Back to Journals » Journal of Asthma and Allergy » Volume 13
Frequency of Tiotropium Bromide Use and Clinical Features of Patients with Severe Asthma in a Real-Life Setting: Data from the Severe Asthma Network in Italy (SANI) Registry
Authors Puggioni F, Brussino L , Canonica GW , Blasi F , Paggiaro P, Caminati M , Latorre M, Heffler E, Senna G
Received 1 August 2020
Accepted for publication 19 October 2020
Published 10 November 2020 Volume 2020:13 Pages 599—604
DOI https://doi.org/10.2147/JAA.S274245
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Francesca Puggioni,1,2 Luisa Brussino,3 Giorgio Walter Canonica,1,2 Francesco Blasi,4,5 Pierluigi Paggiaro,6 Marco Caminati,7,8 Manuela Latorre,6 Enrico Heffler,1,2 Gianenrico Senna7,8 On behalf of the Severe Asthma Network in Italy (SANI) group
1Personalized Medicine, Asthma and Allergy – Humanitas Clinical and Research Center, IRCCS – Rozzano (MI), Milan, Italy; 2Department of Biomedical Sciences, Humanitas University – Pieve Emanuele (MI), Milan, Italy; 3Dipartimento di Scienze Mediche, SSDDU Allergologia e Immunologia Clinica, Università degli Studi di Torino, AO Ordine Mauriziano Umberto I – Torino, Torino, Italy; 4Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy; 5Internal Medicine Department, Respiratory Unit and Adult Cystic Fibrosis Center, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico di Milano, Milan, Italy; 6Department of Surgery, Medicine, Molecular Biology and Critical Care, University of Pisa, Pisa, Italy; 7Department of Medicine, University of Verona, Verona, Italy; 8Allergy Unit and Asthma Center, Verona University Hospital, Verona, Verona, Italy
Correspondence: Enrico Heffler
Personalized Medicine, Asthma and Allergy, Istituto Clinico Humanitas, Milan, Italy
Tel +39 0288247013
Fax + 39 0282246484
Email [email protected]
Purpose: Patients with uncontrolled asthma despite high doses of inhaled corticosteroid therapy plus another controller are defined as severe asthmatics. Tiotropium bromide respimat (TBR) is the only long-acting muscarinic antagonists (LAMA) approved for severe asthma. The aim of this study was to explore the frequency of severe asthmatics treated with TBR and characterize their clinical features in a real-life, registry-based setting.
Materials and Methods: Baseline data from the Severe Asthma Network in Italy (SANI) registry have been analyzed to determine the use of TBR and other LAMA, and to compare clinical, functional and inflammatory features associated with the use of LAMA.
Results: Among a total of 698 enrolled patients, 35.9% were treated with LAMA (23.3% TBR, 4.5% tiotropium bromide handihaler, 4.5% aclidinium, 3.4% glycopyrronium bromide 0.3% umeclidinium bromide). Age of asthma onset was higher in patients taking LAMA, whom, compared to others were more frequently former smokers. They also had a higher annual exacerbation rate, experienced worst asthma control, worst disease-related quality of life and poorer lung function. Bronchiectasis was more frequently found in LAMA users (25.9% vs 13.1%).
Conclusion: TBR is still underused in severe asthma in a real-life setting, while a relevant proportion of patients are treated with other LAMA that are not approved for severe asthma treatment. Patients taking LAMA have features characteristic of even more severe asthma.
