Back to Journals » Drug Design, Development and Therapy » Volume 9
3-Coumaranone derivatives as inhibitors of monoamine oxidase
Authors Van Dyk A, Petzer J, Petzer A, Legoabe L
Received 5 June 2015
Accepted for publication 28 July 2015
Published 3 October 2015 Volume 2015:9 Pages 5479—5489
DOI https://doi.org/10.2147/DDDT.S89961
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Adriaan S Van Dyk,1,2 Jacobus P Petzer,1,2 Anél Petzer,1 Lesetja J Legoabe1
1Centre of Excellence for Pharmaceutical Sciences, 2Pharmaceutical Chemistry, School of Pharmacy, North-West University, Potchefstroom, South Africa
Abstract: The present study examines the monoamine oxidase (MAO) inhibitory properties of a series of 20 3-coumaranone [benzofuran-3(2H)-one] derivatives. The 3-coumaranone derivatives are structurally related to series of α-tetralone and 1-indanone derivatives, which have recently been shown to potently inhibit MAO, with selectivity for MAO-B (in preference to the MAO-A isoform). 3-Coumaranones are similarly found to selectively inhibit human MAO-B with half-maximal inhibitory concentration (IC50) values of 0.004–1.05 µM. Nine compounds exhibited IC50<0.05 µM for the inhibition of MAO-B. For the inhibition of human MAO-A, IC50 values ranged from 0.586 to >100 µM, with only one compound possessing an IC50<1 µM. For selected 3-coumaranone derivatives, it is established that MAO-A and MAO-B inhibition are reversible since dialysis of enzyme–inhibitor mixtures almost completely restores enzyme activity. On the basis of the selectivity profiles and potent action, it may be concluded that the 3-coumaranone derivatives are suitable leads for the development of selective MAO-B inhibitors as potential treatment for disorders such as Parkinson’s disease and Alzheimer’s disease.
Keywords: benzofuran-3(2H)-one, MAO, inhibition, reversible, competitive, Parkinson’s disease
Introduction
The enzyme, monoamine oxidase (MAO), exists as two isoforms, MAO-A and MAO-B, which are encoded by two distinct genes.1,2 The MAOs have been targets in medicinal chemistry and drug discovery for many years since these enzymes metabolize key neurotransmitters in the periphery and in the central nervous system.3,4 For example, MAO-A metabolizes serotonin in the central nervous system, and inhibitors of this isoform (eg, phenelzine, isocarboxazid, tranylcypromine, and moclobemide) are used in the clinic for the treatment of depression. MAO-B, on the other hand, is the main isoform responsible for central dopamine metabolism, and MAO-B inhibitors (eg, selegiline and rasagiline) are thus used for the treatment of Parkinson’s disease.5 A transdermal delivery system of selegiline at higher doses is now also used for major depression.6 On the basis of the observation that MAO-B activity is reduced in smokers, MAO-B inhibitors also represent a potential treatment for smoking cessation. MAO-B inhibitors may mimic the neurochemical effects of smoking by increasing synaptic monoamine levels.7
Besides modulation of monoamine levels, MAO inhibitors are also of clinical value because they reduce the formation of by-products of the MAO catalytic cycle, hydrogen peroxide and aldehyde species. These products may be harmful, particularly in the brain, if not rapidly metabolized by glutathione peroxidase and aldehyde-metabolizing enzymes, respectively.8 The possibility that these enzymes may be dysfunctional in conditions such as Parkinson’s disease has given rise to the theory that excessive or even normal MAO activity may result in neurotoxicity and thus contribute to the degenerative process.8,9 MAO inhibitors have thus been proposed to act as neuroprotective agents in these conditions by reducing the formation of injurious metabolic by-products of the MAO catalytic cycle. MAO-B inhibition seems to be particularly relevant in Parkinson’s disease as MAO-B activity and density increases in most brain regions in an age-related manner.10 Experimental evidence suggests that MAO-generated hydrogen peroxide and aldehyde intermediates in the heart may also be undesirable since these may affect mitochondrial respiration leading to cardiomyocyte death.11 MAO inhibition may therefore be beneficial for the treatment of certain cardiovascular pathologies. Since MAO-A levels and hydrogen peroxide derived from MAO-A are increased in the hearts of aged rats, MAO-A inhibitors appear to be of particular relevance in this regard.12
Although MAO inhibitors have been extensively employed in the clinic, the use of nonselective and selective MAO-A inhibitors declined after the realization that these drugs potentiate the sympathomimetic effects of the dietary amine, tyramine.13,14 This interaction may lead to a potentially fatal increase in blood pressure termed the “cheese effect”. The observation that selective inhibitors of MAO-B do not cause tyramine-induced hypertension and the development of reversible MAO-A inhibitors with low potential for the cheese effect have facilitated the continued use of MAO inhibitors in the clinic.5 Although the older irreversible MAO-A inhibitors are still available for the treatment of certain types of depression, from a drug design and drug safety point of view, reversible inhibition of MAO-A is a more desirable property and such drugs would not require dietary restrictions.15,16 Both reversible and irreversible MAO-B inhibitors, on the other hand, have excellent safety profiles and are not associated with changes in blood pressure.17,18
Based on the clinical utility of MAO inhibitors, the discovery of novel classes of compounds with potent inhibition activity is of interest. Although a number of MAO inhibitors are used in the clinic, none of these may be considered as having both high potency (IC50 [half maximal inhibitory concentration] <0.1 μM) and a reversible mode of action. In this study, a series of twenty 3-coumaranone [benzofuran-3(2H)-one] derivatives have been synthesized and evaluated as inhibitors of human MAO-A and MAO-B. The 3-coumaranone derivatives are structurally related to series of α-tetralone and 1-indanone derivatives, which have recently been shown to potently inhibit MAO-B, and, to a lesser extent, also the MAO-A isoform.19–21 The MAO inhibition potencies of the reported α-tetralone (1–2) and 1-indanone (3–4) derivatives are summarized in Table 1. Based on the activities of these classes of compounds, it is anticipated that 3-coumaranone–derived compounds would also act as MAO inhibitors, particularly of the MAO-B isoform. Since the α-tetralone and 1-indanone derivatives contain alkyloxy substituents, which greatly enhances MAO inhibition, a 3-coumaranone derivative, compound 5a, containing the benzyloxy side chain on the C6 position, was considered as the first member of the series of 3-coumaranone derivatives (Table 2). To explore chemical space and to establish structure–activity relationships (SARs), a total of 20 derivatives were synthesized. The benzyloxy-substituted derivative 5a was substituted on the meta and para positions of the phenyl ring with halogens (F, Cl, Br, I) and alkyl groups (CN, CH3, CF3) to yield 13 derivatives, compounds 5b–n. Derivatives containing the phenylethoxy, phenylpropoxy, and phenoxyethoxy substituents on the C6 position of the 3-coumaranone moiety to yield compounds 5o–q were also synthesized in this study. The phenylethoxy-substituted derivative 5o was further substituted on the phenyl ring with bromine and the methyl group to yield 5r–t. The goal of this study is therefore to explore the possibility that 3-coumaranone derivatives may act as MAO inhibitors. Among compound classes 1–4, the 1-indanone derivatives, 3, are most closely related to the 3-coumaranone derivatives of this study, with substitution in analogous positions in compounds 3 (C5) and 5 (C6). Since series 3 appears to be, in general, weaker MAO-A inhibitors among the reported classes 1–4, it may be expected that 3-coumaranones 5 may exhibit a high degree of MAO-B selectivity. In future studies, other positions of the 3-coumaranone moiety (eg, C5) should be substituted and the effect on MAO isoform selectivity evaluated.
