Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 11
Frequency of sleep disorders in patients with rheumatoid arthritis
Authors Mustafa M , Bawazir Y, Merdad L, Wali S , Attar S , Fathaldin O , Bahlas S, Alhejaili F , Aljohaney A, Jan A , Jadu F
Received 14 January 2019
Accepted for publication 7 May 2019
Published 3 July 2019 Volume 2019:11 Pages 163—171
DOI https://doi.org/10.2147/OARRR.S201556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Chuan-Ju Liu
Mohammad Mustafa,1 Yasser Bawazir,2 Leena Merdad,3 Siraj Wali,4 Suzan Attar,2 Omar Fathaldin,2 Sami Bahlas,2 Faris Alhejaili,4 Ahmed Aljohaney,4 Ahmed Jan,5 Fatima Jadu5
1Rheumatology Unit, Department of Medicine, University of Jeddah, Jeddah, Makkah, Kingdom of Saudi Arabia; 2Rheumatology Unit, Department of Medicine, King Abdulaziz University, Jeddah, Makkah, Kingdom of Saudi Arabia; 3Community Medicine Department, King Abdulaziz University, Jeddah, Makkah, Kingdom of Saudi Arabia; 4Sleep Medicine and Research Center, King Abdulaziz University Hospital, Jeddah, Makkah, Kingdom of Saudi Arabia; 5Faculty of Dentistry, King Abdulaziz University, Jeddah, Makkah, Kingdom of Saudi Arabia
Purpose: To determine the prevalence of common sleep problems among patients with rheumatoid arthritis (RA) and their relationship with the disease activity and quality of life.
Patients and methods: The study sample consisted of 101 patients who attended a rheumatology clinic at a university hospital between October 2015 and May 2016. All subjects were clinically examined and interviewed by physicians using a questionnaire. The collected information included sociodemographic characteristics, the patients’ medical histories, the Disease Activity Score (DAS28), the Berlin questionnaire to assess the risk of obstructive sleep apnea (OSA), the Epworth Sleepiness Scale to assess excessive daytime sleepiness (EDS), the Athens Insomnia Scale to assess insomnia, the International RLS Study Group score to diagnose restless legs syndrome (RLS), and the Health Assessment Questionnaire (HAQ) to assess the quality of life.
Results: The mean age of the participants was 48.7±14.6 years, and 95% of the participants were females. Approximately 60% of the participants were in the remission/low category of disease activity, and the average DAS28 score was 3.3±0.8 years. The prevalence rates of insomnia, EDS, sleep disturbance, risk of OSA, and RLS were 63%, 20%, 20%, 37%, and 63%, respectively. Furthermore, the distribution of sleep disorders was not affected by the disease activity. The association between the HAQ and sleep disorders among the RA patients was not significant.
Conclusion: Sleep disorders are common among RA patients and may require further attention by treating clinicians; nevertheless, these disorders are not associated with disease activity and do not affect the quality of life.
Keywords: prevalence, insomnia, sleep apnea, restless legs syndrome, sleep quality, quality of life
Introduction
Sleep disorders are common in patients with chronic diseases, such as rheumatoid arthritis (RA). Poor sleep quality has been linked to pain, mood, fatigability, stress, and disease activity in the rheumatic disease population.1 Various primary sleep disorders have been observed in RA, including a high prevalence of obstructive sleep apnea (OSA),2 insomnia,3 and restless legs syndrome (RLS).4 Abbasi et al5 determined that the frequency of sleep disorders among 100 RA patients is 72%. More recently, Westhovens et al1 conducted a survey involving 305 patients and concluded that poor control of RA is associated with a reduction in sleep quality, which is likely explained by pain-related arousals.
The risk of OSA among RA patients is reported to be as high as 50%.2 In addition to the well-defined risk factors for OSA, patients with RA may have gross anatomic abnormalities that can cause upper-airway narrowing.6 Redlund-Johnell6 studied 400 patients with RA and found that 70% of those with severe arthritic temporomandibular joint (TMJ) destruction leading to acquired retrognathia experienced episodes of upper-airway obstruction. Hence, TMJ destruction has been observed as a potential risk for OSA in patients with RA.