Keywords: severe asthma, registry, long-acting muscarinic antagonists, real-ligfe
Introduction
Though the prevalence of severe asthma is less than 5% among patients with persistent asthma, this condition accounts for an excessive burden on disease-related healthcare resources and costs.1–5 In order to correctly define a patient as a severe asthmatic, several possible aggravating and/or confounding factors should be systematically addressed (including possible misdiagnosis, proper treatment of relevant comorbidities, treatment adherence and inhaler technique, relevant environmental exposures, etc.) as suggested, for example, by the Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults,6 resulting in a lower prevalence of severe refractory asthma (ie, 3.6% as reported by Hekking et al).1 The recent availability of anti-IgE and anti-IL-5 monoclonal antibodies represents a significant step forward in the treatment of this condition reducing exacerbation rates as well as hospitalizations but their high costs remain their main drawback.2–5 The eligible population for biologic agents is suggested to be around 30–40% for anti-IgE and 25–55% for anti-IL5.7–9
According to the Global Initiative for Asthma (GINA) recommendations,10 severe asthmatic patients can be labelled as step 4 or step 5 depending on their level of asthma control under medium-to-high dose-inhaled corticosteroids (ICS)/long-acting beta2-agonists (LABA). For both step 4 and step 5 Tiotropium bromide Respimat ® (TBR), a long-acting muscarinic antagonist (LAMA) is an add-on therapeutic option. LAMA have long since been approved as a maintenance therapy in chronic obstructive pulmonary disease (COPD),11,12 considering their action on airflow limitation and exercise tolerance improvement.13,14
New generations of selective LAMA molecules act on three out of the five identified muscarinic receptors directly involved in mucus secretion and airway smooth muscle contraction. The binding of acetylcholine (ACh) to the M3 and, in a lesser extent, to the M1 muscarinic receptors, results in smooth muscle contraction and mucus secretion. On the other hand, activation of M2 receptors reduces the release of ACh. Tiotropium has a high selectivity for M3 and M1 receptors, while it rapidly dissociates from M2 receptors,15 making it an optimal anticholinergic drug. Since the approval of tiotropium in COPD, many studies have analyzed its efficacy in asthma, and a single daily dose of Tiotropium was found to be effective in the treatment of nocturnal asthma16–18 as well as in the improvement of lung function tests,19 both in the adult and adolescent population.20
In the 2017 update of GINA recommendations, TBR has been suggested as an add-on treatment for severe asthma.10 Many studies analyzed the comparison between LABA and LAMA as add-on therapy to ICS in asthmatic patients,21 and the efficacy of LAMA in a real-life setting, showing a significant improvement in mean FEV1 values, a reduction in asthma exacerbations, as well as a subjective improvement in exercise tolerance and cough.21–23,23,24
The present study was carried out in a large population of the Severe Asthma Network in Italy (SANI)25 to determine the frequency of TBR treatment in severe asthmatics and to characterize patients’ clinical features in a real-life, registry-based setting.
Materials and Methods
Study Population
SANI is a web-based observatory that collects demographic, clinical and functional data, as well as inflammatory biomarkers of patients with severe asthma, defined according to European Respiratory Society (ERS)/American Thoracic Society (ATS) classification (asthma which requires treatment with high dose ICS plus a second controller, and/or systemic corticosteroids, to prevent it from becoming “uncontrolled” or which remains “uncontrolled” despite this therapy)26 and aged >12 years, recruited by accredited centers homogeneously spread out on the national territory.25 Considering the real-life nature of the SANI registry, no exclusion criteria (including the possibility to have received a diagnosis of asthma-COPD overlap) were present in the protocol.25 In the SANI registry demographic, clinical (ie, allergic sensitizations, comorbidities, information on asthma exacerbations, asthma control, asthma-related quality of life …), functional (lung function parameters), inflammatory (ie, blood eosinophils, serum IgE, exhaled nitric oxide) and asthma-related treatment data are collected.25
Baseline data from the SANI registry have been analyzed to assess the frequency of LAMA treatment as well as the clinical features associated with their use in severe asthmatics.
Patients’ recruitment started in late 2016 – beginning of 2017, just after the first Center received the Ethics Committee approval. Data extraction for this analysis was performed in March 2019.
Ethical Issues
The SANI registry was constructed according to the declarations of Helsinki and Oviedo, and was set up according to the 3rd Edition Recommendation on registries for evaluating patient outcomes published by the Effective Health Care Program of the Agency for Healthcare Research and Quality.27 The protocol has been performed according to the principles and procedures of the Good Clinical Practice28 and in accordance with the Italian laws (Legislative Decree n.211, June 24, 2003; Legislative Decree.n.200 November 6, 2007; Ministry Decree December 21, 2007). The protocol has been approved by the Central Ethics Committee (“Area Vasta Nord-Ovest Toscana” Ethics Committee; protocol number: study number 1245/2016, protocol number: 73714) and the enrollment in the other Center started upon approval of local Ethics Committees. All enrolled patients signed an informed consent.
