Back to Journals » Drug Design, Development and Therapy » Volume 14
1,25(OH)2D3 Strengthens the Vasculogenesis of Multipotent Mesenchymal Stromal Cells from Rat Bone Marrow by Regulating the PI3K/AKT Pathway
Authors Ye B, Weng Y, Lin S, Lin J, Huang Z, Huang W, Cai X
Received 7 July 2019
Accepted for publication 3 March 2020
Published 16 March 2020 Volume 2020:14 Pages 1157—1167
DOI https://doi.org/10.2147/DDDT.S222244
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sukesh Voruganti
Bozhi Ye, 1,* Yawen Weng, 2,* Shuang Lin, 1 Jiahui Lin, 3 Zhouqing Huang, 1 Weijian Huang, 1 Xueli Cai 1
1Department of Cardiology, The Key Lab of Cardiovascular Disease of Wenzhou, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 2Department of Pediatrics, The Second School of Medicine, Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China; 3The First School of Medicine, Wenzhou Medical University, Wenzhou, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xueli Cai
Department of Cardiology, The Key Lab of Cardiovascular Disease of Wenzhou, The First Affiliated Hospital of Wenzhou Medical University, 2 Fuxue Road, ZheJiang 325000, People’s Republic of China
Email [email protected]
Weijian Huang
Department of Cardiology, The Key Lab of Cardiovascular Disease of Wenzhou, The First Affiliated Hospital of Wenzhou Medical University, 2 Fuxue Road, ZheJiang 325000, People’s Republic of China
Email [email protected]
Background: Multipotent mesenchymal stromal cells (MSCs) have recently been reported to promote vasculogenesis by differentiating into endothelial cells and releasing numerous cytokines and paracrine factors. However, due to low cell activity, their potential for clinical application is not very satisfactory. This study aimed to explore the effects and mechanisms of 1,25-dihydroxyvitamin D (1,25(OH) 2D 3) on the vasculogenesis of MSCs.
Methods: MSCs were isolated from the femurs and tibias of rats and characterized by flow cytometry. After treatment with different concentrations of 1,25(OH) 2D 3 (0 μM, 0.1 μM and 1 μM), the proliferation of MSCs was analyzed by Cell Counting Kit-8 (CCK-8), and the migratory capability was measured by Transwell assays and cell scratch tests. Capillary-like structure formation was observed by using Matrigel. Western blotting was used to detect the expression of FLK-1 and vWF to investigate the differentiation of MSCs into endothelial cells. Western blotting and gelatin zymography were used to detect the expression and activities of VEGF, MMP-2 and MMP-9 secreted by MSCs under the influence of 1,25(OH) 2D 3. Finally, the VDR antagonist pyridoxal-5-phosphate (P5P) and the PI3K/AKT pathway inhibitor LY294002 were utilized to test the phosphorylation levels of key kinases in the PI3K/AKT pathway by Western blotting and the formation of capillary-like structures in Matrigel.
Results: The proliferation and migratory capability of MSCs and the ability of MSCs to form a tube-like structure in Matrigel were enhanced after treatment with 1,25(OH) 2D 3. Moreover, MSCs treated with 1,25(OH) 2D 3 showed high expression of vWF and Flk-1. There was a significant increase in the expression of VEGF, MMP-2 and MMP-9 secreted by MSCs treated with 1,25(OH) 2D 3, as well as in the activity of MMP-2 and MMP-9. The phosphorylation level of AKT increased with time after 1,25(OH) 2D 3 treatment, while LY294002 weakened AKT phosphorylation. In addition, the ability to form capillary-like structures was reduced when the VDR and PI3K/AKT pathways were blocked.
Conclusion: This study confirmed that 1,25(OH) 2D 3 treatment can strengthen the ability of MSCs to promote vasculogenesis in vitro, and the mechanism may be related to the activation of the PI3K/AKT pathway.
Keywords: multipotent mesenchymal stromal cells, 1,25-(OH) 2D 3, vasculogenesis, vitamin D receptor, PI3K/AKT pathway
Introduction
Multipotent mesenchymal stromal cells (MSCs) are non-hematopoietic stem cells with multidirectional differentiation and self-replication that can be directly induced to differentiate into various cell types, including osteoblasts, chondrocytes, adipocytes, endotheliocytes and cardiocytes.1 MSCs are easily expanded, retain stem cell characteristics in vitro, and have recently been considered to affect the progression of vasculogenesis, which mainly occurs in two ways.2 First, MSCs can differentiate into vascular endothelial cells and smooth muscle cells, thereby directly contributing to vasculogenesis.3,4 Second, transplanted MSCs release numerous cytokines and paracrine factors. The expression of some paracrine factors, such as vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), matrix metalloprotein 2 (MMP-2), matrix metalloprotein 9 (MMP-9) and p-Cdc42/Rac1, was confirmed to be largely increased in stimulated MSCs.5 However, in most cases, limited by hypoxia and low nutrient levels in the transplanted environment, the cellular viability of MSCs and the density of neovascularization tend to be low, especially in some ischemic diseases.6,7 Thus, to achieve high efficacy of MSCs transplantation, it is important to focus on improving cellular viability and activity.