  | Table 1 The reported IC50 values for the inhibition of recombinant human MAO-A and MAO-B by α-tetralone (1 and 2) and 1-indanone (3 and 4) derivatives (R = alkyl/arylalkyl) |
Materials and methods
General
Chemicals for the synthetic procedures were acquired from Sigma-Aldrich (St Louis, MO, USA). Chemicals for the enzymatic reactions, including kynuramine 2HBr, and microsomes from insect cells expressing recombinant human MAO-A and MAO-B (5 mg/mL) were from Sigma-Aldrich. The use of the recombinant human proteins in this study were approved by the Ethics Committee of the North-West University. Fluorescence spectrophotometry was carried out with a Varian Cary Eclipse fluorescence spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Proton (1H ) and carbon (13C) nuclear magnetic resonance (NMR) spectra were recorded in deuterochloroform on a Bruker Avance III 600 spectrometer (Karlsruhe, Germany) at 600 and 151 MHz, respectively. Chemical shifts (Supplementary materials) are given in parts per million (δ) and spin multiplicities as s (singlet), d (doublet), dd (doublet of doublets), t (triplet), td (triplet of doublets), or m (multiplet). High resolution mass spectra were recorded on a Bruker micrOTOF-Q II mass spectrometer in atmospheric pressure chemical ionization mode. Melting points (mps) were determined with a Büchi B-545 melting point apparatus (Büchi Labortechnik, Flawil, Switzerland) and are uncorrected. Thin-layer chromatography was carried out using silica gel 60 (Merck, Darmstadt, Germany), and the sheets were developed in a mobile phase consisting of one part ethyl acetate and four parts petroleum ether. The developed sheets were examined at a wavelength of 254 nm.
Chemistry
Procedure for the synthesis of 3-coumaranone derivatives 5a–t
To commercially available 6-hydroxy-3-coumaranone (1.998 mmol) in a round bottom flask, 3 mL N, N-dimethylformamide and anhydrous potassium carbonate (3.996 mmol) was added. The appropriate arylalkyl bromide (2.197 mmol) was added to the reaction, and the mixture was stirred for 24 hours at room temperature. Thin layer chromatography was performed to determine the completion of the reaction. Upon completion, water (8 mL) was added to the reaction, and the resulting mixture was filtered. The reaction was extracted to dichloromethane (3×20 mL), and the organic phase was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. After crystallization from cyclohexane, the product was collected via filtration, and the crystals were air dried.22
Enzymology
Determination of IC50 values
The protocol for the determination of IC50 values has been reported in detail in recent publications.19–21 Recombinant human MAO-A and MAO-B served as enzyme sources, and kynuramine was used as substrate for both isoforms.23 The oxidation of kynuramine by the MAOs ultimately yields 4-hydroxyquinoline, which was measured by fluorescence spectrophotometry (λex =310; λem =400 nm). By measuring MAO activities in the presence of various inhibitor concentrations (spanning at least three orders of magnitude), sigmoidal curves were constructed (enzyme activity versus the logarithm of inhibitor concentration), from which IC50 values were determined. These experiments were carried out in triplicate, and the IC50 values are expressed as mean ± standard deviation. We have previously found that the oxidation of kynuramine by the MAO enzymes remains linear throughout the first 20 minutes of the reaction (at 37°C) and that the Km values for the oxidation of kynuramine by recombinant human MAO-A and MAO-B are 16.1±0.21 and 22.7±0.72 μM for MAO-A and MAO-B, respectively.24 These are similar to the values of 41 μM for MAO-A and 29 μM for MAO-B obtained in previous studies.23,25 The conditions of this study, specifically carrying out the reactions at 37°C for 20 minutes, are similar to that reported in the literature.23
Dialysis of enzyme/inhibitor mixtures
The protocol for the dialysis experiments has been reported in detail in recent publications.19,21 The reversibility of MAO-A and MAO-B inhibition by 5k and 5g, respectively, was investigated by measuring the recovery of enzyme activity after mixtures containing the enzyme and test inhibitor (at 4× IC50) were dialyzed. These experiments were carried out in triplicate, and the residual enzyme activities (as percentage of the negative control value) were expressed as mean ± standard deviation.