A recent literature review addressing RLS in RA patients reported that RLS is a frequent comorbidity with prevalence rates of up to 30% and concluded that RLS is an underdiagnosed condition that can seriously impact sleep.4 In another study, chronic insomnia was assessed in a cross-sectional multicenter survey of 689 subjects with RA in Brazil. The results showed that elderly subjects with RA had a 1.6-fold increased risk of experiencing insomnia symptoms compared to subjects without RA.3 Moreover, Purabdollah et al7 studied the effects of variable sleep disorders on the quality of life in 210 patients with RA and concluded that sleep disorders and pain are associated with a poor quality of life in patients with RA.
Although the literature suggests that there may be a link between common sleep disorders and RA, but this relationship is still not well established. Furthermore, the effect of sleep disorders on the disease activity of RA remains undetermined. Therefore, the aims of the present study were to further determine the prevalence of common sleep disorders, namely, sleep disturbance, the risk of OSA, RLS, and insomnia, among patients with RA. In addition we investigated the effect of these sleep disorders on the disease activity and quality of life in patients with RA.
Patients and methods
Subjects
The study sample consisted of 101 patients who attended the rheumatology clinic at King Abdulaziz University Hospital, Jeddah, Saudi Arabia between October 2015 and May 2016. The inclusion criteria for subject enrollment were an age >18 years; and diagnosis of RA according to the American Rheumatology Association (ARA 1987), indicating that at least four of the following seven criteria are required to establish the diagnosis of RA: 1) periarticular morning stiffness for 1 hr or longer; 2) at least three or more joints with arthritis documented by a physician; 3) involvement of proximal interphalangeal joints (PIP), metacarpophalangeal joints (MCP) or the wrist joints; 4) symmetrical distribution of arthritis; 5) presence of rheumatoid nodules; 6) positive rheumatoid factor (RF); and 7) marginal erosions or periarticular osteopenia in radiological studies.8 The first four criteria should be present for a minimum of 6 weeks.9 Ethical approval was obtained from the Ethical Committee of King Abdulaziz University Hospital (KAUH), Jeddah, with reference number 265–14. All patients agreed to participate in this study signed an informed consent.
Data collection
Physicians evaluated all patients using a structured questionnaire and a clinical examination. Furthermore, all questionnaires were translated to Arabic language versions to avoid misunderstandings by the patients. The collected information included sociodemographic characteristics, patient medical histories, Disease Activity Score (DAS28), Berlin questionnaire to assess the risk of OSA, Epworth Sleepiness Scale (ESS) to assess daytime sleepiness, Athens Insomnia Scale (AIS) to assess insomnia, International RLS Study Group (IRLSSG) score to diagnose RLS, and Health Assessment Questionnaire (HAQ) to assess the quality of life. The anthropometric measurements of all patients, including height, weight, body mass index (BMI), neck circumference and blood pressure, were recorded by nurses upon initial recruitment. Laboratory tests for different diseases affecting patient sleep or confirming RA and its assessment, including complete blood counts (CBCs), iron profile, calcium, vitamin D, vitamin B12, thyroid function test, diabetes check-up, renal function test, inflammatory markers [erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP)] and rheumatoid-arthritis-specific antibodies (RF and anti-cyclic citrullinated peptide), were performed for all patients at the KAUH laboratory.
Instruments
Disease Activity Score (DAS28)
The assessment of the RA disease activity was based on the DAS28 score system, which is a global summative and continuous score for disease activity assessment.9 Compared with other tools used for the assessment of disease activity, the DAS28 is one of the most reliable tools recommended by the American College of Rheumatology.9 The DAS28 assessment depends on clinical and biochemical factors and includes a measurement of the number of swollen and tender joints, acute phase reactant (ESR or CRP) and patient’s global assessment of disease activity, which is scored from zero to 100 with higher scores indicating active disease.9 The ranges of DAS28 scores corresponding to high, moderate, and low disease activity and remission have been proposed. High disease activity corresponds to DAS28>5.1, moderate disease activity corresponds to DAS28>3.2 to 5.1, low disease activity corresponds to 2.6–3.2, and remission corresponds to <2.6.10
Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a standardized and valid scale developed to differentiate poor from good sleepers and evaluate the sleep quality in the previous month.11 The PSQI includes 19 self-rated questions. A global PSQI score was calculated by using the PSQI scoring database. The global score ranges from 0 to 21; a normal cut of value of 5 was used. A score of >5 was used to indicate significant sleep disturbance, with higher scores indicating higher levels of sleep disturbance. It was reported that this score had a diagnostic sensitivity of 89.6% and specificity of 86.5% in differentiating poor from good sleepers.11
Epworth Sleepiness Scale (ESS)
The ESS is a validated reliable self-administered questionnaire used to assess subjective daytime sleepiness.12 A subject with a score >10 is considered to have excessive daytime sleepiness (EDS).