The active vitamin D metabolite 1,25-dihydroxyvitamin D (1,25(OH)2D3) is a fat-soluble steroid hormone precursor whose function was recently considered to affect cellular proliferation, differentiation, and immune modulation, and its function is mainly mediated by binding to the vitamin D receptor (VDR), a member of the nuclear hormone receptor superfamily.8 Grundmann9 found that 1,25(OH)2D3 upregulates the expression of VEGF and the activity of MMP-2, thereby enhancing vasculogenesis of cord blood-derived endothelial progenitor cells (EPCs) in vitro. Moreover, it has been reported that 1,25(OH)2D3 promotes capillary-like tubule formation and migration of endothelial colony-forming cells (ECFCs) in culture, minimizing the negative effects of exposure to preeclampsia-related factors.10 However, it is unclear whether 1,25(OH)2D3 is involved in the differentiation and vasculogenesis of MSCs.
In our study, MSCs were treated with different concentrations of 1,25(OH)2D3 to explore their role in vasculogenesis. Furthermore, we explored the possible mechanisms of 1,25(OH)2D3-associated vasculogenesis, which will provide a theoretical basis for clinical treatments using MSCs.
Methods
Primary MSCs Isolation and Culture
Two-week-old male Sprague-Dawley (SD) rats were purchased from the Animal Experiment Center of Wenzhou Medical University and housed under a specific pathogen-free environment. The femur and tibia of the rats were isolated and carefully washed with phosphate-buffered saline (PBS). Bone marrow was then obtained, and every 10 mL of bone marrow was followed by 6 mL of lymphocyte separation (Solarbio, Beijing, China). After centrifugation at 1000 rpm for 20 mins, the white floc layer of the intermediate interface was aspirated, added to the culture medium (Dulbecco’s Modified Eagle’s Medium, HyClone, New York, US. Ten percent foetal bovine serum, GIBCO, Grand Island, US. Penicillin-Streptomycin Solution 100×, Solarbio, Beijing, China), and washed twice. The flushing fluid was seeded into culture medium and cultured in an incubator (Thermo Fisher Scientific, Massachusetts, US) containing 95% air and 5% CO2 at 37°C.
After 2 d of incubation, the culture media were changed to remove non-adherent cells. A fresh and complete medium was added and changed every 2 d until the confluence reached 80–90%. Thereafter, cells were passaged at 1:2 and cultured again. The cells used in this experiment were all harvested from the third passage.
All experiments were performed with the approval of the Wenzhou Medical University Animal Ethics Committee (wydw2016-0170) and in accordance with the China Animal Welfare Legislation, as well as the guide for the care and use of laboratory animals.
Characterization of MSCs by Flow Cytometry
MSCs (3×106 cells) were collected, washed with PBS, and centrifuged at 1000 rpm for 5 mins. According to the manufacturer’s instructions, cells were then incubated with antibodies against CD29 (Biolegend, California, USA), CD90 (Biolegend, California, USA), CD105 (Bioscience, California, USA), CD45 (Abcam, Cambridge, UK) and negative control (Biolegend, California, USA) followed by flow cytometric analysis using the Flow Cytometer System (BD, New Jersey, USA).
Cell Culture and Groups
MSCs cells were treated with complete medium containing 1,25(OH)2D3 at concentrations of 0 µM, 0.1 µM and 1 µM for 48 h, and then cultured for 3–7 d. The cells were cultured under the same conditions (95% air and 5% CO2 at 37°C) and divided into four groups: the normal MSCs group, the 1,25(OH)2D3 (Sigma-Aldrich, St Louis, MO, USA) treatment group, the 1,25(OH)2D3 and pyridoxal-5-phosphate (P5P) (Sigma-Aldrich, St Louis, MO, USA) treatment group, and the 1,25(OH)2D3 and LY294002 (Beyotime, Shanghai, China) treatment group. Each group had three duplicate wells. In addition, in the experiment group, MSCs with 0.1 µM 1,25(OH)2D3 were exposed to the pyridoxal-5-phosphate (0.5 µM) or LY294002 solution at the required concentration for 24 h.
Cell Proliferation Assay
Cell Counting Kit-8 (CCK-8, Signalway Antibody, Maryland, USA) was used for analysis of the influence of 1,25(OH)2D3 on the proliferation of MSCs according to the manufacturer’s instructions. The cells (3×104 cells/well) of different groups were seeded in a 96-well plate with three wells per group and 100 µL per well. The cells were cultured in the incubator for 0, 1 d, 3 d or 7 d. Then, 10 µL of CCK-8 solution was added into each well, and the mixture was incubated for 1 h with 5% CO2 at 37°C. Absorbance was measured at 450 nm using a Microplate Reader (Perlong medical, Beijing, China).
