Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
From “Cure” to “Care”: The Role of the MultiDisciplinary Team on Colorectal Cancer Patients’ Satisfaction and Oncological Outcomes
Authors Lucarini A , Garbarino GM, Orlandi P , Garofalo E, Bragaglia L, Laracca GG , Canali G, Pecoraro A, Mercantini P
Received 17 February 2022
Accepted for publication 24 May 2022
Published 27 June 2022 Volume 2022:15 Pages 1415—1426
DOI https://doi.org/10.2147/JMDH.S362550
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Alessio Lucarini, Giovanni Maria Garbarino, Pierfrancesco Orlandi, Eleonora Garofalo, Lorenzo Bragaglia, Giovanni Guglielmo Laracca, Giulia Canali, Alessandra Pecoraro, Paolo Mercantini On behalf of the Sant’Andrea GLAM collaborative group
Department of Medical and Surgical Sciences and Translational Medicine, Sapienza University of Rome, Sant’Andrea Hospital, Rome, Italy
Correspondence: Alessio Lucarini, Department of Medical and Surgical Sciences and Translational Medicine, Sapienza University of Rome, Sant’Andrea Hospital, Rome, Italy, Email [email protected]
Background: MultiDisciplinary Team (MDT) are held to undertake decisions regarding the whole aspect of oncological diseases. Over the years, they acquired a collaborative approach where clinical decisions are shared by all members. Different guidelines recommend the implementation of MDT, in order to improve the outcomes of these patients. Our aim is to evaluate how the implementation of MDT affects the patients’ satisfaction and adherence to treatment.
Methods: A survey was submitted to every patient affected by colorectal cancer treated by the MDT of Sant’Andrea Hospital (Rome, IT). The investigation period was January 2017–March 2020. Data from patients inside the MDT were compared with patients outside the MDT to evaluate a reduction in waiting times.
Results: A total of 591 patients were collected. A total of 355 patients with colorectal neoplasia were included in our analysis. Cumulative overall survival was 79%. The average waiting time for computed tomography or colonoscopy was 14.9 days for patients in the MDT versus 24.5. A total of 201 patients were eligible for our satisfaction survey. An 89.5% of patients felt followed in their treatment. A 93.5% of patients expressed a high grade of satisfaction for the MDT design.
Conclusion: Our study confirms the importance of a well-structured MDT. Dedicated slots shorten the waiting time, leading to better satisfaction and faster diagnosis. Patients’ satisfaction should be considered as an index of good practice when it comes to oncological patients’ treatment.
Keywords: cancer, multidisciplinary team meeting, patient assessment, patient management, patient outcomes
Introduction
The MultiDisciplinary Team (MDT) meeting in oncology can be defined as a regularly scheduled discussion, usually on a weekly basis, of patients with malignancies. These meetings, also called Tumor Boards (TBs),1 are held in order to undertake decisions by professionals of different specialties, such as surgeons, radiologists, histopathologists, oncologists, clinical nurse specialists and multidisciplinary team coordinators;2 patients discussed may be newly diagnosed, already treated or with disease recurrence and it is essential that they are always presented with complete and comprehensive clinical documentation.3 Over the years, it has been noted that the impact of MDT depends on the type of tumour and especially the stage of the disease at the time of discussion. Certainly, there has been a progressive change in Tumor boards, whose characteristics have evolved over time. Initially created to provide advice from all responsible physicians at a specific point in the cancer treatment pathway,4 they have gradually acquired a more collaborative approach in which clinical decisions and responsibilities are shared by all members, who are the same ones who actually treat patients. In fact, in the guidelines for several types of cancer, multidisciplinary working groups are now specifically included as an essential tool for patient approach and management.5–9 Over the years, key aspects of optimal MDT efficiency have been evaluated. These have been found to be importance of good relationships among team members, adequate nontechnical skills (communication, leadership), and the need for support at organizational level. In the latter area, online tools have been developed in the recent past to enable the continuation of MDTs during the SARS-CoV-2 pandemic.10–12
The literature provides evidence that MDT meetings lead to significant changes in the way patients with malignancies are evaluated, managed, and treated, thereby arriving at shared decisions, improving diagnostic accuracy, achieving accurate staging, and providing the best treatment guaranteeing a greater adherence to guidelines.13–17
It is unclear whether the multidisciplinary approach leads to a measurable benefit for patients. Regarding mortality, there is weak evidence of an association between MDT meetings and survival curves, with fairly mixed results.18–20 Discussion at MDT, as mentioned previously, however, seems to lead to better tumour staging, which in turn produces better outcomes at follow-up.20 Kozak et al demonstrated that MDT reduces treatment time from consultation by approximately 7 days compared to patients not discussed by the same team.21 It is unlikely that the reduction of 7 days affects the prognosis, as in any case it is rational that the reduction of time to start treatment improves patient satisfaction. With regard to patient satisfaction and quality of life, the evidence for improvement in these areas has been weak but promising, so that further investigation has been suggested.16,22 The aim of the present study is to describe how these key points are fundamental when it comes to oncological patients and how can it be improved.