Berlin questionnaire
The Berlin questionnaire is a widely used reliable questionnaire.13 This tool was originally designed as a mean for clinicians to quickly establish apnea risk factors and has been validated in patients aged 18 years or older. The scoring process includes evaluating “yes or no” responses and multiple-choice selections and includes space for calculating the BMI based on the respondent’s measurements. Points are assigned to responses indicating more acute symptoms. For “yes or no” questions, one point is given for each “yes” answer. In the case of the multiple-choice questions, the two answers that correspond to the highest severity of apnea both receive one point. Categories one and two are considered high risk if the individual receives two or more points. The respondent is considered high risk in category three (obesity and blood pressure) if their blood pressure is high or their BMI is >30 kg/m2.
Athens Insomnia Scale (AIS)
The AIS is a validated scale used to assess the severity of insomnia using diagnostic criteria set forth by the International Classification of Diseases (ICD-10).14 This eight-item questionnaire evaluates sleep onset, night and early-morning waking, sleep time, sleep quality, frequency and duration of complaints, distress caused by the experience of insomnia, and interference with daily functioning.
International RLS Study Group (IRLSSG)
The criteria for the diagnosis of RLS were modified in 2014.15 The IRLSSG consists of four “yes/no” questions and is used as a test to screen for RLS. In addition, a fifth criterion for clinically excluding other conditions that may mimic RLS is mandatory to confirm the diagnosis of RLS. All the five criteria must be met to diagnose RLS.
Health Assessment Questionnaire (HAQ)
The HAQ is a patient-oriented questionnaire used to assess the quality of life in different medical fields, such as RA. The HAQ assesses disability, discomfort, medication side effects, costs, and mortality through a questionnaire consisting of 20 questions that assess the upper and lower limbs.16 These questions are organized into the following eight categories: dressing, rising, eating, walking, hygiene, reach, grip, and usual activities. Each question is answered on a four-level scale of impairment ranging from 0 to 3 as follows: 0=no difficulty; 1=some difficulty; 2=much difficulty; and 3=inability to perform the activity. The final result is calculated by summing all categories.
Statistical analysis
The means and standard deviations of the continuous demographic and baseline characteristics and laboratory tests of the participants [age, BMI, RA Disease Activity DAS28, CRP, ESR, RF, anti-cyclic citrullinated peptide, serum ferritin, thyroid-stimulating hormone, vitamin B12, urea, creatinine, and hemoglobin A1c (HbA1c)] are reported. The frequencies and percentages of the categorical characteristics, sleep disorders and risk factors (age classification, gender, smoking status, BMI classification, RA Disease Activity classification DAS28, any comorbidities, hypertension, diabetes, ischemic events, insomnia, EDS, sleep disturbance, sleep apnea, and RLS), are reported.
The association between the RA disease activity and different sleep disorders (insomnia, EDS, sleep disturbance, sleep apnea, and RLS) was tested using a chi-square test. The association between the risk factors and sleep disorders in the RA patients was tested using a chi-square test, Fisher’s exact test or Student’s t-test. The association between the HAQ and sleep disorders was tested using a Student’s t-test. The significance level was set at P<0.05, and all statistical analyses were conducted using STATA Version 13.0 (StataCorp, College Station, TX, USA).
Results
The demographic and baseline characteristics of the 101 participants included in the study are presented in Table 1. The mean age of the participants was 48.7±14.6 years. Approximately 95% and 97% of the participants were female and never smoked, respectively. The mean BMI was 32.1±10.2 kg/m2; more than half of the participants were classified as obese, and approximately 30% of the participants were classified as overweight. Approximately 60% of the participants were in the remission/low category of disease activity, and the average RA Disease Activity Score (DAS28) was 3.3±0.8 years. Regarding comorbidities, approximately half of the participants had at least one comorbidity. The mean values of the laboratory blood tests are illustrated in Table 1.
 |
Table 1 Characteristics of the study population |
The prevalence of insomnia, EDS, sleep disturbance, sleep apnea, and RLS among the participants was 63%, 20%, 20%, 37%, and 63%, respectively. Furthermore, the distribution of insomnia, EDS, sleep disturbance, sleep apnea, and RLS according to the RA Disease Activity was similar between the patients with remission/low disease activity and those with moderate/high disease activity and showed no significant differences (Table 2).