Cell Migration Assay
Cell migration was measured by a Transwell filter system (Corning-Costar, New York, US) with a filter with 8 µm pores. After culture in serum-free medium for 24 h, the cells (2×105 cells/well, 300 µL) of the different groups were seeded into the upper chamber of the insert, and a complete medium (700 µL) was added to the lower chamber. The Transwell filter systems were then incubated for 72 h at 37°C with 5% CO2. The cells were fixed with methanol (Sinpharm, Beijing, China), and the cells that remained on the upper surface of the filter were removed carefully with a cotton swab. The cells were stained with 0.5% crystal violet (Solarbio, Beijing, China) for 30 mins and counted under a microscope (Caikon, Shanghai, China).
Cell scratch test can also be used to examine cell migration. After culture for 24 h, the cells (8×105 cells/well) of the different groups were seeded into the culture medium. A 10 µL pipette tip was used to draw a line in the middle of the dish, and the cells were cultured in an incubator for 24 h. Pictures of the scratches were taken at 0 h and 24 h.
Cell Lumen Formation
Matrigel gel (Corning-Costar, New York, US) was thawed in a refrigerator at 4°C overnight, seeded onto a 12-well plate (80 µL per well), and placed in an incubator for 30 mins to coagulate. Cells were seeded onto the 12-well plate and cultured overnight in the incubator. The cells were then cultured for 3–7 d and a picture was taken. Image J software (NIH Image) was used for quantification of capillary-like structures, and tube length and branching points were analyzed after 6 h by two independent observers. Tube length was measured as the sum of tubular circumference formed by MSCs in one field of vision (microscope at 100×). Branching points were measured as the number of branching points formed by MSCs in one field of vision (magnification 100×).
Western Blotting
The cellular lysates were scraped and collected in eppendorf tubes on ice, then heat at 100°C for 10 mins and centrifuged at 12,000 rpm for 10 mins at 4°C, the supernatants were collected and frozen at −80°C still use. Protein concentration was quantified by BCA protein assays. Equal amounts of protein (25 µg/lane) were separated by electrophoresis through 10% and 12% SDS-PAGE and transferred onto nitrocellulose (NC) membranes (Millipore, Massachusetts, USA), then the membranes were blocked with non-fat milk for 1 h at room temperature. According to the manufacturer’s instructions, the primary antibodies as follows Flk-1 (1:200; Santa Cruz, Dallas, TX, USA), vWF (1:1000, Abcam, Cambridge, UK), VEGF (1:1000, Proteintech, Chicago, USA), AKT (1:1000, Cell Signaling Technology, Boston, USA), p-AKT (1:2000, Cell Signaling Technology, Boston, USA), MMP-2 (1:1000, Abcam, Cambridge, UK), MMP-9 (1:1000, Abcam, Cambridge, UK), GAPDH (1:2000, Cell Signaling Technology, Boston, USA) were incubated with the membrane at 4°C overnight, respectively. After that, washing the membranes and incubating them with secondary antibody conjugated to horseradish peroxidase (HRP; 1:1000) for 1 h at 37°C. After being washed again, protein bands were quantified using the Tanon-5200 Image Analyzer.
Gelatine Zymography
The protein samples (25 µL) extracted from the cells were electrophoretically separated on a 10% SDS-PAGE containing gelatine (1 mg/mL) at 4°C. Next, the gels were washed twice for 30 mins in eluent that included 2.5% Triton X-100, 50 mM Tris-HCl and 5 mM CaCl2 (pH 7.6, Jrdun Biotechnology, Shanghai, China). The gels were washed three times in pure water for 20 mins, and then incubated in the medium (pH 7.6, 50 mM Tris-HCl, 5 mM CaCl2, 0.02% Brij-35) at 37°C for 20 h. After staining with 0.05% Coomassie Brilliant Blue (Jrdun Biotechnology, Shanghai, China), the gels were photographed by a Tanon-5200 Image Analyzer.
Statistical Analysis
All experiments were repeated three times. Statistical analyses were performed using Graphpad prism 8 software (GraphPad, San Diego, CA, USA). The results of the experiments are displayed as the mean±SEM, and normality was determined using the Shapiro–Wilk test. Statistical evaluation of the data was performed by using the unpaired Student’s test to compare variables between two groups, and One-way analysis of variance (ANOVA) followed by post hoc tests was used for the comparisons of multiple groups. A P-value of <0.05 was considered to indicate a statistically significant result.