Statistical Analysis
Statistical analysis was performed using SPSS 21.0 software (SPSS, Chicago, IL, USA). The Kolmogorov–Smirnov test was used to evaluate the normality of distribution of each continuous variable, and depending on the result of this test, the Student’s t-test or Mann–Whitney test was used to compare variables. Categorical variables were compared with the Fisher exact test. A p-value < 0.05 was considered statistically significant.
Results
Among a total of 698 enrolled patients by March 2019, 35.9% were treated with LAMA (23.3% TBR, 4.5% Tiotropium bromide Handihaler, 4.5% Aclidinium, 3.4% Glycopyrronium bromide 0.3% Umeclidinium bromide) (Figure 1). As shown in Table 1 patients taking LAMA showed a significantly higher age of asthma onset, and were more frequently former smokers. They reported a higher annual exacerbation rate, showed worst asthma control (assessed with both Asthma Control Test, ACT,29 and Asthma Control Questionnaire, ACQ)30 as well as worst disease-related quality of life (assessed by Asthma Quality of Life Questionnaire, AQLQ)31 (Table 2). LAMA users had a significantly lower basal FEV1% of predicted value (Table 3), had more frequently concomitant bronchiectasis, and were more frequently treated with oral corticosteroids and biological agents (Table 2). No significant difference has been recorded in regard to biomarkers of type-2 inflammation, such as serum total IgE, blood eosinophil count and exhaled nitric oxide (FENO) (Table 2).
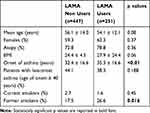 |
Table 1 Demographic Characteristics of Patients with Severe Asthma Treated with or without Long-Acting Anti-Muscarinic Agents (LAMA) |
 |
Table 2 Clinical and Inflammatory Characteristics of Patients with Severe Asthma Treated with or without Long-Acting Anti-Muscarinic Agents (LAMA) |
 |
Table 3 Functional Characteristics of Patients with Severe Asthma Treated with or without Long-Acting Anti-Muscarinic Agents (LAMA) |
 |
Figure 1 Prevalence of patients with severe asthma treated with long-acting anti-muscarinic agents (LAMA). |
No significant difference was found, for any parameter, between patients using TBR and those treated with other LAMA molecules and devices.
Discussion
Our study highlights that within the population of SANI registry, around one out of three patients were undergoing LAMA therapy; this is in line with what was reported by other national registries for severe asthma, with LAMA treatment being used in 13–45% of patients depending on the country.32 That group was overall characterized by more severe disease in terms of lung function, exacerbations, patients’ reported outcomes and required therapy; in fact, the prescription rate of biological agents, in particular anti-IL5 agents, and the use of systemic corticosteroids were higher in patients under LAMA therapy. Worthy of mention, the distribution of inflammatory biomarkers in that sub-population was very similar to the overall severe asthmatics population sample.
TBR has been proven to be an effective add-on therapy in adult patients with asthma treated with ICS/LABA combination reducing the exacerbation rate as well as ameliorating lung function.21,22 Its clinical efficacy was confirmed in adolescents even if at a lesser extent.20 Similar positive results were also reported in a real-life setting where the significant reduction of exacerbations was confirmed by a reduction of antibiotic course and oral steroid usage.24
That body of evidence provided the rationale for including TBR as an add-on recommended treatment for GINA step 4 and 5 patients,10 when medium-to-high dose ICS-LABA treatment is not associated with an optimal disease control. Interestingly, GINA recommendations place TBR before the biologic treatment option. The ERS/ATS guidelines on the management of severe asthma also suggest the use of TBR in patients with severe uncontrolled asthma.26
Despite these findings and the evidence of its mechanisms of action, which may account for its efficacy in asthma, our study reports that TBR is still underused, while a relevant proportion of patients are treated with other LAMA that are not currently approved for severe asthma. Similarly, a recent investigation conducted in the United States highlighted that a triple combination including ICS, LABA and LAMA had been prescribed to no more than 27% of patients undergoing biologic treatment.33 The reason for the low prescription rate of TBR is not easy to understand. When looking at the study population, both in our dataset and in real-life literature,33 it is quite apparent that severe asthmatics treated with LAMA are affected by a more difficult-to-control disease, in terms of exacerbations, lung function and quality of life.