 |
Table 2 Insomnia, EDS, sleep apnea, sleep disturbance and RLS according to RA disease activity |
The associations between the different risk factors and insomnia, sleep disturbance, EDS, RLS, and sleep apnea are summarized in Tables 3 and 4. However, insomnia showed no association with any of the suggested risk factors except for gender (P=0.039) (Table 3). Age, BMI classification, any comorbidity, hypertension, and diabetes showed significant associations with sleep apnea. Additionally, 49% of the participants with any comorbidity were categorized as high risk for sleep apnea, while only 25% of those with no comorbidity were categorized as high risk (P=0.012). The high-risk group had increased percentages of hypertension and diabetes (P≤0.001, 0.001, respectively) (Table 4). The associations between the different risk factors and RLS in the RA patients were non-significant except for serum ferritin level (Table 4). A lower level of serum ferritin was detected in patients who met the IRLSSG criteria (P=0.022). Finally, the association between HAQ and sleep disorders among the RA patients was non-significant (Table 5).
 |
Table 3 Risk factors for insomnia; sleep disturbance and excessive daytime sleepiness among RA patients |
 |
Table 4 Risk factors for sleep apnea and RLS among RA patients |
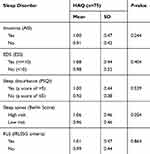 |
Table 5 Association between the HAQ and sleep disorders in RA patients |
Discussion
This study showed that sleep disorders are actually common among patients with RA. Insomnia and RLS were the most common sleep disorders, both of which affected 63% of the study population. Furthermore, a high risk of OSA was reported in 37% of the subjects, while poor sleep quality and EDS affected one-fifth of the study population. Moreover, sleep disorders in conjunction with severe RA had no effect on the quality of life.
In this study, the prevalence of RLS in the RA patients (63%) was much higher than that in the general Saudi population (8.4%).17 This higher prevalence can be explained by the high risk of iron deficiency anemia secondary to the chronic use of NSAIDs in the RA patients,18 which was identified as the main risk factor for secondary RLS.19 Iron deficiency was further confirmed in our study population by the presence of low serum ferritin in patients with RLS. In addition, the prevalence of RLS reported in this study was higher than that reported among RA patients in other studies. Reynolds et al20 reported a strong link between RLS and RA disease with a prevalence of 5–15%; however, the previous study did not use the standard IRLSSG criteria. This discrepancy was explained by iron deficiency anemia and chronic inflammatory conditions with the secretion of multiple cytokines and inflammatory mediators.21,22 Taylor-Gjevre et al22 used the standard IRLSSG criteria to screen RA patients and found that approximately a quarter of RA patients had RLS significantly affecting their quality of life. However, most patients with RLS were underdiagnosed due to the lack of awareness of health care professionals.4
The risk of insomnia based on AIS in the Saudi population is approximately 77%.23 This study showed that two-thirds of the study population had insomnia, which is close to that reported in the general population. Furthermore, this percentage is higher than the globally reported percentage among RA patients. Freitas et al3 used the Nottingham Health Profile and revealed that 55% of rheumatoid patients suffered from insomnia. This finding was explained by pain, functional disability, and depression. Moreover, Westhovens et al1 confirmed the positive relationship between active RA and insomnia by using the AIS. These results were explained by the presence of attentiveness secondary to pain.
The relationship between RA and OSA has been reported in several studies conducted worldwide. Reading et al2 showed that half of the rheumatoid patients have OSA. Furthermore, Sugahara et al24 demonstrated a high risk of OSA (75%) in RA patients compared to that in non-RA subjects. In our study, a high risk of OSA was found in 37% of patients, which is much higher than the estimated prevalence of clinically significant OSA in a sample of the Saudi population (8.5%; 12.4% in men and 4.8% in women).25 Furthermore, we found that the association between high disease activity and sleep apnea was not statistically significant. A larger sample size may be needed to identify a significant association. However, aging, diabetes, hypertension, and obesity were found to be significant risk factors for OSA, which is consistent with the reported literature.25 Furthermore, acquired retrognathia secondary to TMJdestruction in RA has been proposed as a risk factor for OSA and probably attributes to the high risk of OSA in RA.26 Hence, the risk of OSA is particularly high in patients with RA, and our study population had predisposing factors similar to those previously reported despite the limited data.