Results
Characterization of MSCs
To verify whether the acquired cells were MSCs, we used flow cytometric analysis to determine the expression of the BMSC-specific molecules CD29, CD90, and CD105 and the hematopoietic marker CD45. The acquired cells exhibited strong positive signals for CD29, CD90 and CD105 (Figure 1A–C) but were negative for CD45 (Figure 1D). The negative control for flow cytometry is shown in Figure 1E. These results suggest that the cells we acquired were MSCs.
1,25(OH)2D3 Promotes the Proliferation and Migration of MSCs
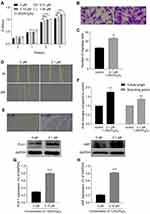
The proliferation of MSCs was detected by CCK-8. Compared with the viability in the control group, the viability of the cells was significantly increased after the 0.1 μM and 1 μM 1,25(OH)2D3 treatment, although the effect of 0.01 μM 1,25(OH)2D3 was not obvious (Figure 2A).
Next, we used the Transwell system to detect the migration of MSCs. The number of cells treated with 0.1 μM 1,25(OH)2D3 that migrated from the upper layer to the lower layer in the Transwell chamber was greater than that in the control group, suggesting that the migratory ability of MSCs was strengthened (Figure 2B and C). Similarly, after 24 h of wound healing, the images showed that the number of cells that migrated to the middle was significantly increased and almost fused the scratch with 0.1 µM 1,25(OH)2D3 treatment compared to that of the control group (Figure 2D). These results suggest that 1,25(OH)2D3 promotes the proliferation and migration of MSCs.
1,25(OH)2D3 Promotes the Vasculogenesis and Differentiation of MSCs into Endothelial in vitro
1,25(OH)2D3 treatment promoted the formation of capillary-like structures by MSCs in vitro. MSCs incubated with 0.1 μM 1,25(OH)2D3 showed significantly more endothelial tube formation and neovascularization in Matrigel than those in the control group (Figure 2E). The tube lengths were 1.757 higher and the branching points were 1.363 times higher (1.757 ± 0.065 and 1.363 ± 0.026; P < 0.05; n = 3) (Figure 2F).
Endothelial cells are polygonal or flat and form the inner wall of the blood vessel. Research has shown that stimulated human adipose mesenchymal stem cells (ASCs) enhance neovascularization by differentiation into endothelial cells, manifested by high expression of endothelium-specific genes such as von Willebrand factor (vWF) and Flk-1 and robust in vitro microvascular formation.11 After treatment with 0.1 μM 1,25(OH)2D3, we detected the expression of Flk-1 and vWF in the MSCs culture. As expected, Western blotting indicated high expression of Flk-1 and vWF compared with that of the control group, which suggests that the MSC-derived cells are indeed functional endothelial cells (Figure 2G and H).
VEGF, MMP-2 and MMP-9 are Involved in the Vasculogenesis Effect of 1,25(OH)2D3 on MSCs
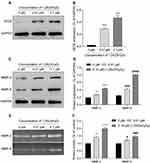
VEGF, MMP-2 and MMP-9 have been reported to play an important role in the process of vasculogenesis.12,13 To explore whether VEGF, MMP-2 and MMP-9 are involved in the vasculogenesis effect of 1,25(OH)2D3 on MSCs, we detected their expression and activity in MSCs treated with different concentrations of 1,25(OH)2D3 (0 µM, 0.1 µM and 1 µM). Western blotting analysis showed that 1,25(OH)2D3 induced a significant dose-dependent increase, with a maximal response at 0.1 µM, in the protein expression of VEGF in cell lysates (Figure 3A and B), and both MMP-2 and MMP-9 showed the same tendency (Figure 3C and D). Gelatine zymography analysis showed that the activity of MMP-2 and MMP-9 in the conditioned media was significantly upregulated, with maximal activity at 0.1 µM 1,25(OH)2D3 treatment (Figure 3E and F).
Effect of VDR Blockade or PI3K/AKT Pathway Activation on the Vasculogenesis of 1,25(OH)2D3-Treated MSCs
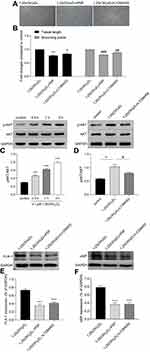
The genomic action of 1,25(OH)2D3 is mediated through the interaction of the VDR with vitamin D response elements (VDREs) of the target genes.14 To explore whether VDR is involved in the 1,25(OH)2D3-promotion of the vasculogenesis of MSCs, we tested the capillary-like structures formation of MSCs after P5P blocking. Matrigel showed a decrease in the formation of capillary-like structures compared with the 0.1µM 1,25(OH)2D3 treatment group (Figure 4A), with 0.773 times lower tube length and 0.796 times lower branching points (0.773 ± 0.032 and 0.796 ± 0.024; P < 0.05; n = 3) (Figure 4B).