The construction of Lineweaver–Burk plots
The protocol for the construction of Lineweaver–Burk plots has been reported in detail in recent publications.19,21 A set of six Lineweaver–Burk plots was constructed for the inhibition of MAO-B by 5g. The first plot was constructed in the absence of inhibitor, and five plots were constructed in the presence of different concentrations of the test inhibitor (0.25 × IC50, 0.5 × IC50, 0.75 × IC50, 1 × IC50 and 1.25 × IC50). The Ki value was estimated by global (shared) fitting of the inhibition data directly to the Michaelis–Menten equation using the Prism 5 software package (GraphPad, San Diego, CA, USA) as well as by plotting of the slopes of the Lineweaver–Burke plots versus inhibitor concentration (x-axis intercept equals −Ki).
Results and discussion
Chemistry
The synthesis of the 3-coumaranone derivative 5a has previously been reported.22 This protocol was employed in the present study. Commercially available 6-hydroxy-3-coumaranone (6; 1 equiv) was reacted with an appropriately substituted arylalkyl bromide (1.1 equiv), with N, N-dimethylformamide serving as solvent and potassium carbonate (2 equiv) as base (Figure 1). After 24 hours reaction at room temperature, water was added and the product was extracted to dichloromethane. Recrystallization of the crude from cyclohexane yielded the target 3-coumaranone derivatives in acceptable yields (45%–66%). The 3-coumaranone derivatives, 5a–t, were characterized by 1H NMR, 13C NMR, and mass spectrometry.
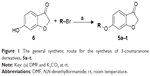  | Figure 1 The general synthetic route for the synthesis of 3-coumaranone derivatives, 5a–t. |
IC50 values for the inhibition of MAO
The MAO inhibitory properties of the 3-coumaranone derivatives were evaluated by using the recombinant human enzymes. The catalytic activities of both MAO isoforms were measured by using the MAO-A/B mixed substrate, kynuramine, according to the protocol already described in the literature.21,23 The MAO-catalyzed oxidation product of kynuramine, 4-hydroxyquinoline, may be conveniently measured by fluorescence spectrophotometry. By measuring MAO activities in the presence of various concentrations of the test inhibitor, sigmoidal dose–response curves (catalytic rate versus logarithm of inhibitor concentration) were constructed from which the IC50 values, as a measure of the inhibition potencies, were calculated.24
The MAO inhibition potencies of the 3-coumaranone derivatives (5a–t) are given in Table 2. From these data, the following observations may be made and conclusions drawn:
- The 3-coumaranone derivatives are selective for MAO-B over the MAO-A isoform. In all instances, the 3-coumaranone derivatives are more potent MAO-B inhibitors than MAO-A inhibitors, with the selectivity index (SI) values >3.5. The most selective inhibitors are compounds 5h, 5l, 5n, and 5q, which did not inhibit MAO-A, even at a concentration of 100 μM.
- Although the 3-coumaranone derivatives are selective for the MAO-B isoform, potent MAO-A inhibitors have been discovered. For example, compound 5k inhibits MAO-A with an IC50 value of 0.586 μM. This value is in the submicromolar range and may thus be viewed as potent inhibition. For comparison, toloxatone, a clinically used MAO-A inhibitor exhibits a reported IC50 value of 3.92 μM under identical experimental conditions.26 Compound 5k is thus a sixfold more potent MAO-A inhibitor than toloxatone. Other 3-coumaranones with MAO-A inhibition potencies comparable to that of toloxatone are 5d (IC50=2.19 μM), 5f (IC50=2.80 μM), 5g (IC50=3.92 μM), and 5r (IC50=2.65 μM).
- The 3-coumaranones are highly potent MAO-B inhibitors. With the exception of 5s, all of the 3-coumaranones display IC50 values in the submicromolar range (0.004–0.564 μM). The most potent MAO-B inhibitor discovered is compound 5g, with an IC50 value of 0.004 μM. For comparison, lazabemide and safinamide, reference MAO-B inhibitors, exhibit reported IC50 values of 0.091 and 0.048 μM, respectively, under identical experimental conditions.26 Compound 5g is thus a 22-fold more potent MAO-B inhibitor than lazabemide. Besides 5g, four other highly potent MAO-B inhibitors with IC50 values <0.020 μM were also discovered, compounds 5d–f and 5i.
- Limited SARs for the inhibition of the MAOs by the benzyloxy-substituted derivatives (5a–n) may be derived. Since derivatives 5a–n are all potent MAO-B inhibitors, few clear SARs are apparent. For the benzyloxy-substituted derivatives, no clear correlation between the position (meta and para) of the substituent on the benzyloxy ring and MAO-B inhibition potency is apparent, and high potency inhibitors are represented by compounds substituted on both the meta and para positions of the benzyloxy ring. Definitive correlations between the nature (alkyl and halogen) of the substituent and MAO-B inhibition potency also are not apparent, and high-potency inhibitors are represented by compounds substituted with halogens (F, Cl, Br, I) and alkyl groups (CN, CH3, CF3) on the benzyloxy ring. It is, however, noticeable that among the five most potent MAO-B inhibitors, the four compounds containing chlorine or bromine as substituents are included. As for MAO-A, no clear correlation between the position (meta and para) or nature (alkyl and halogen) of the substituent and MAO-A inhibition potency is apparent. Since only one of the 20 derivatives, compound 5k, inhibited MAO-A with an IC50 value in the submicromolar range, this particular substitution pattern (the 3-CN group) on the benzyloxy ring is more appropriate for MAO-A inhibition than the others considered in this study.
- It is interesting that the phenylethoxy-substituted derivatives, 5o and 5r–t, exhibit reduced MAO-B inhibition potency compared to the benzyloxy-substituted homologues. For example, 5o (IC50=0.107 μM) is a weaker MAO-B inhibitor than its benzyloxy-substituted homologue 5a (IC50=0.062 μM). In each instance, the benzyloxy-substituted homologues are more potent MAO-B inhibitors than the corresponding phenylethoxy-substituted compounds (compare 5r with 5f; 5s with 5l; 5t with 5m). It may thus be concluded that, for 3-coumaranones, phenylethoxy substitution is less appropriate for MAO-B inhibition than benzyloxy substitution.