EDS is strongly related to the female gender and has been observed in 20% of the general population,27 which is comparable to our finding that 20% of our study population had EDS. Moreover, this value is similar to that reported in the literature. Abbasi et al5 showed that 24% of rheumatoid patients complain of EDS.
Poor sleep quality affects 20% of RA patients regardless of disease activity, which may be due to the effect of pain in active RA that can lead to frequent awakening episodes as shown in a previous study.1 To the best of our knowledge, no local data addressing the prevalence of sleep disturbances among Saudis are available. However, Abbasi et al5 showed decreased sleep quality in three-quarters of RA patients due to the presence of pain.
Despite the known detrimental effect of sleep disorders on the quality of life, we did not observe a significant relationship between these disorders and the quality of life in the RA patients. This discrepancy could be explained by the fact that RA patients already have a lower standard quality of life, and sleep disorders do not substantially decrease their quality of life. Another explanation for this nonsignificant result could be related to the distribution of our patient population. Most RA patients recruited in this study were in the remission/low-risk group.
The strengths of our study include a study population representative of patients with a confirmed diagnosis of RA based on validated criteria (ARA 1987).14 Furthermore, although RA is an uncommon disease, a reasonable sample size of 101 patients was included. In addition, the distribution of disease activity was approximately even, and low and high disease activity represented 60% and 40% of the patients, respectively. However, this study has several limitations. First, this study adopted a cross-sectional design. Second, a confirmatory test using polysomnography to diagnose OSA was not used. Third, we did not include a control group for comparison. Finally, information regarding medications, which may play a role in sleep disorders, was not collected in our study.
Conclusion
Sleep disorders are common among RA patients and may require further attention by treating clinicians. This study showed no association between sleep disorders and the quality of life in RA patients, but this association could be masked by the lower quality of life experienced by RA patients due to pain and activity levels.
Abbreviation list
AIS, Athens Insomnia Scale; ARA, American Rheumatology Association; BMI, body mass index; CBCs, complete blood counts; CCP, anti-cyclic citrullinated peptide; CRP, C-reactive protein; DAS28, Disease Activity Score; EDS, excessive daytime sleepiness; ESR, erythrocyte sedimentation rate; ESS, Epworth Sleepiness Scale; HAQ, Health Assessment Questionnaire; HbA1c, hemoglobin A1c; ICD-10, International Classification of Diseases; IRLSSG, International RLS Study Group; KAUH, King Abdulaziz University Hospital; MCP, metacarpophalangeal joints; OSA, obstructive sleep apnea; PIP, proximal interphalangeal joints; RA, rheumatoid arthritis; RF, rheumatoid factor; RLS, restless legs syndrome; TMJ, temporomandibular joint; TSH, thyroid-stimulating hormone.
Acknowledgments
We would like to express our gratitude to Dr Mohamed Hamed for participating in the data collection process and Mrs Walaa Abuzahra for coordinating the data collection process and arranging the procedures. We also appreciate greatly the assistance of Dr Heba Mominkhan, Dr Alshaima Alghamdi, and Dr Rasha Taha Baqais in writing the proposal of this project. This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. RG-01-140-38. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
Author contributions
All authors participated equally in contributions to conception and design, acquistion of data, or analysis and interpretation of data. Drafting the article or revising it critically for important intellectual content. All authors provided final approval of the version to be published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
Professor Siraj Wali reports grants from the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Westhovens R, Van der Elst K, Matthys A, Tran M, Gilloteau I. Sleep problems in patients with rheumatoid arthritis. J Rheumatol. 2014;41(1):31–40. doi:10.3899/jrheum.140167
2. Reading SR, Crowson CS, Rodeheffer RJ, Fitz-Gibbon PD, Maradit-Kremers H, Gabriel SE. Do rheumatoid arthritis patients have a higher risk for sleep apnea? J Rheumatol. 2009;36(9):1869–1872. doi:10.3899/jrheum.080430
3. Freitas DC, Schlosser TC, dos Santos AA, Neri AL, Ceolim MF. Association between insomnia and rheumatoid arthritis in elderly. Rev Esc Enferm USP. 2013;47(4):869–875. doi:10.1590/S0080-623420130000400014
4. Gjevre JA, Tayler Gjevre RM. Restless legs syndrome as a comorbidity in rheumatoid arthritis. Autoimmune Dis. 2013;2013:352782.