Furthermore, in our cohort of severe asthmatics, those treated with LAMA were not characterized by more severe Type-2 inflammation, at least according to the evaluated biomarkers (total IgE, blood eosinophils count, FENO), even if the entire study population showed high frequency of Type-2 late-onset severe asthma. Considering these results together, the prescription of LAMA does not seem oriented by specific Type-2 biomarkers. The high proportion of Type-2 late-onset severe asthmatics in our registry population might be interpreted as a potential trait accounting for less efficacy of LAMA; however, patients requiring LAMA for achieving a better asthma control seem to express a different “sub-phenotype”, characterized by lower Type-2 inflammatory traits, such as former smoking habit like in our data, prompting the clinical suspect of a possible Asthma-COPD overlap at least in a proportion of these patients. In this regard, the long-standing use of LAMA in COPD and some similarities between neutrophilic phenotype of severe asthma and COPD may account for this finding. Unfortunately, information on the concomitant presence of asthma and COPD (Asthma-COPD overlap) is missing in our registry.
The efficacy of TBR has been proved independently of age, atopic status34 or body mass index,35 and therefore its use should be implemented as an add-on treatment strategy in order to improve lung function and exacerbation rate in different patient groups, independently of the indication for biological agents’ treatment.
It is worthy of mention that the current guidelines on severe asthma management recommend the use of TBR rather than any LAMA.10,26 This recommendation is partially disregarded in our dataset. Especially when talking about severe obstructive syndromes, the relevance of the device is not negligible. In fact, different devices require peculiar conditions in terms of patients’ inhalation flow and manual skills in order to obtain an optimal drug delivery. According to the available evidence,36 TBR is suitable for different patient groups regardless of the inhalation flow (>30 or <30 L/min) and specific manual skills without impacting the drug delivery efficiency.
Conclusion
In conclusion, our data highlights a low prescription rate of TBR for severe asthma patients, despite the evidence that this treatment option can have a significant impact on severe asthma control.
Limits of our study include the intrinsic nature of real-life registry-based studies because of their heterogeneity in terms of treated patients and clinical settings, but the strength of data derived from disease registries is in their ability to provide a precise picture of real clinical practice,37 therefore suggesting possible interventions (ie, educational interventions for clinicians) in order to improve the adherence to guidelines and international recommendations.
Acknowledgments
SANI is supported by Unrestricted Grants from AstraZeneca, Glaxo Smith Kline, Novartis & Sanofi Genzyme. SANI Group: Bonavia M, Caiaffa MF, Calabrese C, Camiciottoli G, Caruso C, Centanni S, Conte ME, Corsico AG, Cosmi L, Costantino MT, Crimi N, D’Alo S, D’Amato M, Del Giacco S, Farsi A, Favero E, Foschino BMP, Guarnieri G, Guida G, Yacoub MR, Lombardi C, Macchia L, Mazza F, Menzella F, Milanese M, Montuschi P, Nucera E, Paoletti G, Parente R, Passalacqua G, Patella V, Pelaia G, Pini L, Ricciardi L, Ricciardolo FLM, Richeldi L, Ridolo E, Rolla G, Santus P, Scichilone N, Solidoro P, Spadaro G, Spanevello A, Vianello A, Zappa MC. Concetta Sirena, Daniela Morrone & Silvia Rabotti for their unvaluable job in setting SANI and in collecting data. The results of the present study have been also presented and discussed as an abstract at the European Respiratory Society (ERS) 2019 annual congress.
