Back to Journals » Drug Design, Development and Therapy » Volume 14
A Comparison of Dexmedetomidine and Midazolam for the Prevention of Postoperative Nausea and Vomiting Caused by Hemabate in Cesarean Delivery: A Randomized Controlled Trial
Authors Hu B , Zhou H, Zou X, Shi J, Li X, Tan L
Received 27 February 2020
Accepted for publication 12 May 2020
Published 28 May 2020 Volume 2020:14 Pages 2127—2133
DOI https://doi.org/10.2147/DDDT.S251525
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Anastasios Lymperopoulos
Bailong Hu,1,* Haiyan Zhou,2,* Xiaohua Zou,1 Jing Shi,1 Xingyu Li,1 Li Tan1
1Department of Anesthesiology, The Affiliated Hospital of Guizhou Medical University, Guiyang,People’s Republic of China; 2Department of Clinical Research Centre, The Affiliated Hospital of Guizhou Medical University, Guiyang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bailong Hu Email [email protected]
Objective: To compare the efficacy of dexmedetomidine and midazolam in the prevention of postoperative nausea and vomiting (PONV) caused by hemabate in postpartum hemorrhage during cesarean delivery.
Methods: One hundred and five parturients with American Society of Anesthesiology (ASA) physical status I and II, aged 20– 40 years, undergoing elective cesarean delivery under epidural anesthesia were randomly allocated into dexmedetomidine group (group D, n=35), midazolam group (group M, n=35) and control group (group C, n=35). Patients received an intrauterine injection of 250 μg hemabate and continuous intravenous infusion of 5 units oxytocin immediately following the delivery of the infant. At the same time, patients in group D received 1μg/kg intravenous dexmedetomidine, group M received 0.02 mg/kg intravenous midazolam and group C received 20 mL intravenous saline. Parameters such as the PONV, other adverse reactions (chest distress, flush, etc.) caused by hemabate, patient satisfaction, the sedation (OAA/S) scores, and the hemodynamic parameters were recorded in both groups.
Results: The PONV incidence in group D and group M was significantly lower compared with group C (6%, 17%, and 71% for group D, group M, and group C, respectively, P< 0.05). The sedation (OAA/S) scores in group D and group M was significantly higher compared with group C (1.62± 0.28, 1.75± 0.31, and 1.00± 0.00 for group D, group M, and group C, respectively, P< 0.05). The patient satisfaction in group D and group M was significantly higher compared with group C (94%, 69%, and 46% for group D, group M, and group C, respectively, P< 0.05). Furthermore, there were more patients satisfied with group D than group M (94% vs.69%, P< 0.05).
Conclusion: Intravenous dexmedetomidine (1 μg/kg) and midazolam (0.02 mg/kg) were equally effective in preventing PONV introduced by hemabate and dexmedetomidine is superior to midazolam in patient satisfaction.
Keywords: dexmedetomidine, midazolam, PONV, hemabate, cesarean delivery
Introduction
Background
Hemabate, a synthetic 15-methyl analog of prostaglandin F2α, has been discovered to be more powerful than oxytocin in preventing and reducing postpartum hemorrhage (PPH) in high-risk patients undergoing cesarean delivery.1 However, due to the effect on gastrointestinal smooth muscle, hemabate may cause severe nausea and vomiting,2 which may reduce patient satisfaction and delay discharge from hospital.
Dexmedetomidine is a highly selective α2 adrenergic agonist that has several functions, including sedation, anti-anxiety, analgesia, cardiovascular stability, and less inhibition of respiration.3,4 Recently, many studies have shown that it has antiemetic effect,5,6 and Liu et al have shown that low dose dexmedetomidine can effectively prevent the adverse reactions induced by hemabate.7 On the other hand, previous studies showed that midazolam was also effective in the reduction of PONV during cesarean delivery.8–10 However, no studies have focused on comparing dexmedetomidine and midazolam for preventing the PONV induced by hemabate. In this trial, we will compare the efficacy of dexmedetomidine and midazolam in the prevention of PONV induced by hemabate in postpartum hemorrhage during cesarean delivery. To the best of our knowledge, this is the first trial that compared the efficacy and safety of dexmedetomidine and midazolam in the prevention of PONV induced by hemabate in postpartum hemorrhage during cesarean delivery.