- Increasing the length of the C6 substituent from benzyoxy (5a) to phenylethoxy (5o) to phenylpropoxy (5p) enhances MAO-A inhibition potency. This suggests that for 3-coumaranone derivatives, a larger C6 side chain is more appropriate for MAO-A inhibition, at least with respect to chain length. For MAO-B inhibition, a clear trend is not apparent, since the benzyoxy- and phenylpropoxy-substituted derivatives are more potent than the phenylethoxy-substituted derivative. Among these three homologues, the phenylpropoxy-substituted derivative is the most potent MAO-B inhibitor with an IC50 value of 0.055 μM.
- For the inhibition of MAO-B, the phenoxyethoxy-substituted derivative, 5q (IC50=0.050 μM), is approximately equipotent to the phenylpropoxy-substituted derivative 5p (IC50=0.055 μM). For the inhibition of MAO-B, the phenoxy oxygen of 5q may thus be viewed as isosteric with the CH2 of the phenylpropoxy substituent. The phenoxyethoxy-substituted derivative, in contrast, is not an inhibitor of MAO-A, up to a maximal tested concentration of 100 μM. Since the phenylpropoxy-substituted derivative is a MAO-A inhibitor with an IC50 value of 11.8 μM, it may tentatively be concluded that the phenoxy oxygen reduces MAO-A inhibition activity. A possible explanation for this observation is that the phenoxy oxygen confers more rigidity to the inhibitor structure, which may, as a result, be less suited for binding to the smaller MAO-A active site cavity.
- 6-Hydroxy-3-coumaranone (6) proved to be a weak MAO-A and MAO-B inhibitor, with IC50 values of >100 and 12.0 μM, respectively. Compared to the benzyloxy-substituted compound, 5a (IC50=0.062 μM), compound 6 is 193-fold weaker as a MAO-B inhibitor. This demonstrates that an appropriate C6 substituent is a requirement for MAO-B inhibition by the 3-coumaranone class of compounds.
MAO inhibition is reversible
Based on the potent MAO-B inhibition activities of the 3-coumaranone derivatives, the reversibility of MAO-B inhibition was investigated. For this purpose, a representative inhibitor, compound 5g, was combined with the MAO-B enzyme for 15 minutes and subsequently dialyzed for 24 hours. The concentration of 5g employed was equal to 4× IC50 for the inhibition of MAO-B. After dialysis, the residual MAO-B activity was measured and compared to the activity of MAO-B dialyzed in the absence of inhibitor (negative control). A similar dialysis study was conducted with the irreversible inhibitor, (R)-deprenyl, and served as a positive control. For reversible inhibition, enzyme activity is expected to recover to the level of the negative control after dialysis, while for irreversible inhibition, enzyme activity is not recovered. As shown in Figure 2, after dialysis of MAO-B/5g mixtures, MAO-B activity is recovered to 87% of the negative control. In contrast, the MAO-B activity of undialyzed mixtures of MAO-B and 5g is 42% of the negative control. After dialysis of MAO-B/(R)-deprenyl mixtures, the residual enzyme activity is only 3.1% of the negative control. These data suggest that 5g is a reversible MAO-B inhibitor since dialysis partially restores MAO-B inhibition.
Similar dialysis experiments (as for MAO-B) show that MAO-A inhibition by compound 5k also is reversible. Compound 5k (at 4× IC50) was selected as a representative MAO-A inhibitor and was combined with MAO-A for 15 minutes and subsequently dialyzed for 24 hours. Dialysis experiments with MAO-A in the absence of inhibitor and in the presence of the irreversible inhibitor, pargyline (at 4× IC50), served as negative and positive controls, respectively. After dialysis of MAO-A/5k mixtures, MAO-A activity is recovered to 109% of the negative control value (Figure 3). In contrast, the MAO-A activity of undialyzed mixtures of MAO-A and 5k is 65% of the negative control. After dialysis of MAO-A/pargyline mixtures, the residual enzyme activity is only 1.6% of the negative control. These data are consistent with a reversible interaction between 5k and MAO-A.
MAO inhibition is competitive
To determine the mode of inhibition (eg, competitive), Lineweaver–Burk plots were constructed for the inhibition of MAO-B by 5g. For this purpose, MAO-B activities were measured at eight different kynuramine concentrations (15–250 μM) in the absence of inhibitor and in the presence of five different inhibitor concentrations (0.001–0.005 μM). As shown in Figure 4, the set of Lineweaver–Burk plots is indicative of competitive inhibition since the plots intersect on the y-axis. Thus, it may be concluded that compound 5g most likely acts as a competitive inhibitor of human MAO-B. By constructing a plot of the slopes of the Lineweaver–Burk plots versus inhibitor concentration, a Ki value of 0.0045 μM for the inhibition of MAO-B by 5g is estimated. Global (shared) fitting of the inhibition data directly to the Michaelis–Menten equation yields a similar Ki value of 0.0034±0.0003 μM (r2 =0.99).