5. Abbasi M, Yazdi Z, Rezaie N. Sleep disturbances in patients with rheumatoid arthritis. Niger J Med. 2013;22(3):181–186.
6. Redlund-Johnell I. Upper airway obstruction in patients with rheumatoid arthritis and temporomandibular joint destruction. Scand J Rheumatol. 1988;17(4):273–279. doi:10.3109/03009748809098796
7. Purabdollah M, Lakdizaji S, Rahmani A, Hajalilu M, Ansarin K. Relationship between sleep disorders, pain and quality of life in patients with rheumatoid arthritis. J Caring Sci. 2015;4(3):233–241. doi:10.15171/jcs.2015.024
8. Arnett FC, Edworthy SM, Bloch DA, et al. The American rheumatism association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi:10.1002/art.1780310302
9. Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: american college of rheumatology recommendations for use in clinical practice. Arthritis Care Res (Hoboken). 2012;64(5):640–647. doi:10.1002/acr.21649
10. Aletaha D, Ward MM, Machold KP, Nell VP, Stamm T, Smolen JS. Remission and active disease in rheumatoid arthritis: defining criteria for disease activity states. Arthritis Rheum. 2005;52(9):2625–2636.
11. Buysse DJ, Reynolds CF
12. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi:10.1093/sleep/14.6.540
13. Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American society of anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108(5):822–830. doi:10.1097/ALN.0b013e31816721fa
14. Soldatos CR, Dikeos DG, Paparrigopoulos TJ. Athens insomnia scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–560. doi:10.1016/S0022-3999(00)00095-7
15. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi:10.1016/S1389-9457(03)00010-8
16. Bruce B, Fries JF. The Stanford health assessment questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. doi:10.1186/1477-7525-1-20
17. Wali SO, Abaalkhail B. Prevalence of restless legs syndrome and associated risk factors among middle-aged Saudi population. Ann Thorac Med. 2015;10(3):193–198. doi:10.4103/1817-1737.160839
18. Al-Ghamdi A, Attar SM. Extra-articular manifestations of rheumatoid arthritis: a hospital-based study. Ann Saudi Med. 2009;29(3):189–193. doi:10.4103/0256-4947.51774
19. Alsafadi S, Abaalkhail B, Wali SO, et al. Risk factors of primary and secondary restless legs syndrome among a middle-aged population in SaudiArabia: a community-based study. Ann Thorac Med. 2018;13:175–181. doi:10.4103/atm.ATM_344_17
20. Reynolds G, Blake DR, Pall HS, Williams A. Restless leg syndrome and rheumatoid arthritis. Br Med J (Clin Res Ed). 1986;292(6521):659–660. doi:10.1136/bmj.292.6521.659
21. Hennessy MD, Zak RS, Gay CL, Pullinger CR, Lee KA, Aouizerat BE. Polymorphisms of interleukin-1 Beta and interleukin-17Alpha genes are associated with restless legs syndrome. Biol Res Nurs. 2013;16(2):143–151. doi:10.1177/1099800413478827
22. Taylor-Gjevre RM, Gjevre JA, Skomro R, Nair B. Restless legs syndrome in a rheumatoid arthritis patient cohort. J Clin Rheumatol. 2009;15(1):12–15. doi:10.1097/RHU.0b013e318190f94c
23. Ahmed AE, AL-Jahdali H, Fatani A, et al. The effects of age and gender on the prevalence of insomnia in a sample of the Saudi population. Ethn Health. 2017;22:285–294. doi:10.1080/13557858.2016.1244624
24. Sugahara T, Mori Y, Kawamoto T, Sakuda M. Obstructive sleep apnea associated with temporomandibular joint destruction by rheumatoid arthritis. J Oral Maxillofac Surg. 1994;52:876–880.
25. Wali SO, Abalkhail B, Krayem A. Prevalence and risk factors of obstructive sleep apnea syndrome in a Saudi Arabian population. Ann Thorac Med. 2017;12:88–94. doi:10.4103/1817-1737.203746
26. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi:10.1513/pats.200709-155MG
27. Fatani A, Al-Rouqi K, Al Towairky J, et al. Effect of age and gender in the prevalence of excessive daytime sleepiness among a sample of the Saudi population. J Epidemiol Glob Health. 2015;5(4 Suppl. 1):S59–S66. doi:10.1016/j.jegh.2015.05.005
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