Disclosure
Prof. Dr. Francesco Blasi reports grants, personal fees from AstraZeneca and Insmed; received lecture-advisory board fees from Chiesi, GSK, Guidotti, Grifols, Menarini, Novartis, Pfizer, Vertex, and Zambon, outside the submitted work. Prof Pierluigi Paggiaro reports institutional support and personal grants for advisory boards and lectures from ALK-Abellò, AstraZeneca, Chiesi, GlaxoSmithKline, Guidotti, Menarini, Mundipharma, Novartis, and Sanofi. Prof. Giorgio Walter Canonica reports grants, personal fees from Menarini, Alk-Abellò, Anallergo, Boehringer Ingelheim, Chiesi, Circassia, Genentech, Guidotti Malesci, GSK, Meda, Merck, Merck Sharp & Dome, Novartis, Recordati-InnuvaPharma, Roche, Sanofi, Stallergenes, UCB Pharma, Teva, AstraZeneca, ThermoFischer, Valeas, Vibor Pharma. Dr. Enrico Heffler reports grants, personal fees and/or grants from AstraZeneca, Sanofi, Novartis, GSK, Circassia, and Nestlè Purina, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Hekking PP, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135:90–896. doi:10.1016/j.jaci.2014.08.042
2. Zeiger RS, Schatz M, Dalal AA, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016;4:120–129. doi:10.1016/j.jaip.2015.08.003
3. Bourdin A, Fabry-Vendrand C, Ostinelli J, et al. The burden of severe asthma in France: a case-control study using a medical claims database. J Allergy Clin Immunol Pract. 2019;7:1477–1487. doi:10.1016/j.jaip.2018.12.029
4. Vianello A, Caminati M, Andretta M, et al. Prevalence of severe asthma according to the drug regulatory agency perspective: an Italian experience. World Allergy Organ J. 2019;12(4):100032. doi:10.1016/j.waojou.2019.100032
5. O’Neill S, Sweeney J, Patterson CC, et al. The cost of treating severe refractory asthma in the UK: an economic analysis from the British thoracic society difficult asthma registry. Thorax. 2015;70(4):376–378. doi:10.1136/thoraxjnl-2013-204114
6. Porsbjerg C, Ulrik C, Skjold T, et al. Nordic consensus statement on the systematic assessment and management of possible severe asthma in adults. Eur Clin Respir J. 2018;5(1):1440868.
7. Albers FC, Müllerová H, Gunsoy NB, et al. Biologic treatment eligibility for real-world patients with severe asthma: the IDEAL study. J Asthma. 2018;55(2):152–160. doi:10.1080/02770903.2017.1322611
8. Ilmarinen P, Tuomisto LE, Niemelä O, Kankaanranta H. Prevalence of patients eligible for anti-il-5 treatment in a cohort of adult-onset asthma. J Allergy Clin Immunol Pract. 2019;7(1):165–174.e4. doi:10.1016/j.jaip.2018.05.032
9. Schleich F, Brusselle G, Louis R, et al. Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir Med. 2014;108(12):1723–1732. doi:10.1016/j.rmed.2014.10.007
10. Global initiative on asthma. Available from: http://ginasthma.org/.
11. Global initiative for chronic obstructive lung diseases. Available from: https://goldcopd.org/.
12. Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22(22):672–688. doi:10.1183/09031936.03.00040703
13. Gosens R, Groos N. The mode of action of anticholinergics in asthma. Eur Resp J. 2018;52:1701247. doi:10.1183/13993003.01247-2017
14. Canning BJ, Reflex regulation of airway smooth muscle tone. J Appl Physiol. 2006;101:971–985. doi:10.1152/japplphysiol.00313.2006
15. Barnes PJ. The pharmacological properties of tiotropium. Chest. 2000;117(2_suppl):63S–66S. doi:10.1378/chest.117.2_suppl.63S
16. Coe CI, Barnes PJ. Reduction of nocturnal asthma by an inhaled anticholinergic drug. Chest. 1986;90:485–488. doi:10.1378/chest.90.4.485
17. Morrison JF, Pearson SB, Dean HG. Parasympathetic nervous system in nocturnal asthma. Br Med J. 1988;296(6634):1427–1429. doi:10.1136/bmj.296.6634.1427
18. O’Connor BJ, Towse LJ, Barnes PJ. Prolonged effect of tiotropium bromide on methacholine-induced bronchoconstriction in asthma. Am J Resp Crit Care Med. 1996;154(4 Pt 1):876–880. doi:10.1164/ajrccm.154.4.8887578
19. Terzano C, Petroianni A, Ricci A, D’Antoni L, Allegra L. Early protective effects of tiotropium bromide in patients with airways hyperresponsiveness. Eur Rev Med Pharmacol Sci. 2004;8(6):259–264.