Conclusion
In conclusion, this study shows that 3-coumaranone derivatives are in general potent inhibitors of MAO-B. For example, five (5d–g and 5i) of the 20 compounds evaluated exhibited IC50 values <0.020 μM. With the exception of one compound (5s), all IC50 values were <1 μM for the inhibition of MAO-B. These good activities have been anticipated since series of structurally related α-tetralone and 1-indanone derivatives have recently been shown to potently inhibit MAO-B (Table 1).19–21 It is evident that potent MAO-B inhibitors are present among these reported series as well as among the 3-coumaranone derivatives. This study also finds that the 3-coumaranone derivatives are selective MAO-B inhibitors with comparatively weaker MAO-A inhibition potencies. SI values were >3.5. Only one compound (5k) inhibits MAO-A with an IC50<1 μM. Four compounds (5h, 5l, 5n, and 5q) did not inhibit MAO-A, even at a concentration of 100 μM (SI >1,852). These compounds are, however, potent MAO-B inhibitors (IC50=0.028–0.054 μM) and may be viewed as particularly appropriate where selective inhibition of MAO-B is required. Since the reported α-tetralone and 1-indanone derivatives exhibit IC50<10.75 μM for the inhibition of MAO-A, compounds 5h, 5l, 5n, and 5q may be viewed as superior with respect to isoform selectivity. As mentioned in the “Introduction” section, MAO-A inhibitors may cause tyramine-induced changes in blood pressure, and selective MAO-B inhibition could be a desirable trait. The finding that representative 3-coumaranones are reversible MAO inhibitors further diminishes the possibility that these compounds may provoke the cheese effect. This study thus shows that 3-coumaranone derivatives are potent and selective MAO-B inhibitors with existing clinical utilities (eg, Parkinson’s disease) and potential for new application for various disease states (Alzheimer’s disease, smoking cessation).27 Based on the significant MAO inhibitory potencies of 3-coumaranones, future studies should explore the effect of substitution at other positions, C4, C5, and C7 on MAO inhibition.
Acknowledgments
The NMR and MS spectra were recorded by André Joubert and Johan Jordaan of the SASOL Center for Chemistry, North-West University. This work is based on the research supported in part by the Medical Research Council and National Research Foundation of South Africa (grant specific unique reference numbers [UID] 96180 and 85642). The grantholders acknowledge that opinions, findings, and conclusions or recommendations expressed in any publication generated by the NRF-supported research are that of the authors and that the NRF accepts no liability whatsoever in this regard.
Disclosure
The authors report no conflicts of interest in this work.
References
Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999;22:197–217. | ||
Bach AW, Lan NC, Johnson DL, et al. cDNA cloning of human liver monoamine oxidase A and B: molecular basis of differences in enzymatic properties. Proc Natl Acad Sci U S A. 1988;85(13):4934–4938. | ||
Ramsay RR. Monoamine oxidases: the biochemistry of the proteins as targets in medicinal chemistry and drug discovery. Curr Top Med Chem. 2012;12(20):2189–2209. | ||
Ramsay RR. Inhibitor design for monoamine oxidases. Curr Pharm Des. 2013;19(14):2529–2539. | ||
Finberg JP. Update on the pharmacology of selective inhibitors of MAO-A and MAO-B: focus on modulation of CNS monoamine neurotransmitter release. Pharmacol Ther. 2014;143(2):133–152. | ||
Frampton JE, Plosker GL. Selegiline transdermal system: in the treatment of major depressive disorder. Drugs. 2007;67(2):257–265. | ||
George TP, Weinberger AH. Monoamine oxidase inhibition for tobacco pharmacotherapy. Clin Pharmacol Ther. 2008;83(4):619–621. | ||
Youdim MB, Bakhle YS. Monoamine oxidase: isoforms and inhibitors in Parkinson’s disease and depressive illness. Br J Pharmacol. 2006;147(Suppl 1):S287–S296. | ||
Edmondson DE. Hydrogen peroxide produced by mitochondrial monoamine oxidase catalysis: biological implications. Curr Pharm Des. 2014;20(2):155–160. | ||
Fowler JS, Volkow ND, Wang GJ, et al. Age-related increases in brain monoamine oxidase B in living healthy human subjects. Neurobiol Aging. 1997;18(4):431–435. | ||
Kaludercic N, Mialet-Perez J, Paolocci N, Parini A, Di Lisa F. Monoamine oxidases as sources of oxidants in the heart. J Mol Cell Cardiol. 2014;73:34–42. | ||
Maurel A, Hernandez C, Kunduzova O, et al. Age-dependent increase in hydrogen peroxide production by cardiac monoamine oxidase A in rats. Am J Physiol Heart Circ Physiol. 2003;284(4):H1460–H1467. | ||
Da Prada M, Zürcher G, Wüthrich I, Haefely WE. On tyramine, food, beverages and the reversible MAO inhibitor moclobemide. J Neural Transm Suppl. 1988;26:31–56. | ||
Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatr. 2012;73(Suppl 1):17–24. | ||
Bonnet U. Moclobemide: therapeutic use and clinical studies. CNS Drug Rev. 2003;9(1):97–140. | ||