Methods
A retrospective study was conducted on a cohort of patients who were prospectively discussed by the Tumor Board (TB) held at the Sant’Andrea Hospital and University of Rome from the official start date of the project, January 26 2017 to March 05 2020. Only colorectal cancer patients older than 18 years of age, of any sex, and with any comorbidity were included. We excluded all patients who presented to our MDT with the sole purpose of a second opinion, all patients who were exclusively placed on a therapeutic indication by the TB and then redirected to other centers, and all those who did not present at the first post-discussion appointment.
Patient demographics, tumor location and pathologic staging, date of discussion at the MDT, and date of first treatment were collected in temporal order from the internal MDT registry and the hospital intranet. Internal data from the hospital’s Single Booking Center (SBC) were analyzed for the items “Computerized Axial Tomography (CT) Of Abdomen” (ICD-9 code: 88.01.6) and Closed [Endoscopic] Biopsy Of Large Intestine (ICD-9 code: 45.25) in patients diagnosed with colon and rectal cancer (ICD-9 codes: 153 and 154) who request these procedures in the years 2019 and 2020. Subsequently, the time between the request and the execution of the examination in these patients was compared with the times of the patients discussed at our TB, to whom the same procedures were requested.
Regarding the degree of satisfaction, this was measured through telephone interviews conducted between October 19 2020 and February 15 2021, using a Likert scale23 with scores ranging from 1 to 5, consisting of the following questions24 reported in Figure 1.
 |
Figure 1 Likert scales. |
Our primary outcome was the degree of satisfaction of patients treated with our TB. Secondary outcomes regarded the waiting time for the proposed exams and the Overall Survival.
Data were collected in a dedicated Microsoft Excel® (2018) database. Statistical analysis was performed with SPSS v25 (IBM, Armonk, USA) and GraphPad Prism v8.0.2 (GraphPad Software, La Jolla, California, USA) for Windows 10 and MacOS 11.
Survival analyses were conducted using the Kaplan–Meier method with Log rank test and Cox regression analysis (stepwise method). Categorical variables are presented as frequency or percentage and were compared with the use of the chi-square test or Fisher’s exact test, as appropriate. Categorical variables with multiple categories obtained from the questionnaire were analyzed, regarding the proposed treatment, with the Post-Hoc analysis method. Continuous variables are presented as mean (±standard deviation) or median (range) and were compared with the use of Student’s t test. The Mann–Whitney U-test was used for continuous and not normally distributed outcomes.
All reported p values were two-tailed, and p values of less than 0.05 were considered to indicate statistical significance.
The study is conducted in accordance with the principles of the Declaration of Helsinki and “good clinical practice” guidelines. Informed consent has been obtained from the patients. Ethical approval has been completed by the ethics committee at Sant’Andrea Hospital.