To further explore the underlying mechanisms by which 1,25(OH)2D3 promotes vasculogenesis of MSCs, we evaluated cell lysates for the phosphorylation of key kinases involved in the PI3K/AKT pathway, which is known to regulate MMPs and VEGF and plays a role in angiogenesis. After treatment with 1,25(OH)2D3 for 30 mins, 1 h or 3 h, the expression of p-AKT showed a time-dependent increase, and there was no difference in the expression of total AKT compared with that of the control group (Figure 4C). 1,25(OH)2D3-activated phosphorylation was significantly inhibited by the specific PI3K inhibitor LY294002. After LY294002 treatment, the expression of p-AKT was obviously decreased compared with that of the 1,25(OH)2D3 treatment group (Figure 4D).
The blocking differences could also clearly and significantly be seen in Matrigel, in which the formation of capillary-like structures was decreased (Figure 4A). The tube lengths were 0.836 times lower and the branching points were 0.876 times lower compared with the 0.1 μM 1,25(OH)2D3 treatment group (0.836 ± 0.024 and 0.876 ± 0.024; P<0.05; n=3) (Figure 4B).
In addition, the expression of FLK-1 and vWF obviously decreased after blocking the VDR or PI3K pathway, which indicated that VDR binding and the PI3K pathway were involved in the 1,25(OH)2D3-mediated differentiation of MSCs to endothelial cells (Figure 4E and F).
Discussion
This study successfully isolated MSCs from rat bone marrow and identified that 1,25(OH)2D3 treatment markedly promoted cell proliferation and MSC migration. Further studies illustrated that 1,25(OH)2D3 strengthens the ability of MSCs to promote vasculogenesis in vitro by paracrine stimulation and activation of VEGF, MMP-2 and MMP-9 secreted by as the MSCs. Subsequently, after blocking the VDR and the PI3K pathway, we found that 1,25(OH)2D3-mediated vasculogenesis was associated with VDR binding and the PI3K/AKT pathway.
MSCs are characterized by high expression of specific markers, such as CD29, CD90 and CD105, and by the absence of markers specific to hematopoietic and endothelial cell lineages, such as CD45.15 Hence, using the corresponding antibodies, we identified whether the cells we isolated from rat bone marrow were MSCs by flow cytometry. The results showed obvious CD29+, CD90+, CD105+ and CD45−, which confirmed that the isolated cells were MSCs.
Previous literature has shown that transplanted MSCs exhibit strong vasculogenesis activity.16,17 Ikhapoh et al18 demonstrated that atherogenic cytokines regulate VEGF-A-induced differentiation of MSCs into endothelial cells in vitro. Moreover, transplanted MSCs prelease paracrine factors such as VEGF, which regulate vasculogenesis to promote the formation of novel vasoganglions in the surrounding infarcted area.19 VEGF is a highly specific provascular endothelial growth factor that has the ability to stimulate both angiogenesis and vasculogenesis, thereby improving blood supply.20 Matrix metalloproteinases (MMPs) are important proteolytic systems that degrade extracellular matrices. MMP-2 can recognize various extracellular matrix components as substrates21 and enhance vasculogenesis and metastasis of aberrant tumors.22 MMP-9 contributes to strengthening the proliferation of vascular epithelial cells and inhibiting their apoptosis after injury.23 Both MMP-2 and MMP-9 play important roles in regulating the hair cycle by inducing VEGF, IGF-1, and TGF-β expression.24 Thus, these three factors are commonly considered as specific factors for vasculogenesis. In our study, we found that the proliferation and migratory capabilities of MSCs were enhanced after treatment with 1,25(OH)2D3. The improvement accompanied a dose-dependent increase in the expression of VEGF, which was also true for the expression and activity of MMP-2 and MMP-9. Interestingly, compared with the expression and activity of MMP-2, the results showed that MMP-9 was expressed more highly in cell lysates but showed reduced activity in conditioned media. This result indicated that MMP-9 activity may interfere with some factors stimulated by 1,25(OH)2D3 during the secretion into the extracellular milieu, but MMP-2 was not affected. Adya et al25 also corroborated our results, but the underlying mechanism is currently unclear and requires further research. Western blotting analysis showed that the expression of vWF and FLK-1 were both significantly increased in the 1,25(OH)2D3 treatment group. These results indicated that the vasculogenic effect of 1,25(OH)2D3 on MSCs occurred by promoting not only its paracrine effects and activation of VEGF, MMP-2 and MMP-9 but also the differentiation of MSCs into endothelial cells.