20. Hamelmann E, Bateman ED, Vogelberg C, et al. Tiotropium add-on therapy in adolescents with moderate asthma: A 1-year randomized controlled trial. J Allergy Clin Immunol. 2016;138(2):441–450. doi:10.1016/j.jaci.2016.01.011
21. Kew KM, Evans DJ, Allison DE, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long-acting beta2-agonists (LABA) for adults with asthma. Cochrane Database Syst Rev. 2015;6:CD011438.
22. Abadoglu O, Berk S. Tiotropium may improve asthma symptoms and lung function in asthmatic patients with irreversible airway obstruction: the real-life data. Clin Respir J. 2016;10(4):421–427. doi:10.1111/crj.12230
23. Cook AL, Kinane TB, Nelson BA. Tiotropium use in pediatric patients with asthma or chronic cough: a case series. Clin Pediatr. 2014;53(14):1393–1395. doi:10.1177/0009922814525836
24. Price D, Kaplan A, Jones R, et al. Long-acting muscarinic antagonist use in adults with asthma: real-life prescribing and outcomes of add-on therapy with tiotropium bromide. J Asthma Allergy. 2015;14:1–13.
25. Senna G, Guerriero M, Paggiaro PL, et al. SANI-Severe Asthma Network in Italy: a way forward to monitor severe asthma. Clin Mol Allergy. 2017;15(1):9. doi:10.1186/s12948-017-0065-4
26. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
27. 3rd edition recommendation on registries for evaluating patient outcomes – effective health care program of the agency for healthcare research and quality. Available from: https://effectivehealthcare.ahrq.gov/topics/registries-guide-3rd-edition/research/.
28. Commission directive 91/507/EEC of 19 July 1991 modifying the annex to council directive 75/318/eec on the approximation of the laws of member states relating to analytical, pharmacotoxicological and clinical standards and protocols in respect of the testing of medicinal products. Available from: https://op.europa.eu/en/publication-detail/-/publication/98ea0b12-c69c-4608-a406-001e48ed29a5/language-en.
29. Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113(1):59–65. doi:10.1016/j.jaci.2003.09.008
30. Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14(4):902–907. doi:10.1034/j.1399-3003.1999.14d29.x
31. Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the asthma quality of life questionnaire. Chest. 1999;115(5):1265–1270. doi:10.1378/chest.115.5.1265
32. Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157(4):790–804. doi:10.1016/j.chest.2019.10.053
33. Averell CM, Laliberté F, Duh MS, Wu JW, Germain G, Faison S. Characterizing real-world use of tiotropium in asthma in the USA. J Asthma Allergy. 2019;12:309–321. doi:10.2147/JAA.S216932
34. Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367:1198–1207. doi:10.1056/NEJMoa1208606
35. Khurana S, Paggiaro P, Buhl R, et al. Tiotropium reduces airflow obstruction in asthma patients, independent of body mass index. J Allergy Clin Immunol Pract. 2019;7(7):2425–2428. doi:10.1016/j.jaip.2019.03.007
36. Dekhuijzen PN, Vincken W, Virchow JC, et al. Prescription of inhalers in asthma and COPD: towards a rational, rapid and effective approach. Respir Med. 2013;107(12):1817–1821. doi:10.1016/j.rmed.2013.09.013
37. Heffler E, Paoletti G, Giorgis V, et al. Real-life studies of biologics used in asthma patients: key differences and similarities to trials. Expert Rev Clin Immunol. 2019;15(9):951–958. doi:10.1080/1744666X.2019.1653758
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