Results
A total of 591 patients discussed at the Sant’Andrea Hospital’s MDT between January 26, 2017 and March 05, 2020 were retrieved from the internal database. Three hundred and fifty-five patients with colorectal neoplasia were collected. The mean age of our population was 73.06 ± 11.73 years, with a prevalence of tumours in the right colon (117, 32.96%), the rectum (102, 28.73%) and the sigmoid colon (72, 20.28%). Most patients were found to be in an intermediate stage of disease (according to AJCC 2018 eighth edition25), 101 (28.5%) stage II, 113 (31.8%) stage III, 48 stage I and 58 stage IV (Table 1).
 |
Table 1 Clinical Characteristics |
Amongst the visits requested by the multidisciplinary team, the most requested was the oncological consultation (58%). The mean waiting time between the date of discussion and the start of the treatment was 14.9 days (range 1–60 days). Most patients were not asked for any additional instrumental exams (61.8%) and, amongst the additional exams the most requested was the CT (9.2%) see Table 2.
 |
Table 2 Next Steps |
In patients discussed at the TB, the average waiting time for CT scan of the abdomen was 12.1 days, the average waiting time for colonoscopy was 9.1 days. The waiting times were compared with those from SBC (a total of 373 patients included in the analysis) requiring the same exams with a previous diagnosis of colon and rectal cancer (ICD-9 codes: 153 and 154) and, respectively, an average of 24.5 (p 0.0041) and 24.6 days (p 0.0455) was found, see Table 3.
 |
Table 3 Waiting Times |
Overall survival for stage 0 was 100%, for stage I 91.6% (4/48), for stage II 92% (8/101), for stage III 82.3% (20/113) and for stage IV 48.27% (30/58). The mean follow-up was 27.5 months with a maximum follow-up of 49 months (Table 4, Figure 2).
 |
Table 4 Overall Survival per Stage |
 |
Figure 2 Overall survival. |
Seventy-four out of 355 patients were excluded from the questionnaire study because they died at the time of the interview period. Additional 80 patients were excluded because they did not respond to the interview due to refusal or inability to contact them by telephone. The remaining 201 patients were included in the questionnaire (Figure 3).
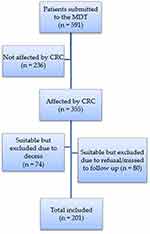 |
Figure 3 Flowchart of included patients. |
As a result, most patients felt followed in their treatment (180 responses between 4 and 5 to question 1, 89.5%) and found the indications given regarding the examinations and treatment to be clear (192 responses between 4 and 5 to question 3, 95.5%).
Regarding the difficulty in keeping appointments for proposed examinations and treatments, the data showed substantial ease by the participants (150 responses between 1 and 2 to question 2, 74.6%). The majority of patients would have appreciated psychological support in their pathway (120 responses partially or totally agreed to question 4, 59.7%), with a fair part of “indifferent” response (69 responses, 34.3%).
Regarding the possibility to choose the physician (question 5) and personal involvement in therapeutic decisions (question 6), patients were contrary (162 responses totally or partially disagreed to question 5, 80.6% and 176 responses totally or partially disagreed to question 6, 87.6%).
With regard to the general practitioner, the majority of the interviewees were indifferent about the possibility, on our part, of informing them about the treatment or the examinations to which the MDT had referred them (152 indifferent responses to question 7, 75.6%).
Finally, almost all patients were satisfied with the overall management of the multidisciplinary team (188 responses between 4 and 5 to question 8, 93.5%) see Table 5.
 |
Table 5 Answers to the Questionnaire |
The 201 patients who responded to the questionnaire were then divided according to the type of treatment to which they were referred:
- Surgery (15 patients)
- Oncological examination (125 patients)
- Radiotherapy (11 patients)
- Follow-up (50 patients).
In accordance with this subdivision, differences between groups were analysed. For questions 1, 6, 7, and 8, no statistically significant differences were found. For question 2, a difference but non-significant (p 0.09) was found in score 1 (ease in keeping appointments and proposed treatments) for patients referred for oncologic visits.
A strong difference (p 0.05) was also found for question 4 in patients referred to surgery, who were more likely to receive psychological support than the other groups. A statistically significant difference between the groups (p 0.03) was present for question 5, with most patients referred to an oncologic visit not wishing they could have personally chosen the physicians who followed them through the process.