In recent years, with the continuous development of cellular biotechnology, MSC transplantation has become another breakthrough treatment for ischemic diseases after angioplasty. Lee et al26 found that intracoronary infusion of human MSCs was tolerable and safe and led to modest improvements in cardiac contraction at a 6-month follow-up in MI patients. Unfortunately, the effect of MSCs transplantation therapy is still not satisfactory due to the low cellular viability of transplanted MSCs. Currently, improving the survival of MSCs in the ischemic environment after transplantation is a hot topic, and many efforts have been made. Hu et al27 showed that Tongxinluo stimulation promotes tube formation by MSCs, and the underlying mechanisms were associated with increased migration ability of MSCs and the upregulation of MMP-2 and VEGF expression. Moreover, concentration-dependent Tongxinluo also decreases apoptosis of MSCs under hypoxic and serum deprivation conditions.28 In our study, we stimulated MSCs with different doses of 1,25(OH)2D3 and discovered that cell proliferation and migration were both significantly promoted with 0.1 μM 1,25(OH)2D3 stimulation.
1,25(OH)2D3 is essential for mineral homeostasis and skeletal integrity, but it has also played an increasingly important role in influencing cell growth and differentiation in recent years, which is mediated by binding to the VDR.29 VDR-mediated vitamin D significantly enhances the proliferation of neural stem cells (NSCs) and enhances their differentiation into neurons and oligodendrocytes.30 It has been reported that 1,25(OH)2D3 stimulates the differentiation of cultured mouse myeloid leukemia cells into macrophages,31 as well as cardiac cells and colorectal microadenoma.32,33 However, it has not been determined whether 1,25(OH)2D3 can promote the proliferation and differentiation of MSCs, especially into endothelial cells. We confirmed that 1,25(OH)2D3 treatment promotes the proliferation and migration of MSCs and their differentiation into endothelial cells.
Recently, some studies found associations between 1,25(OH)2D3 and cardiovascular disease (CVD), lung disease, and tuberculosis.34,35 Panizo et al36 found that in uremic cardiomyopathy, activation of 1,25(OH)2D3 receptors weakens myocardial fibrosis by increasing the expression of some proteins, including MMP-2. In addition, supplementation of vitamin D in streptozotocin-induced diabetic mice improves endothelial function,37 and induces increased expression of VEGF in vascular smooth muscle cells (VSMCs) by binding to the VDR, which acts as a transcription factor for the VEGF promoter.38 Similarly, the role of vitamin D in restoring angiogenic balance by increasing VEGF levels has also been demonstrated in a pregnancy-induced hypertension rat model.39 In our study, the size of the official cavities and the number of official cavity-like structures formed by MSCs treated with 1,25(OH)2D3 were significantly increased. After blocking the VDR channel with pyridoxal-5-phosphate, the cell-to-tube capacity of MSCs was significantly weakened, demonstrating that 1,25(OH)2D3 promotes vasculogenesis of MSCs, which is mediated by the VDR.
The PI3K/AKT pathway is widely present in eukaryotic animal cells and is involved in mediating many important cellular processes.40 In recent years, it has been found that PI3K/AKT can promote the proliferation of MSCs and inhibit their apoptosis. Lv et al41 overexpressed Hif-1α in MSCs by transfection with a Hif-1α-expressing vector in an oxygen-glucose deprivation (OGD)-induced injury model. The results indicated that Hif-1α overexpression improved cell viability and suppressed apoptosis, in which the PI3K/AKT/mTOR pathway is implicated. Xue et al42 also found that lentivirus-mediated overexpression of miR-486 resulted in an increase in VEGF at both the mRNA and protein levels and reduced apoptosis of MSCs. Furthermore, PTEN-PI3K/AKT was shown to be involved in changes in BMSC biological functions that are regulated by miR-486. These findings demonstrate that the survival and vasculogenesis of MSCs may be related to the PI3K/AKT pathway. To further explore whether the mechanism by which 1,25(OH)2D3 promotes vasculogenesis in MSCs is related to the PI3K pathway, we tested the main factors and blocked the pathway by using the PI3K-specific inhibitor LY294002. Western blotting showed that the expression of pAKT in MSCs increased with time after 1,25(OH)2D3 treatment but was significantly reduced in the 1,25(OH)2D3 and LY294002 treatment groups. The cell-to-tube capacity of MSCs was also significantly weakened after LY294002 treatment. These results suggest that 1,25(OH)2D3-mediated vasculogenesis by MSCs may be associated with the PI3K/AKT pathway.
In conclusion, this study shows that 1,25(OH)2D3 strengthened the vasculogenesis of MSCs, among which 1,25(OH)2D3 promoted the expression of VEGF, and enhanced the activity of MMP-2 and MMP-9. This promotion is mediated by VDR and is achieved by activating the PI3K pathway (Figure 5).
 |
Figure 5 A schematic model of the role of 1,25(OH)2D3 in the vasculogenesis of MSCs. |
Acknowledgments
This study was funded by the Wenzhou Science and Technology Bureau (Grant No Y20160030).
Disclosure
The authors confirm that they have no conflicts of interest in this work.