Methods
Study Design
A total of 105 parturients with American Society of Anesthesiology (ASA) physical status I–II, aged 20–40 years, undergoing elective cesarean delivery under epidural anesthesia were randomly allocated into three groups: Group D (n=35), Group M (n=35) and Group C (n=35). Randomization was carried out by a computer-generated list of random numbers which were sealed in an envelope. Before anesthesia, an anesthesiologist who was blinded to the study opened the sealed envelope and performed the epidural anesthesia.
Selection of Participants
Patients who had mental illnesses, neuromuscular sicknesses, reactive bronchial diseases, diabetes, body mass index (BMI﹥30kg/m2), abnormal liver and renal function, the sensory block level was higher than that of T4, known allergies to any anesthetic agent, a history of PONV in previous surgery, or severe sinus bradycardia (<50 beats per min [bpm]) were excluded. Patients were also excluded from the study if they had a history of long-term opioid, NSAIDs or sedative utilization.
Ethics Issues
This study was conducted in accordance with the Declaration of Helsinki and approved by the Biological-Medical Ethical Committee of the Affiliated Hospital of Guizhou Medical University Hospital, Guiyang, Guizhou, China. Written informed consent was obtained from all patients. This study was also registered at www.chictr.org (identifier: ChiCTR-INR-17013500).
Intervention
All parturients were maintained nil per os (nothing by mouth, NPO) 8 hours prior to anesthesia. Standard monitoring (the electrocardiography (ECG), noninvasive blood pressure (NIBP), and pulse oximetry saturation (SpO2) ) were applied for each patient after they entered the operating room. All parturients were treated with lactated Ringer’s solution (10 mL/kg) for 20–30 min to prevent hypotension. If the blood pressure decreased more than 20% from baseline pressure or systolic blood pressure decreased less than 90 mmHg, 10 mg of ephedrine was injected. A continuous epidural anesthesia (CEA) technique was performed for cesarean delivery procedures. The epidural anesthesia was performed at the L1–L2 interspace. As high as 0.75% ropivacaine 7–10 mL was infused. The vital signs of each parturient were monitored for every 5 min. Pinprick test was used to confirm adequate sensory block up to T4 level. Surgery was started when the sensory nerve block reached at T4–T6. Patients received an intrauterine injection of 250 μg hemabate (Pharmacia & Upjohn Company, Kalamazoo, MI, USA) and continuous intravenous infusion of 5 units oxytocin during the cesarean delivery, immediately following the delivery of the infant. After hemabate injection, patients in group D received 1μg/kg intravenous dexmedetomidine (Jiang Su Heng Rui Medicine Co. Ltd, Jiangsu Province, China) diluted to 20 mL with physiological saline, group M received 0.02 mg/kg intravenous midazolam (Jiang Su Nhua Pharma. Co. Ltd, Jiangsu Province, China) diluted to 20 mL with physiological saline, and group C received 20 mL intravenous physiological saline. The infusion of above mentioned three group liquids were completed in 15 minutes. After surgery, the patient was transported to the PACU, and 1 h after the study medicine infusion, the parturient was transported to the ward. Patients got epidural analgesia with 0.1% ropivacaine+0.5 μg/mL sufentanil (continuous, 8 mL/h; bolus, 2 mL; lockout interval, 15 min; 1-h limitation, 20 mL) in a patient-controlled analgesia device for the postoperative 48 h.
Outcome Measures
Hemodynamic parameters such as heart rate (HR), systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded at the following time points: 1 min prior to hemabate administration (T0); 5 min (T1); 20 min (T2); 40 min (T3) and 60 min (T4) following hemabate injection. Parameters such as the adverse reactions caused by hemabate, the grade of patient satisfaction, the sedation (OAA/S) scores, and the hemodynamic parameters were recorded in both groups. The investigators, responsible anesthesiologists, surgeons, nurse, the patients, and the staff who registered patients and gathered study results were blinded to group allocation.
Primary Outcome
The primary outcome was the incidence of PONV. Nausea was defined as an uneasy feeling in the stomach while vomiting refers to the forceful expulsion of gastric contents.11 PONV was evaluated by means of Bellville scoring score (0: no symptoms, 1: nausea 2: retching 3: vomiting).12 Patients were treated with 10 mg of metoclopramide in case of two or more emesis episodes.