As mentioned previously, most respondents were indifferent about the possibility of informing their GP (question 7), however we found a statistically significant difference (p 0.02) in patients referred for surgery; the vast majority, in fact, reported to be indifferent to such possibility (see Table 6).
 |
Table 6 Statistical Analysis of the Questionnaire |
Discussion
The clinical management of patients by MDT has now entered clinical practice and is recommended by several oncologic guidelines,26,27 although the implementation is heterogenous worldwide,28 especially in developing countries.29 It is well established that the accuracy of diagnosis is improved, with the indication to perform examinations to guarantee a more complete staging14–17,30 and cancer care can be improved using newly developed electronic health record and cloud-based software.11,12,31 Consequently, treatment is also modified and integrated among all the specialists taking part in the discussion, thus increasing the adherence to the guidelines.13–17 Regarding the impact on survival, data are not homogeneous, but it seems logical that survival also benefits from better diagnosis and treatment. In our study sample, survival results in the early stages of the disease overlap with those found in the literature, and even better in the advanced stages, where an accurate diagnosis and a concerted treatment by all available resources could make the difference. This could be due to the shorter follow-up time of patients (maximum follow-up 49 months and medium 27.5 months) compared to the literature (60-month follow-up).32 The patient’s quality of life, satisfaction and possible involvement in decision-making processes are certainly the least clear aspects of the actual works in literature as stated by a recent review from Specchia et al.15,17,22
In our study, we analysed these aspects, finding that – regardless of the treatment proposed – patients felt highly guided and declared to be fully satisfied with the MDT (about 90% of responses with scores between 4 and 5 for both items). The indications provided by the MDT regarding diagnostic and therapeutic choices were also found to be very clear (about 95% of patients with a response between 4 and 5) and the respondents also declared a certain ease in respecting the timing of the appointments (75% with a response between 1 and 2). This last aspect had an important – though not reaching statistical – difference (p 0.09) for patients referred for oncological examination, which compared to the other groups found easier to keep assigned appointments. This could be due to the scheduling of appointments by the case-manager (a TB figure fully dedicated to management and planning) who best expresses her role in this subset of patients, where a greater number of exams are requested by specialists.33–35 Regarding the possibility of taking part in the decision-making process, about 87% of respondents totally disagreed with this possibility. This highlights the deep trust that patients place in the physician involved to make decisions in their interests, without feeling the need to participate, as they evidently feel their needs and preferences are well represented by the specialists who treat them.
The other major objective of this study was to evaluate the average treatment time from discussion at our TB to the first visit proposed. Of the 355 colorectal cancer patients analysed, the mean time was 14.9 days, 7 days shorter than the one reported by Kozak et al21 who reported that a multidisciplinary approach to the CRC patients reduced the mean time of treatment by 7.8 days (21.5 days vs 29.3 days). In addition, thanks to the analysis of the average waiting time between request and instrumental examination (abdomen CT and/or colonoscopy in our case) for patients diagnosed with colorectal cancer (ICD-9 codes 153 and 154), we demonstrate that our multidisciplinary approach reduces the average waiting time of 9.5 days (14.9 days instead of 24.5 days, p < 0.0001). More specifically, patients discussed at TB compared to outpatients have a mean gain of about 12 days when they are asked to perform a CT scan (12.1 days vs 24.5 days, p 0.0041) and about 15 days if they are asked to perform a colonoscopy (9.1 days instead of 24.6 days, p 0.0455). These results reflect the high efficiency of the multidisciplinary pathway, with dedicated slots for exams, and may also explain the high satisfaction and feeling of care in patients.
Through our analysis, we then looked at other aspects involved in the relationship between MDT and patients. We asked the patients if they preferred to have psychological support during their course, about 60% agreed with this option, with 30% of them indifferent. A greater consensus was found with an important difference (p 0.05) in the group undergoing surgery.
In conclusion, we noted that patients referred to the oncological visit were contrary – compared to the other groups – regarding the possibility to choose a different doctor (p 0.03). In fact, it is known that a strong bond is established between oncologist and patient over time and this, leading to higher levels of psychosocial well-being and treatment adherence rates, would explain the response.36,37
During the interviews, a potential bias was identified in the interviewer’s learning curve and his knowledge of the purpose of the study. On the other hand, with regard to the patients interviewed, a bias due to the heterogeneity of their schooling and the response set phenomenon38 has been identified. To avoid such systematic deviations, the interviews were conducted by a single interviewer calling patients in random order (instead of temporal order) from their date of presentation to the TB. The 8 questions were also asked randomly between interviews, with an objective and accurate explanation given by the interviewer. A randomly chosen time point for the interviews is another bias we recognize in our study.