Provost JC, Funck-Brentano C, Rovei V, D’Estanque J, Ego D, Jaillon P. Pharmacokinetic and pharmacodynamic interaction between toloxatone, a new reversible monoamine oxidase-A inhibitor, and oral tyramine in healthy subjects. Clin Pharmacol Ther. 1992;52(4):384–393. | ||
Pae CU, Bodkin JA, Portland KB, Thase ME, Patkar AA. Safety of selegiline transdermal system in clinical practice: analysis of adverse events from postmarketing exposures. J Clin Psychiatr. 2012;73(5):661–668. | ||
Finberg JP, Gillman K. Selective inhibitors of monoamine oxidase type B and the “cheese effect”. Int Rev Neurobiol. 2011;100:169–190. | ||
Legoabe LJ, Petzer A, Petzer JP. α-Tetralone derivatives as inhibitors of monoamine oxidase. Bioorg Med Chem Lett. 2014;24(12):2758–2763. | ||
Legoabe LJ, Petzer A, Petzer JP. The Synthesis and evaluation of C7-substituted α-tetralone derivatives as inhibitors of monoamine oxidase. Chem Biol Drug Des. Epub January 8, 2015. | ||
Mostert S, Petzer A, Petzer JP. Indanones as high-potency reversible inhibitors of monoamine oxidase. Chem Med Chem. 2015;10(5):862–873. | ||
Łączkowski KZ, Pakulski MM, Krzemiński MP, Jaisankar P, Zaidlewicz M. Asymmetric synthesis of N-substituted N-hydroxyureas. Tetrahedron Asymmetry. 2008;19(7):788–795. | ||
Novaroli L, Reist M, Favre E, Carotti A, Catto M, Carrupt PA. Human recombinant monoamine oxidase B as reliable and efficient enzyme source for inhibitor screening. Bioorg Med Chem. 2005;13(22):6212–6127. | ||
Strydom B, Malan SF, Castagnoli N Jr, Bergh JJ, Petzer JP. Inhibition of monoamine oxidase by 8-benzyloxycaffeine analogues. Bioorg Med Chem. 2010;18(3):1018–1028. | ||
Dunn RV, Marshall KR, Munro AW, Scrutton NS. The pH dependence of kinetic isotope effects in monoamine oxidase A indicates stabilization of the neutral amine in the enzyme-substrate complex. FEBS J. 2008;275(15):3850–3858. | ||
Petzer A, Pienaar A, Petzer JP. The inhibition of monoamine oxidase by esomeprazole. Drug Res (Stuttg). 2013;63(9):462–467. | ||
Cai Z. Monoamine oxidase inhibitors: promising therapeutic agents for Alzheimer’s disease (Review). Mol Med Rep. 2014;9(5):1533–1541. |
Supplementary materials
5a: 6-(Benzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 56%. mp 91.4°C–96.6°C. 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J =8.6 Hz, 1H), 7.40 (m, 4H), 7.35 (m, 1H), 6.71 (dd, J =8.6, 2.1 Hz, 1H), 6.60 (d, J =2.0 Hz, 1H), 5.11 (s, 2H), 4.60 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 70.6, 75.5, 97.4, 112.2, 114.5, 125.2, 127.5, 128.4, 128.8, 135.5, 167.2, 176.4, 197.5. APCI-HRMS m/z: calcd for C15H13O3 (MH+), 241.0859, found 241.0852.
5b: 6-(4-Fluorobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 55%. mp 121.6°C–126.5°C. 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J =8.6 Hz, 1H), 7.41–7.35 (m, 2H), 7.08 (t, J =8.7 Hz, 2H), 6.69 (dd, J =8.6, 2.1 Hz, 1H), 6.58 (d, J =2.1 Hz, 1H), 5.06 (s, 2H), 4.60 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.9, 75.5, 97.3, 99.9, 112.1, 114.6, 115.8, 125.2, 129.4, 131.3, 161.9, 163.5, 166.9, 176.3, 197.5. APCI-HRMS m/z: calcd for C15H12FO3 (MH+), 259.0765, found 259.0764.
5c: 6-(3-Fluorobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 58%. mp 56.2°C–56.3°C. 1H NMR (600 MHz, CDCl3) δ 7.57 (d, J =8.6 Hz, 1H), 7.35 (td, J =7.9, 5.8 Hz, 1H), 7.17 (d, J =8.1 Hz, 1H), 7.12 (d, J =9.4 Hz, 1H), 7.03 (td, J =8.4, 2.2 Hz, 1H), 6.71 (dd, J =8.6, 2.1 Hz, 1H), 6.58 (d, J =2.0 Hz, 1H), 5.11 (s, 2H), 4.61 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.7, 75.5, 97.4, 112.1, 114.2, 114.8, 115.4, 122.7, 125.3, 130.3, 138.1, 162.2, 166.8, 176.3, 197.5. APCI-HRMS m/z: calcd for C15H12FO3 (MH+), 259.0765, found 259.0752.
5d: 6-(4-Chlorobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 51%. mp 137.5°C–141.7°C. 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J =8.6 Hz, 1H), 7.37–7.33 (m, 4H), 6.69 (dd, J =8.6, 2.1 Hz, 1H), 6.57 (d, J =2.1 Hz, 1H), 5.07 (s, 2H), 4.60 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.7, 75.5, 97.4, 112.1, 114.7, 125.2, 128.8, 128.9, 134.0, 134.3, 166.9, 176.3, 197.5. APCI-HRMS m/z: calcd for C15H12ClO3 (MH+), 275.0469, found 275.0452.
5e: 6-(3-Chlorobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 57%. mp 108.7°C–112.3°C. 1H NMR (600 MHz, CDCl3) δ 7.47 (d, J =8.6 Hz, 1H), 7.31 (s, 1H), 7.24–7.17 (m, 3H), 6.61 (dd, J =8.6, 2.0 Hz, 1H), 6.48 (d, J =2.0 Hz, 1H), 4.98 (s, 2H), 4.51 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.6, 75.5, 97.4, 112.0, 114.8, 125.2, 125.3, 127.4, 128.5, 130.0, 134.7, 137.5, 166.8, 176.3, 197.5. APCI-HRMS m/z: calcd for C15H12ClO3 (MH+), 275.0469, found 275.0453.
5f: 6-(4-Bromobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 51%. mp 140.0°C–142.1°C.1H NMR (600 MHz, CDCl3) δ 7.56 (d, J =8.6 Hz, 1H), 7.52 (d, J =8.4 Hz, 2H), 7.28 (d, J =8.4 Hz, 2H), 6.69 (dd, J =8.6, 2.1 Hz, 1H), 6.57 (d, J =2.0 Hz, 1H), 5.06 (s, 2H), 4.60 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.8, 75.5, 97.4, 112.1, 114.7, 122.4, 125.2, 129.1, 131.9, 134.5, 166.8, 176.3, 197.5. APCI-HRMS m/z: calcd for C15H12BrO3 (MH+), 318.9964, found 318.9963.