Secondary Outcomes
The secondary outcomes include the following: 1) Other adverse reactions induced by the hemabate, which include the following: (a) flush, (b) diarrhea, (c) headache, (d) elevated blood pressure, and (e) chest congestion; 2) the hemodynamic changes at each point; 3) the patient satisfaction: the patients completed a simple questionnaire (0, very satisfied; 1, satisfied; 2, not satisfied) while they leave the PACU. If the patient can not recall what happened during the procedure, then the sedation cannot be considered complete at the time of evaluation; 4) the sedation scores (Observer’s Assessment of Alertness⁄Sedation (OAA⁄S) scale) (where 1 =awake ⁄ alert and 5 = deep sleep).13
Statistical Analysis
The sample size was estimated based on the adverse reactions with a type I error of 0.05 and a power of 80%. Based on our pilot study, a sample size of 29 patients in each group was necessary. To allow for missing cases and dropouts due to various reasons, we calculated that we would need 35 patients for each group. Statistical analysis was conducted with SPSS for Windows version 22.0 (SPSS Inc, Chicago, IL, USA). Continuous data were presented as mean ± standard deviation, and categorical data were presented as percentage. Normality assessment of continuous data was performed with Kolmogorov‐Smirnov and/or Shapiro‐Wilk tests. Continuous data were compared with analysis of variance (ANOVA) for parametric data or Kruskal–Wallis test for non‐parametric data. Post hoc comparisons among the repeated measures in each group were performed by the Tukey HSD and/or LSD method, if appropriate. Percentage data comparison was performed using the Chi-square test or Fisher’s exact test. An analysis of ordinal data was performed using the Mann–Whitney U-test. Differences in the hemodynamic changes were compared by repeated-measures ANOVA. A P value less than 0.05 was considered statistically significant.
Results
Of the 109 patients, 2 patients refused to participate and 2 patients did not meet inclusion criteria. The remaining 105 parturients completed the study (Figure 1). There were no significant differences between groups in demographic, clinical, and intraoperative characteristics (Table 1).
 |
Table 1 Patients Demographic Data |
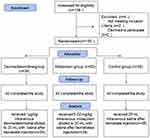 |
Figure 1 Flow diagram based on Consolidated Standards of Reporting Trials (CONSORT) statement. |
The PONV incidence in group D and group M was significantly lower compared with group C (6%, 17% and 71% for group D, group M, and group C, respectively, P<0.05), whereas the PONV incidence did not differ between group D and group M (P>0.05) (Table 2). The antiemetic agent metoclopramide was administered to 15 (43%) patients in group C, compared with 2 (6%) patients in group M and no patients in group D, which was statistically significant (P<0.05) (Table 2).
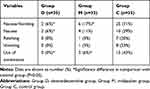 |
Table 2 Incidence of PONV in 3 Groups |
The incidence of chest distress in group D and group M was significantly lower compared with group C (P<0.05) (Table 3). Other side effects induced by hemabate such as flush, diarrhea, and headache did not differ between the groups.
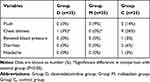 |
Table 3 Incidence of Other Adverse Reactions in 3 Groups |
No patients could not recall what happened during the operation, and all patients completed the satisfaction questionnaire assessment. The sedation (OAA/S) scores in group D and group M were significantly higher compared with group C (1.62±0.28, 1.75±0.31, and 1.00±0.00 for the group D, group M, and group C, respectively, P<0.05). The patient satisfaction score in group D and group M was significantly higher compared with group C (94%, 69%, and 46% for group D, group M, and group C, respectively, P<0.05). Furthermore, there were more patients satisfied with group D than group M (94% vs.69%, P<0.05) (Table 4). The hemodynamic changes did not differ between the three groups (Figure 2).
 |
Table 4 Patient Satisfaction and OAA/S in 3 Groups |
Discussion
In this study, the high incidence of PONV caused by hemabate during cesarean delivery was confirmed similar to the previous studies.6 In addition, the incidence of PONV in group D and group M were significantly lower compared with group C. To our knowledge, this is the first study comparing the effect of dexmedetomidine and midazolam for the prevention of PONV introduced by hemabate during cesarean delivery.
The overall incidence of PONV during regional anesthesia for cesarean delivery is estimated to 21–79%.14 PONV can increase the potential risk of aspiration, reduce patient satisfaction, and prolong hospitalization.15 In addition, PONV may increase procedure time and inadvertent surgery trauma, causing electrolyte imbalance and aggravate bleeding.16 Hematate has a strong and lasting contractile effect on uterine smooth muscle, which can effectively prevent and reduce PPH during cesarean deliver. However, one of the most distressing side effects after its administration is severe nausea and vomiting.7 Therefore, PONV should be prevented as soon as possible to enhance recovery after surgery according to the guidelines,14 especially for those who receive hemabate. The common causes of PONV during cesarean delivery including age, surgical procedure, anesthetic technique, opioid administration, and hypotension.14 In this study, all patients and groups were identical regarding the operation and their anesthetic management.