Patients presenting to the TB after March 05, 2020, were excluded from data collection to avoid confounding effects related to the SARS-CoV-2 pandemic, which has negatively impacted cancer patient outcomes, waiting times, newly diagnosed cancer and patient relationships with healthcare settings.39,40 Regarding patient characteristics, those with a more advanced disease stage may have received an appointment to begin treatment more quickly than patients with a lower disease stage who were assigned to follow-up by the MDT, thus confounding outcomes regarding wait times. Our oncologic long-term results should be confirmed with an extended follow-up at 60 months, actually not possible due to the recent implementation of our MDT.
Data collected during our study are self-reported and obtained through telephone interview. Participants may have felt compelled to provide socially acceptable responses. The retrospective design of the study, with diagnosis and prognosis already known, may have influenced patients’ perceptions of waiting times. The study included only patients from a single treatment center. Studies of colorectal cancer patients discussed by other MDTs and treated in different centres, perhaps using different guidelines, are needed to obtain more solid results. More difficult would be to prospectively compare on a large scale the aspects analysed by our study between patients outside and patients inside an MDT, as this is now present in many of the treatment centres. Finally, the study examined exclusively the patients’ perspective. Data from health care providers and administrators could shed light on additional causes of wait times.
Despite these limitations, our study represents one of the few papers in literature that analyses the clinical, physical, and mental aspects of the impact of MDT on patients. We objectively and statistically analysed difficult-to-assess qualitative parameters, such as patient satisfaction and needs.
Conclusion
Our study confirms the importance of a well-structured MDT, where patients followed by this group of specialists may benefit from an advantage in time to “first treatment” or “first exam”. Dedicated slots in the endoscopy or radiology department do shorten the waiting time for patients discussed in the TB, leading to a better patients’ satisfaction and a faster and better diagnosis. Patients’ satisfaction should be considered as an index of good practice when it comes to oncological patients’ treatment, due to the psychological implications that these pathologies have on the patients and their families. The figure of a case manager, who took care (and not only cure) of the patients’ needs, is fundamental to obtain high satisfaction results.
Ethics Approval
The study is conducted in accordance with the principles of the Declaration of Helsinki and “good clinical practice” guidelines. Informed consent has been obtained from the patients. Ethical approval has been completed by the ethics committee at Sant’Andrea Hospital.
Consent to Participate
Informed consent was acquired after a complete explanation of the study and its purposes. If the participants denied their consent, they were automatically halted from continuing the survey. The participants can withdraw their consent at any time.
Consent for Publication
Informed consent included consent for publication.
Acknowledgment
Sant’Andrea GLAM collaborative group: Genoveffa Balducci, Tommaso Bocchetti, Mario Ferri, Francesco D’Angelo, Giuseppe Nigri, Marcello Gasparrini, Giorgio Castagnola, Niccolò Petrucciani, Paolo Aurello, Giulia Arrivi, Michela Roberto, Francesca Matilde Schipilliti, Federica Mazzuca, Paolo Marchetti, Bruno Annibale, Maria Rinzivillo, Francesco Panzuto, Mattia Falchetto Osti, Elsa Iannicelli, Francesca Di Gregorio, Michela Polici, Elisa Rosati, Andrea Laghi, Michele Rossi, Daniela Prosperi, Paola Mazzarelli, Federico Mainardi, Emanuela Pilozzi, Cristiana Giacani.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors disclose no conflict of interest. No funding has been received for the preparation of this manuscript.
References
1. Multidisciplinary Team [Internet]; [cited June 6, 2021]. Available from: https://datadictionary.nhs.uk/nhs_business_definitions/multidisciplinary_team.html.