References
1. Baksh D, Song L, Tuan RS. Adult mesenchymal stem cells: characterization, differentiation, and application in cell and gene therapy. J Cell Mol Med. 2004;8(3):301–316. doi:10.1111/jcmm.2004.8.issue-3
2. Karpov AA, Udalova DV, Pliss MG, Galagudza MM. Can the outcomes of mesenchymal stem cell-based therapy for myocardial infarction be improved? Providing weapons and armour to cells. Cell Prolif. 2017;50:2. doi:10.1111/cpr.12316
3. Silva GV, Litovsky S, Assad JA, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi:10.1161/01.CIR.0000151812.86142.45
4. Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi:10.1161/hc0102.101442
5. Zhang Q, Zhou M, Wu X, et al. Promoting therapeutic angiogenesis of focal cerebral ischemia using thrombospondin-4 (TSP4) gene-modified bone marrow stromal cells (BMSCs) in a rat model. J Transl Med. 2019;17(1):111. doi:10.1186/s12967-019-1845-z
6. Williams AR, Hare JM, Dimmeler S, Losordo D. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res. 2011;109(8):923–940. doi:10.1161/CIRCRESAHA.111.243147
7. Narita T, Suzuki K. Bone marrow-derived mesenchymal stem cells for the treatment of heart failure. Heart Fail Rev. 2015;20(1):53–68. doi:10.1007/s10741-014-9435-x
8. Umar M, Sastry KS, Chouchane AI. Role of vitamin D beyond the skeletal function: a review of the molecular and clinical studies. Int J Mol Sci. 2018;19(6):6. doi:10.3390/ijms19061618
9. Grundmann M, Haidar M, Placzko S, et al. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Cell Physiol. 2012;303(9):C954–C962.
10. Nema J, Sundrani D, Joshi S. Role of vitamin D in influencing angiogenesis in preeclampsia. Hypertens Pregnancy. 2019;38(4):201–207. doi:10.1080/10641955.2019.1647231
11. Min Y, Han S, Aae Ryu H, Kim SW. Human adipose mesenchymal stem cells overexpressing dual chemotactic gene showed enhanced angiogenic capacity in ischaemic hindlimb model. Cardiovasc Res. 2018;114(10):1400–1409.
12. Crafts TD, Jensen AR, Blocher-smith EC, Markel TA. Vascular endothelial growth factor: therapeutic possibilities and challenges for the treatment of ischemia. Cytokine. 2015;71(2):385–393. doi:10.1016/j.cyto.2014.08.005
13. Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705(2):69–89. doi:10.1016/j.bbcan.2004.09.006
14. Patel SR, Ke HQ, Vanholder R, Koenig RJ, Hsu CH. Inhibition of calcitriol receptor binding to vitamin D response elements by uremic toxins. J Clin Invest. 1995;96(1):50–59. doi:10.1172/JCI118061
15. Casado-díaz A, Pérez GD, Quesada-gómez JM. Comprehensive Biotechnology. 2011:455–466.
16. Wang HH, Cui YL, Zaorsky NG, et al. Mesenchymal stem cells generate pericytes to promote tumor recurrence via vasculogenesis after stereotactic body radiation therapy. Cancer Lett. 2016;375(2):349–359. doi:10.1016/j.canlet.2016.02.033
17. Gomes SA, Rangel EB, Premer C, et al. S-nitrosoglutathione reductase (GSNOR) enhances vasculogenesis by mesenchymal stem cells. Proc Natl Acad Sci U S A. 2013;110(8):2834–2839. doi:10.1073/pnas.1220185110
18. Ikhapoh IA, Pelham CJ, Agrawal DK. Atherogenic cytokines regulate VEGF-A-induced differentiation of bone marrow-derived mesenchymal stem cells into endothelial cells. Stem Cells Int. 2015;2015:498328. doi:10.1155/2015/498328
19. Tang YL, Zhao Q, Zhang YC, et al. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117(1):3–10. doi:10.1016/j.regpep.2003.09.005
20. Tang Y, Gan X, Cheheltani R, et al. Targeted delivery of vascular endothelial growth factor improves stem cell therapy in a rat myocardial infarction model. Nanomedicine. 2014;10(8):1711–1718. doi:10.1016/j.nano.2014.06.001
21. Barascuk N, Vassiliadis E, Larsen L, et al. Development and validation of an enzyme-linked immunosorbent assay for the quantification of a specific MMP-9 mediated degradation fragment of type III collagen–A novel biomarker of atherosclerotic plaque remodeling. Clin Biochem. 2011;44(10–11):900–906. doi:10.1016/j.clinbiochem.2011.04.004