The incidence of PONV can be lowered with some sedatives such as dexmedetomidine and midazolam. As a highly selectiveα2 adrenergic receptor agonist, dexmedetomidine exhibits sedative, analgesic, and sympatholytic properties.17 Recently, the incidence of PONV induced by dexmedetomidine has caused extensive concern. A recent meta-analysis indicated that dexmedetomidine significantly reduced the occurrence of PONV.18 Our study showed that the incidence and severity of PONV induced by hemabate were significantly reduced in the dexmedetomidine group. The mechanism of dexmedetomidine reducing the incidence of PONV may be related to the reduction of sympathetic tone and catecholamine release.19
Furthermore, the incidence of chest distress in the dexmedetomidine group was significantly reduced compared with control group, which may be related to the inhibition of airway smooth muscle contraction.20 However, some research work is needed to clarify its mechanism in the future.
Several investigations have reported that midazolam, used in a subhypnotic dose, was effective in treating PONV.21,22 Our study found that patients who received midazolam experienced significantly fewer episodes of PONV compared with the control group, which was similar to the finding of O. Tarhan et al.8 The detailed antiemetic mechanism of midazolam still remains unknown. Midazolam related antiemetic effect may be attributed to reducing synthesis, release, and postsynaptic effect of dopamine by acting on CRTZ-mediated regions.7,23
Parturients who are awake during surgery may suffer anxiety, fear, and stress, and a certain degree of sedation can increase the patient satisfaction and affect PONV.24 In the present study, patient satisfaction was significantly higher in group D and group M, in which patients experienced a higher degree of sedation and a lower incidence of PONV, compared with group C. Although there was no significant difference in the incidence of PONV between the group D and group M, the patient satisfaction in group D was higher compared with group M, which may be related to the fact that the number of PONV and other adverse reactions (such as flushing and chest distress) in group D was less than that in group M.
Conclusions
In conclusion, intravenous dexmedetomidine (1 μg/kg) and midazolam (0.02 mg/kg) were equally effective in preventing PONV introduced by hemabate and improve satisfaction in patients undergoing cesarean delivery, furthermore, dexmedetomidine is superior to midazolam in patient satisfaction.
Data Sharing Statement
We are willing to share all relevant data in this study. Readers can contact the corresponding author to obtain data by email after 6 months of publication of this article. The study protocol, statistical analysis plan, and clinical study report will also be available.
Funding
This work was supported by the Pain Management Branch of Chinese Society of Cardiothoracic and Vascular Anesthesiology: CSCVA-PM-2017005, the National Natural Science Foundation of China (No.81904319), the Fund of Guiyang Science and Technology department ([2019] 9-1-24), the Guizhou Provincial Natural Science Foundation (qiankehejichu[2020]1Y298), the Science and Technology Fund of Guizhou Provincial Health Department (qiankehe pingtairencai[2018]5779-52, qiankehepingtairencai[2018] 5779-38), and Traditional Chinese Medicine Project of Guizhou Administration of Traditional Chinese Medicine (QZYY-2019-013).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Buttino L, Garite TJ. The use of 15 methyl F2αProstaglandin (Prostin 15M) for the control of postpartum hemorrhage. Am J Perinatol. 1986;3(03):241–243. doi:10.1055/s-2007-999875
2. Bai J, Sun Q, Zhai H. A comparison of oxytocin and carboprost tromethamine in the prevention of postpartum hemorrhage in high-risk patients undergoing cesarean delivery. Exp Ther Med. 2014;7(1):46–50. doi:10.3892/etm.2013.1379
3. Piao G, Wu J. Systematic assessment of dexmedetomidine as an anesthetic agent: a meta-analysis of randomized controlled trials. Arch Med Sci. 2011;10(1):19–24.
4. Bajwa SJS, Gupta S, Kaur J, et al. Reduction in the incidence of shivering with perioperative dexmedetomidine: a randomized prospective study. J Anaesthesiol Clin Pharmacol. 2012;28(1):86. doi:10.4103/0970-9185.92452
5. Khasawinah TA, Ramirez A, Berkenbosch JW, et al. Preliminary experience with dexmedetomidine in the treatment of cyclic vomiting syndrome. Am J Ther. 2003;10(4):303–307. doi:10.1097/00045391-200307000-00012
6. Zaben KRA, Mohsen WA, Basha AS, Al-Zaben KR, Al-Mustafa MM, Alghanem SM. A balanced anesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Med J. 2009;30(12):1537–1541.