5g: 6-(3-Bromobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 50%. mp 120.0°C–122.6°C. 1H NMR (600 MHz, CDCl3) δ 7.59–7.54 (m, 2H), 7.47 (d, J =7.9 Hz, 1H), 7.32 (d, J =7.7 Hz, 1H), 7.26 (d, J =7.8 Hz, 1H), 6.70 (dd, J =8.6, 2.1 Hz, 1H), 6.57 (d, J =2.1 Hz, 1H), 5.07 (s, 2H), 4.61 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.5, 75.5, 97.4, 112.0, 114.8, 122.8, 125.3, 125.8, 130.3, 130.3, 131.5, 137.8, 166.8, 176.3, 197.5. APCI-HRMS m/z: calcd for C15H12BrO3 (MH+), 318.9964, found 318.9955.
5h: 6-(4-Iodobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 52%. mp 136.0°C–138.5°C. 1H NMR (600 MHz, CDCl3) δ 7.72 (d, J =8.1 Hz, 2H), 7.56 (d, J =8.6 Hz, 1H), 7.15 (d, J =8.1 Hz, 2H), 6.68 (dd, J =8.6, 1.5 Hz, 1H), 6.56 (d, J =1.6 Hz, 1H), 5.05 (s, 2H), 4.60 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.9, 75.5, 94.0, 97.4, 112.1, 114.7, 125.3, 129.2, 135.2, 137.9, 166.8, 176.3, 197.5. APCI-HRMS m/z: calcd for C15H12IO3 (MH+), 366.9826, found 366.9805.
5i: 6-(3-Iodobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 51%. mp 161.2°C–164.5°C. 1H NMR (600 MHz, CDCl3) δ 7.77 (s, 1H), 7.68 (d, J =8.0 Hz, 1H), 7.57 (d, J =8.6 Hz, 1H), 7.36 (d, J =8.1 Hz, 1H), 7.12 (t, J =7.8 Hz, 1H), 6.70 (dd, J =8.6, 2.1 Hz, 1H), 6.57 (d, J =2.0 Hz, 1H), 5.05 (s, 2H), 4.61 (s, 2H).13C NMR (151 MHz, CDCl3) δ 69.5, 75.6, 94.5, 97.4, 112.0, 114.8, 125.3, 126.5, 130.4, 136.3, 137.5, 137.8, 166.8, 176.3, 197.5. APCI-HRMS m/z: calcd for C15H12IO3 (MH+), 366.9826, found 366.9809.
5j: 6-(4-Cyanobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 59%. mp 185.8°C–187.2°C. 1H NMR (600 MHz, CDCl3) δ 7.68 (d, J =8.2 Hz, 2H), 7.57 (d, J =8.6 Hz, 1H), 7.52 (d, J =8.3 Hz, 2H), 6.70 (dd, J =8.6, 2.1 Hz, 1H), 6.56 (d, J =2.0 Hz, 1H), 5.17 (s, 2H), 4.60 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.3, 75.5, 97.5, 111.8, 112.2, 115.0, 118.4, 125.4, 127.5, 132.5, 140.9, 166.4, 176.2, 197.4. APCI-HRMS m/z: calcd for C16H12NO3 (MH+), 266.0812, found 266.0812.
5k: 6-(3-Cyanobenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 59%. mp 148.4°C–155.9°C. 1H NMR (600 MHz, CDCl3) δ 7.72 (s, 1H), 7.67–7.61 (m, 2H), 7.58 (d, J =8.6 Hz, 1H), 7.51 (t, J =7.8 Hz, 1H), 6.71 (dd, J =8.6, 2.1 Hz, 1H), 6.58 (d, J =2.1 Hz, 1H), 5.14 (s, 2H), 4.61 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.1, 75.6, 97.5, 111.9, 113.0, 115.0, 118.4, 125.4, 129.6, 130.7, 131.5, 132.0, 137.2, 166.4, 176.2, 197.5. APCI-HRMS m/z: calcd for C16H12NO3 (MH+), 266.0812, found 266.0797.
5l: 6-(4-Methylbenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 52%. mp 104.9°C–109.2°C. 1H NMR (600 MHz, CDCl3) δ 7.55 (d, J =8.6 Hz, 1H), 7.29 (d, J =8.0 Hz, 2H), 7.20 (d, J =7.8 Hz, 2H), 6.69 (dd, J =8.6, 2.1 Hz, 1H), 6.59 (d, J =2.1 Hz, 1H), 5.06 (s, 2H), 4.59 (s, 2H), 2.35 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 21.2, 70.6, 75.5, 97.3, 112.2, 114.4, 125.1, 127.7, 129.4, 132.4, 138.3, 167.3, 176.4, 197.5. APCI-HRMS m/z: calcd for C16H15O3 (MH+), 255.1016, found 255.1003.
5m: 6-(3-Methylbenzyloxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 54%. mp 158.4°C–161.7°C. 1H NMR (600 MHz CDCl3,) δ 7.56 (d, J =8.6 Hz, 1H), 7.15 (d, J =7.5 Hz, 1H), 7.23–7.19 (m, 2H), 7.28 (t, J =7.5 Hz, 1H), 6.71 (dd, J =8.6, 2.1 Hz, 1H), 6.60 (d, J =2.0 Hz, 1H), 5.07 (s, 2H), 4.60 (s, 2H), 2.36 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 21.4, 70.7, 75.5, 97.4, 112.2, 114.5, 124.6, 125.1, 128.2, 128.6, 129.2, 135.4, 138.5, 167.3, 176.4, 197.6. APCI-HRMS m/z: calcd for C16H15O3 (MH+), 255.1016, found 255.1020.
5n: 6-[4-(Trifluoromethyl)benzyloxy]-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 45%. mp 137.5°C–143.8°C. 1H NMR (600 MHz, CDCl3) δ 7.65 (d, J =8.1 Hz, 2H), 7.57 (d, J =8.6 Hz, 1H), 7.53 (d, J =8.0 Hz, 2H), 6.71 (dd, J =8.6, 2.1 Hz, 1H), 6.58 (d, J =2.1 Hz, 1H), 5.17 (s, 2H), 4.61 (s, 2H). 13C NMR (151 MHz, CDCl3) δ 69.6, 75.5, 97.5, 112.0, 114.9, 123.0, 124.8, 125.3, 125.7 (q), 127.4, 130.5 (q), 139.5, 166.7, 176.3, 197.5. APCI-HRMS m/z: calcd for C16H12F3O3 (MH+), 309.0733, found 309.0720.