22. Ghosh A, Dasgupta D, Ghosh A, et al. MiRNA199a-3p suppresses tumor growth, migration, invasion and angiogenesis in hepatocellular carcinoma by targeting VEGFA, VEGFR1, VEGFR2, HGF and MMP2. Cell Death Dis. 2017;8(3):e2706. doi:10.1038/cddis.2017.123
23. Lange C, Storkebaum E, de Almodovar CR, Dewerchin M, Carmeliet P. Vascular endothelial growth factor: a neurovascular target in neurological diseases. Nat Rev Neurol. 2016;12(8):439–454. doi:10.1038/nrneurol.2016.88
24. Hou C, Miao Y, Wang J, et al. Collagenase IV plays an important role in regulating hair cycle by inducing VEGF, IGF-1, and TGF-beta expression. Drug Des Devel Ther. 2015;9:5373–5383. doi:10.2147/DDDT.S89124
25. Adya R, Tan BK, Punn A, Chen J, Randeva HS. Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis. Cardiovasc Res. 2008;78(2):356–365. doi:10.1093/cvr/cvm111
26. Lee JW, Lee SH, Youn YJ, et al. A randomized, open-label, multicenter trial for the safety and efficacy of adult mesenchymal stem cells after acute myocardial infarction. J Korean Med Sci. 2014;29(1):23–31. doi:10.3346/jkms.2014.29.1.23
27. Hu XY, Wang WX, Yu MJ, et al. Tongxinluo promotes mesenchymal stem cell tube formation in vitro. J Zhejiang Univ Sci B. 2011;12(8):644–651. doi:10.1631/jzus.B1101005
28. Li N, Yang YJ, Cui HH, et al. Tongxinluo decreases apoptosis of mesenchymal stem cells concentration-dependently under hypoxia and serum deprivation conditions through the AMPK/eNOS pathway. J Cardiovasc Pharmacol. 2014;63(3):265–273. doi:10.1097/FJC.0000000000000044
29. Fathi N, Ahmadian E, Shahi S, et al. Role of vitamin D and vitamin D receptor (VDR) in oral cancer. Biomed Pharmacother. 2019;109:391–401. doi:10.1016/j.biopha.2018.10.102
30. Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang GX. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol. 2015;98(2):240–245. doi:10.1016/j.yexmp.2015.02.004
31. Abe E, Miyaura C, Sakagami H, et al. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78(8):4990–4994. doi:10.1073/pnas.78.8.4990
32. Groschel C, Aggarwal A, Tennakoon S, et al. Effect of 1,25-dihydroxyvitamin D3 on the Wnt pathway in non-malignant colonic cells. J Steroid Biochem Mol Biol. 2016;155(Pt B):224–230. doi:10.1016/j.jsbmb.2015.02.011
33. Kim IM, Norris KC, Artaza JN. Vitamin D and cardiac differentiation. Vitam Horm. 2016;100:299–320.
34. Huang H, Porpodis K, Zarogoulidis P, et al. Vitamin D in asthma and future perspectives. Drug Des Devel Ther. 2013;7:1003–1013. doi:10.2147/DDDT.S50599
35. Huang SJ, Wang XH, Liu ZD, et al. Vitamin D deficiency and the risk of tuberculosis: a meta-analysis. Drug Des Devel Ther. 2017;11:91–102. doi:10.2147/DDDT.S79870
36. Panizo S, Carrillo-lopez N, Naves-diaz M, et al. Regulation of miR-29b and miR-30c by vitamin D receptor activators contributes to attenuate uraemia-induced cardiac fibrosis. Nephrol Dial Transplant. 2017;32(11):1831–1840. doi:10.1093/ndt/gfx060
37. Wong MS, Leisegang MS, Kruse C, et al. Vitamin D promotes vascular regeneration. Circulation. 2014;130(12):976–986. doi:10.1161/CIRCULATIONAHA.114.010650
38. Cardus A, Panizo S, Encinas M, et al. 1,25-dihydroxyvitamin D3 regulates VEGF production through a vitamin D response element in the VEGF promoter. Atherosclerosis. 2009;204(1):85–89. doi:10.1016/j.atherosclerosis.2008.08.020
39. Song J, Li Y, An R. Vitamin D restores angiogenic balance and decreases tumor necrosis factor-alpha in a rat model of pre-eclampsia. J Obstet Gynaecol Res. 2017;43(1):42–49. doi:10.1111/jog.13186
40. Yu JS, Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143(17):3050–3060. doi:10.1242/dev.137075
41. Lv B, Hua T, Li F, et al. Hypoxia-inducible factor 1 alpha protects mesenchymal stem cells against oxygen-glucose deprivation-induced injury via autophagy induction and PI3K/AKT/mTOR signaling pathway. Am J Transl Res. 2017;9(5):2492–2499.
42. Shi XF, Wang H, Xiao FJ, et al. MiRNA-486 regulates angiogenic activity and survival of mesenchymal stem cells under hypoxia through modulating Akt signal. Biochem Biophys Res Commun. 2016;470(3):670–677. doi:10.1016/j.bbrc.2016.01.084
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.