7. Liu Y, Chen HX, Kang DL, et al. Influence of dexmedetomidine on incidence of adverse reactions introduced by hemabate in postpartum hemorrhage during cesarean section. Int J Clin Exp Med. 2015;8(8):13776.
8. Tarhan O, Canbay O, Celebi N, et al. Subhypnotic doses of midazolam prevent nausea and vomiting during spinal anesthesia for cesarean section. Minerva Anestesiol. 2007;73(12):629–633.
9. Lee Y, Wang JJ, Yang YL, et al. Midazolam vs ondansetron for preventing postoperative nausea and vomiting: a randomised controlled trial. Anaesthesia. 2007;62(1):18–22. doi:10.1111/j.1365-2044.2006.04895.x
10. Rasooli S, Moslemi F, Khaki A. Effect of sub hypnotic doses of propofol and midazolam for nausea and vomiting during spinal anesthesia for cesarean section. Anesthesiology Pain Med. 2014;4(4):e19384. doi:10.5812/aapm.19384
11. Lacy BE, Parkman HP, Camilleri M, et al. Chronic nausea and vomiting: evaluation and treatment. Am J Gastroenterol. 2018;113(5):647–659. doi:10.1038/s41395-018-0039-2
12. Bellville JW, Bross ID, Howland WS. A method for the clinical evaluation of antiemetic agents. Anesthesiology. 1959;20(6):753–760. doi:10.1097/00000542-195911000-00002
13. Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer’s Assessment of Alertness ⁄ Sedation (OAA ⁄ S) scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990;10(4):244–251.
14. Macones GA, Caughey AB, Wood SL, et al. Guidelines for postoperative care in cesarean delivery: Enhanced Recovery After Surgery (ERAS) Society recommendations (part 3). Am J Obstet Gynecol. 2019;221(3):
15. Danielak-Nowak M, Musioł E, Arct-Danielak D, et al. A comparison of subhypnotic doses of propofol and midazolam during spinal anaesthesia for elective caesarean section. Anaesthesiol Intensive Ther. 2016;48(1):13–18. doi:10.5603/AIT.2016.0003
16. Rashiq S, Bray P. Relative value to surgical patients and anesthesia providers of selected anesthesia related outcomes. BMC Med Inform Decis Mak. 2003;3(1):3. doi:10.1186/1472-6947-3-3
17. Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62(1):118–133. doi:10.1016/S0034-7094(12)70110-1
18. Jin S, Liang DD, Chen C, et al. Dexmedetomidine prevent postoperative nausea and vomiting on patients during general anesthesia: a PRISMA-compliant meta analysis of randomized controlled trials. Medicine. 2017;96(1):e5770. doi:10.1097/MD.0000000000005770
19. Mo Y, Qiu S. Effects of dexmedetomidine in reducing post-cesarean adverse reactions. Exp Ther Med. 2017;14(3):2036–2039. doi:10.3892/etm.2017.4759
20. Groeben H, Mitzner W, Brown RH. Effects of the α2-adrenoceptor agonist dexmedetomidine on bronchoconstriction in dogs. Anesthesiology J ASA. 2004;100(2):359–363. doi:10.1097/00000542-200402000-00026
21. Unlugenc H, Guler T, Gunes Y, et al. Comparative study of the antiemetic efficacy of ondansetron, propofol and midazolam in the early postoperative period. Eur J Anaesthesiol. 2004;21(1):60–65. doi:10.1097/00003643-200401000-00010
22. Bauer KP, Dom PM, Ramirez AM, et al. Preoperative intravenous midazolam: benefits beyond anxiolysis. J Clin Anesth. 2004;16(3):177–183. doi:10.1016/j.jclinane.2003.07.003
23. Deka B, Talukdar B, Laha AK, et al. Effects of intravenous midazolam during spinal anaesthesia for caesarian section. Int J Basic Clin Pharmacol. 2016;5(3):754–757. doi:10.18203/2319-2003.ijbcp20161514
24. Cheng YJ, Wang YP, Fan SZ, et al. Intravenous infusion of low dose propofol for conscious sedation in cesarean section before spinal anesthesia. Acta Anaesthesiol Sin. 1997;35(2):79–84.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