5o: 6-(2-Phenylethoxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 50%. mp 77.1°C–84.8°C. 1H NMR (600 MHz, CDCl3) δ 7.53 (d, J =8.6 Hz, 1H), 7.33–7.30 (m, 2H), 7.27–7.23 (m, 3H), 6.62 (dd, J =8.6, 2.0 Hz, 1H), 6.51 (d, J =2.0 Hz, 1H), 4.59 (s, 2H), 4.22 (t, J =7.0 Hz, 2H), 3.11 (t, J =7.0 Hz, 2H). 13C NMR (151 MHz, CDCl3) δ 35.4, 69.3, 75.5, 96.9, 112.0, 114.3, 125.1, 126.8, 128.6, 128.9, 137.5, 167.4, 176.5, 197.6. APCI-HRMS m/z: calcd for C16H15O3 (MH+), 255.1016, found 255.1011.
5p: 6-(3-Phenylpropoxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 53%. mp 84.4°C–96.7°C. 1H NMR (600 MHz CDCl3,) δ 7.54 (d, J =8.6 Hz, 1H), 7.30–7.27 (m, 2H), 7.21–7.18 (m, 3H), 6.63 (dd, J =8.6, 2.1 Hz, 1H), 6.48 (d, J =2.0 Hz, 1H), 4.59 (s, 2H), 3.99 (t, J =6.3 Hz, 2H), 2.80 (t, J =7.6 Hz, 2H), 2.16–2.09 (m, 2H). 13C NMR (151 MHz, CDCl3) δ 30.3, 31.9, 67.6, 75.5, 96.8, 112.0, 114.2, 125.0, 126.1, 128.4, 128.5, 140.9, 167.6, 176.5, 197.5. APCI-HRMS m/z: calcd for C17H17O3 (MH+), 269.1172, found 269.1180.
5q: 6-(2-Phenoxyethoxy)-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 53%. mp 156.2°C–158.2°C. 1H NMR (600 MHz, CDCl3) δ 7.56 (d, J =8.6 Hz, 1H), 7.30 (td, J =7.4, 2.0 Hz, 2H), 6.97 (t, J =7.4 Hz, 1H), 6.94 (d, J =7.9 Hz, 2H), 6.68 (dd, J =8.6, 2.1 Hz, 1H), 6.58 (d, J =2.0 Hz, 1H), 4.62 (s, 2H), 4.39–4.36 (m, 2H), 4.35–4.33 (m, 2H). 13C NMR (151 MHz, CDCl3) δ 65.9, 67.2, 75.5, 97.1, 112.0, 114.6, 114.6, 121.3, 125.2, 129.6, 158.3, 167.1, 176.4, 197.6. APCI-HRMS m/z: calcd for C16H15O4 (MH+), 271.0965, found 271.0953.
5r: 6-[2-(4-Bromophenyl)ethoxy]-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 45%. mp 40.7°C–40.8°C. 1H NMR (600 MHz, CDCl3) δ 7.53 (d, J =8.6 Hz, 1H), 7.43 (d, J =8.4 Hz, 2H), 7.14 (d, J =8.4 Hz, 2H), 6.60 (dd, J =8.6, 2.1 Hz, 1H), 6.49 (d, J =2.0 Hz, 1H), 4.59 (s, 2H), 4.19 (t, J =6.7 Hz, 2H), 3.06 (t, J =6.7 Hz, 2H). 13C NMR (151 MHz, CDCl3) δ 34.8, 68.9, 75.5, 96.9, 111.9, 114.5, 120.6, 125.1, 130.7,131.7, 136.6, 167.2, 176.4, 197.5. APCI-HRMS m/z: calcd for C16H14BrO3 (MH+), 333.0121, found 333.0129.
5s: 6-[2-(4-Methylphenyl)ethoxy]-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 56%. mp 98.6°C–104.2°C. 1H NMR (600 MHz, CDCl3) δ 7.53 (d, J =8.6 Hz, 1H), 7.15 (d, J =8.1 Hz, 2H), 7.12 (d, J =8.0 Hz, 2H), 6.62 (dd, J =8.6, 2.1 Hz, 1H), 6.50 (d, J =2.0 Hz, 1H), 4.59 (s, 2H), 4.19 (t, J =7.1 Hz, 2H), 3.07 (t, J =7.0 Hz, 2H), 2.32 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 21.0, 35.0, 69.5, 75.5, 96.8, 112.0, 114.3, 125.1, 128.8, 129.3, 134.3, 136.3, 167.4, 176.5, 197.6. APCI-HRMS m/z: calcd for C17H17O3 (MH+), 269.1172, found 269.1159.
5t: 6-[2-(3-Methylphenyl)ethoxy]-2H-1-benzofuran-3-one
The title compound was prepared in a yield of 66%. mp 209.3°C–212.2°C. 1H NMR (600 MHz, CDCl3) δ 7.54 (d, J =8.6 Hz, 1H), 7.20 (t, J =7.5 Hz, 1H), 7.09–7.04 (m, 3H), 6.63 (dd, J =8.6, 2.0 Hz, 1H), 6.51 (d, J =2.0 Hz, 1H), 4.60 (s, 2H), 4.20 (t, J =7.1 Hz, 2H), 3.07 (t, J =7.1 Hz, 2H), 2.33 (s, 3H). 13C NMR (151 MHz, CDCl3) δ 21.4, 35.3, 69.4, 75.5, 96.9, 112.0, 114.3, 125.1, 125.9, 127.5, 128.5, 129.7, 137.3, 138.2, 167.4, 176.5, 197.6. APCI-HRMS m/z: calcd for C17H17O3 (MH+), 269.1172, found 269.1159.
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




