancer Res. 2009;7:1613–1621. | ||
Matsusue R, Kubo H, Hisamori S, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009;16:2645–2653. | ||
Richert MM, Vaidya KS, Mills CN, et al. Inhibition of CXCR4 by CTCE-9908 inhibits breast cancer metastasis to lung and bone. Oncol Rep. 2009;21:761–767. | ||
Sciume G, Santoni A, Bernardini G. Chemokines and glioma: invasion and more. J Neuroimmunol. 2010;224:8–12. | ||
Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–329. | ||
Wald O, Weiss ID, Galun E, Peled A. Chemokines in hepatitis C virus infection: pathogenesis, prognosis and therapeutics. Cytokine. 2007; 39:50–62. | ||
Eferl R. A dual role for interferon gamma signalling in hepatocellular carcinoma. J Hepatol. 2012;57:940–942. | ||
Fu J, Xu D, Liu Z, et al. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology. 2007;132:2328–2339. | ||
Hirano S, Iwashita Y, Sasaki A, Kai S, Ohta M, Kitano S. Increased mRNA expression of chemokines in hepatocellular carcinoma with tumor-infiltrating lymphocytes. J Gastroenterol Hepatol. 2007; 22:690–696. | ||
Li P, Du Q, Cao Z, et al. Interferon-gamma induces autophagy with growth inhibition and cell death in human hepatocellular carcinoma (HCC) cells through interferon-regulatory factor-1 (IRF-1). Cancer Lett. 2012;314:213–222. | ||
Shen X, Li N, Li H, Zhang T, Wang F, Li Q. Increased prevalence of regulatory T cells in the tumor microenvironment and its correlation with TNM stage of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2010;136:1745–1754. | ||
Tang L, Hu HD, Hu P, et al. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Ther. 2007;14:1226–1234. | ||
Yang XH, Yamagiwa S, Ichida T, et al. Increase of CD4+ CD25+ regulatory T-cells in the liver of patients with hepatocellular carcinoma. J Hepatol. 2006;45:254–262. | ||
Liang Z, Brooks J, Willard M, et al. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. | ||
Li X, Li P, Chang Y, et al. The SDF-1/CXCR4 axis induces epithelial-mesenchymal transition in hepatocellular carcinoma. Mol Cell Biochem. 2014;392:77–84. | ||
Yoshitake N, Fukui H, Yamagishi H, et al. Expression of SDF-1 alpha and nuclear CXCR4 predicts lymph node metastasis in colorectal cancer. Br J Cancer. 2008;98:1682–1689. | ||
Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–1924. | ||
Nikkhoo B, Jalili A, Fakhari S, et al. Nuclear pattern of CXCR4 expression is associated with a better overall survival in patients with gastric cancer. J Oncol. 2014;2014:808012. | ||
Spano JP, Andre F, Morat L, et al. Chemokine receptor CXCR4 and early-stage non-small cell lung cancer: pattern of expression and correlation with outcome. Ann Oncol. 2004;15:613–617. | ||
Zhao BC, Wang ZJ, Mao WZ, et al. CXCR4/SDF-1 axis is involved in lymph node metastasis of gastric carcinoma. World J Gastroenterol. 2011;17:2389–2396. | ||
Yopp AC, Shia J, Butte JM, et al. CXCR4 expression predicts patient outcome and recurrence patterns after hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2012;19(suppl 3):S339–S346. | ||
Sakai N, Yoshidome H, Shida T, et al. CXCR4/CXCL12 expression profile is associated with tumor microenvironment and clinical outcome of liver metastases of colorectal cancer. Clin Exp Metastasis. 2012;29:101–110. | ||
Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1alpha)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin Cancer Res. 2011;17: 2074–2080. | ||
De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. | ||
Donzella GA, Schols D, Lin SW, et al. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat Med. 1998;4:72–77. | ||
Monnier J, Boissan M, L’Helgoualc’h A, et al. CXCR7 is up-regulated in human and murine hepatocellular carcinoma and is specifically expressed by endothelial cells. Eur J Cancer. 2012;48:138–148. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