2. Wright FC, De Vito C, Langer B, Hunter A. Multidisciplinary cancer conferences: a systematic review and development of practice standards. Eur J Cancer. 2007;43(6):1002–1010. doi:10.1016/j.ejca.2007.01.025
3. Lesslie M, Parikh J. Implementing a multidisciplinary tumor board in the community practice setting. Diagnostics. 2017;7(4):55. doi:10.3390/diagnostics7040055
4. Fennell ML, Das IP, Clauser S, Petrelli N, Salner A. The organization of multidisciplinary care teams: modeling internal and external influences on cancer care quality. J Natl Cancer Inst Monogr. 2010;2010(40):72–80. doi:10.1093/jncimonographs/lgq010
5. Overview | lung cancer: diagnosis and management | guidance. NICE; [cited June 6, 2021]. Available from: https://www.nice.org.uk/guidance/ng122.
6. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, The ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii1–9.
7. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26:v56–68. doi:10.1093/annonc/mdv295
8. Argilés G, Tabernero J, Labianca R, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291–1305. doi:10.1016/j.annonc.2020.06.022
9. Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Cancer Netw. 2019;17(5.5):603–605. doi:10.6004/jnccn.2019.5007
10. Mercantini P, Lucarini A, Mazzuca F, Osti MF, Laghi A. How technology can help in oncologic patient management during COVID-19 outbreak. Eur J Surg Oncol. 2020;46(6):1189–1191. doi:10.1016/j.ejso.2020.04.050
11. Hammer RD, Prime MS. A clinician’s perspective on co-developing and co-implementing a digital tumor board solution. Health Informatics J. 2020;26(3):2213–2221. doi:10.1177/1460458219899841
12. Janssen A, Robinson T, Brunner M, Harnett P, Museth KE, Shaw T. Multidisciplinary teams and ICT: a qualitative study exploring the use of technology and its impact on multidisciplinary team meetings. BMC Health Serv Res. 2018;18(1). doi:10.1186/s12913-018-3242-3
13. Coory M, Gkolia P, Yang IA, Bowman RV, Fong KM. Systematic review of multidisciplinary teams in the management of lung cancer. Lung Cancer. 2008;60(1):14–21. doi:10.1016/j.lungcan.2008.01.008.
14. Basta YL, Baur OL, van Dieren S, Klinkenbijl JHG, Fockens P, Tytgat KMAJ. Is there a benefit of multidisciplinary cancer team meetings for patients with gastrointestinal malignancies? Ann Surg Oncol. 2016;23(8):2430–2437. doi:10.1093/annonc/mdu260
15. Lamb BW, Brown KF, Nagpal K, Vincent C, Green JSA, Sevdalis N. Quality of care management decisions by multidisciplinary cancer teams: a systematic review. Ann Surg Oncol. 2011;18(8):2116–2125. doi:10.1245/s10434-011-1675-6
16. Prades J, Remue E, van Hoof E, Borras J. Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes. Health Policy (New York). 2015;119(4):464–474. doi:10.1016/j.healthpol.2014.09.006
17. Pillay B, Wootten AC, Crowe H, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: a systematic review of the literature. Cancer Treat Rev. 2016;42:56–72. doi:10.1016/j.ctrv.2015.11.007
18. Wille-Jørgensen P, Sparre P, Glenthøj A, et al. Result of the implementation of multidisciplinary teams in rectal cancer. Color Dis. 2013;15(4):410–413. doi:10.1111/codi.12013
19. MacDermid E, Hooton G, Macdonald M, et al. Improving patient survival with the colorectal cancer multi-disciplinary team. Color Dis. 2009;11(3):291–295. doi:10.1111/j.1463-1318.2008.01580.x
20. Palmer G, Martling A, Cedermark B, Holm T. Preoperative tumour staging with multidisciplinary team assessment improves the outcome in locally advanced primary rectal cancer. Colorectal Dis. 2011;13(12):1361–1369. doi:10.1111/j.1463-1318.2010.02460.x
21. Kozak VN, Khorana AA, Amarnath S, Glass KE, Kalady MF. Multidisciplinary clinics for colorectal cancer care reduces treatment time. Clin Colorectal Cancer. 2017;16(4):366–371. doi:10.1016/j.clcc.2017.03.020
22. Specchia ML, Frisicale EM, Carini E, et al. The impact of tumor board on cancer care: evidence from an umbrella review. BMC Health Serv Res. 2020;20(1). doi:10.1186/s12913-020-4930-3.
23. Likert R. A technique for the measurement of attitudes. - PsycNET. PsycNET [Internet]; 1932 [cited June 6, 2021]. Available from: https://psycnet.apa.org/record/1933-01885-001.
24. Crow R, Gage H, Hampson S, et al. The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature. Health Technol Assess (Rockv). 2002;6(32) doi:10.3310/hta6320
25. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99.
26. Borras J, Albreht T, Audisio R, et al. Policy statement on multidisciplinary cancer care. Eur J Cancer. 2014;50(3):475–480. doi:10.1016/j.ejca.2013.11.012
27. Atun R, Ogawa T, Martin-Moreno JM. Analysis of National Cancer Control Programmes in Europe; 2009.
28. Lamprell K, Arnolda G, Delaney GP, Liauw W, Braithwaite J. The challenge of putting principles into practice: resource tensions and real-world constraints in multidisciplinary oncology team meetings. Asia Pac J Clin Oncol. 2019;15(4):199–207. doi:10.1111/ajco.13166
29. Barrios C, Sánchez-Vanegas G, Villarreal-Garza C, et al. Barriers and facilitators to provide multidisciplinary care for breast cancer patients in five Latin American countries: a descriptive-interpretative qualitative study. Lancet Reg Health. 2022;11:100254.
30. Kočo L, Weekenstroo HHA, Lambregts DMJ, et al. The effects of multidisciplinary team meetings on clinical practice for colorectal, lung, prostate and breast cancer: a systematic review. Cancers. 2021;13(16):4159. doi:10.3390/cancers13164159
31. Huilgol YS, Adler‐Milstein J, Ivey SL, Hong JC. Opportunities to use electronic health record audit logs to improve cancer care. Cancer Med. 2022. doi:10.1002/cam4.4690
32. Colorectal cancer survival rates | Colorectal Cancer Prognosis [Internet]. [cited July 19, 2021]. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html.
33. Lamb B, Sevdalis N, Mostafid H, Vincent C, Green J. Quality improvement in multidisciplinary cancer teams: an investigation of teamwork and clinical decision-making and cross-validation of assessments. Ann Surg Oncol. 2011;18(13):3535–3543. doi:10.1245/s10434-011-1773-5
34. Lamb B, Taylor C, Lamb J, et al. Facilitators and barriers to teamworking and patient centeredness in multidisciplinary cancer teams: findings of a national study. Ann Surg Oncol. 2013;20(5):1408–1416. doi:10.1245/s10434-012-2676-9
35. Lamb B, Sevdalis N, Arora S, Pinto A, Vincent C, Green J. Teamwork and team decision-making at multidisciplinary cancer conferences: barriers, facilitators, and opportunities for improvement. World J Surg. 2011;35(9):1970–1976. doi:10.1007/s00268-011-1152-1
36. Thomas T, Althouse A, Sigler L, et al. Stronger therapeutic alliance is associated with better quality of life among patients with advanced cancer. Psychooncology. 2021;30(7):1086–1094. doi:10.1002/pon.5648
37. Fuertes J, Toporovsky A, Reyes M, Osborne J. The physician-patient working alliance: theory, research, and future possibilities. Patient Educ Couns. 2017;100(4):610–615. doi:10.1016/j.pec.2016.10.018
38. Piedmont RL. Acquiescence response set. 2014 [cited June 6, 2021]: 11. Available from: https://link.springer.com/referenceworkentry/10.1007/978-94-007-0753-5_12.
39. Morris EJA, Goldacre R, Spata E, et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: a population-based study. Lancet Gastroenterol Hepatol. 2021;6(3):199–208. doi:10.1016/S2468-1253(21)00005-4
40. Kempf E, Priou S, Lamé G, et al. Impact of two waves of Sars-Cov2 outbreak on the number, clinical presentation, care trajectories and survival of patients newly referred for a colorectal cancer: a French multicentric cohort study from a large group of university hospitals. Int J Cancer. 2022;150(10):1609–1618. doi:10.1002/ijc.33928
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
