Back to Journals » International Journal of Nanomedicine » Volume 10 » Issue 1
Free radical scavenging in vitro and biological activity of diphenyl diselenide-loaded nanocapsules: DPDS-NCS antioxidant and toxicological effects
Authors Stefanello ST, Dobrachinski F, de Carvalho NR, Amaral GP, Barcelos RP, Oliveira VA, Oliveira CS, Giordani CFA, Pereira ME, Rodrigues OED, Soares F
Received 23 April 2015
Accepted for publication 28 May 2015
Published 4 September 2015 Volume 2015:10(1) Pages 5663—5670
DOI https://doi.org/10.2147/IJN.S87190
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Thomas Webster
Sílvio Terra Stefanello,1 Fernando Dobrachinski,1 Nélson Rodrigues de Carvalho,1 Guilherme Pires Amaral,1 Rômulo Pillon Barcelos,1 Vitor Antunes Oliveira,1 Cláudia Sirlene Oliveira,1 Camila Ferrazza Alves Giordani,2 Maria Ester Pereira,1 Oscar Endrigo Dorneles Rodrigues,2 Félix Alexandre Antunes Soares1
1Departamento de Bioquímica e Biologia Molecular, 2Departamento de Química, Centro de Ciências Naturais e Exatas, Universidade Federal de Santa Maria, Santa Maria, Brazil
Abstract: Selenium compounds, such as diphenyl diselenide (DPDS), have been shown to exhibit biological activity, including antioxidant effects. However, the use of DPDS in pharmacology is limited due to in vivo pro-oxidative effects. In addition, studies have shown that DPDS-loaded nanocapsules (DPDS-NCS) have greater bioavailability than free DPDS in mice. Accordingly, the aim of this study was to investigate the antioxidant properties of DPDS-NCS in vitro and biological activity in mice. Our in vitro results suggested that DPDS-NCS significantly reduced the production of reactive oxygen species and Fe(II)-induced lipid peroxidation (LPO) in brain. The administration of DPDS-NCS did not result in death or change the levels of endogenous reduced or oxidized glutathione after 72 hours of exposure. Moreover, ex vivo assays demonstrated that DPDS-NCS significantly decreased the LPO and reactive oxygen species levels in the brain. In addition, the highest dose of DPDS-NCS significantly reduced Fe(II)- and sodium nitroprusside-induced LPO in the brain and Fe(II)-induced LPO in the liver. Also, δ-aminolevulinate acid dehydratase within the brain was inhibited only in the highest dose of DPDS-NCS. In conclusion, our data demonstrated that DPDS-NCS exhibited low toxicity in mice and have significant antioxidant characteristics, indicating that nanoencapsulation is a safer method of DPDS administration.
Keywords: diphenyl diselenide, DPDS-NCS, lipid peroxidation, antioxidant properties, δ-ALA-D
Introduction
Selenium (Se) is an essential trace element for mammals found within selenoproteins and has important health benefits.1,2 However, several studies have reported that there is a narrow margin between the beneficial and the toxic effects of Se, stimulating the creation of new Se compounds, including diphenyl diselenide (DPDS) and ebselen.3,4
Accordingly, investigators have described that DPDS, a highly lipophilic organic selenium compound, has promising beneficial effects, which include neurological and hepatic protection against oxidative stress conditions.5,6 In addition, most of the antioxidant properties of DPDS are associated with its ability to mimic the enzyme glutathione peroxidase.7
In contrast, studies have shown that DPDS has pro-oxidative effects in vivo, such as oxidation of sulfhydryl (–SH) groups in proteins, reduction of glutathione levels (GSH), and inhibition of δ-aminolevulinic acid dehydratase (δ-ALA-D).8 In addition, the lethal dose (LD50) for a single intraperitoneal (ip) administration of DPDS in mice is 210 μmol/kg.9
In this regard, nanotechnology is widely applied to produce significant advantages over prodrugs, including improving the bioavailability of lipophilic molecules and increasing the safe dose of toxic compounds.10,11 In addition, nanoparticles help to enhance the stability of drugs and can efficiently control the drug release in a system, which can provide pharmacological efficacy by entrapping the drug molecules until they reach their target.12
Studies have shown that DPDS-loaded-nanocapsules (DPDS-NCS) have greater bioavailability than free DPDS in mice, which suggests an increase in drug absorption after nanoencapsulation.13 However, DPDS-NCS antioxidant effects and the toxicity in mice remain unknown. Thus, the present study was designed to evaluate the antioxidant properties of DPDS-NCS against the generation of reactive oxygen or nitrogen species (ROS and RNS) in vitro and ex vivo and the biological effects following 72-hour exposure of DPDS-NCS exposure in mice.
Materials and methods
Materials
Thiobarbituric acid (TBA), malonldialdehyde (MDA), O-phthalaldehyde (OPT), N-ethylmaleimide, Tris-HCl, trichloroacetic acid, and sodium dodecyl sulfate (SDS) were obtained from Sigma-Aldrich Co. (St Louis, MO, USA). Fe(II) sulfate, sodium nitroprusside (SNP), and acetic acid were obtained from Merck (Rio de Janeiro, RJ, Brazil). All other chemicals were of analytical grade and obtained from standard commercial suppliers.
Compound
DPDS was prepared following the method previously described by Paulmier, and the chemical purity of this compound (99.9%) was accessed by hydrogen and carbon nuclear magnetic resonance and gas chromatography.14
Preparation of nanocapsules
Nanocapsule suspensions were prepared by the interfacial deposition of preformed polymer method as described by Fessi et al.15 Briefly, an organic solution consisted of the oily phase – canola oil (1.551 g), a low HLB (hydrophilic–lipophilic balance) surfactant – Span 80® (0.383 g), polymer (polycaprolactone [PCL] with molecular weight 70,000–90,000 Da) (0.5 g), and acetone (133.5 mL) at 40°C was added under moderated magnetic stirring to an aqueous solution (266.5 mL of Milli-Q water) containing a high HLB surfactant – Tween 80® (0.383 g) at 35°C. DPDS-NCS were prepared by dissolving DPDS in the organic solution during the preparation of the nanocapsules, according to the method described by Giordani et al.13
Nanocapsules characteristics
The particle size, polydispersity indices, and zeta potential were determined using Zetasizer Nano Series (Malvern Instruments, Malvern, UK). The pH values were determined using a Digimed DM-20 calibrated potentiometer (Digimed, São Paulo, SP, Brazil). The encapsulation efficiency was determined by high-performance liquid chromatography as described by Giordani et al.13
Animals
Male adult swiss albino mice (25–30 g) were used. They were obtained from a local breeding colony. The animals were maintained on a 12-hour light and 12-hour dark cycle at a room temperature of 22°C±2°C, with free access to food and water. The animals were used according to the guidelines of the Committee on Care and Use of Experimental Animal Resources. The protocol was approved by the Committee on the Ethics of Animal Experiments of the Federal University of Santa Maria, Brazil (permit number 024745/2009).
In vitro experiments
Tissue preparation
The mice were sacrificed by cervical dislocation. The brains and livers were removed and immediately placed on ice. Both tissues were homogenized in 10 mM Tris-HCl and centrifuged for 10 minutes at 2,000 rpm. The supernatant fraction (S1) was collected immediately for the in vitro assays.
Lipid peroxidation assay
Lipid peroxidation (LPO) was determined by measuring TBA- reactive substances as previously described by Ohkawa et al.16 Aliquots of brain and liver supernatants (100 μL of S1) were incubated for 60 minutes with freshly prepared Fe(II) (10 μM) in the absence or presence of unloaded nanocapsules (vehicle) and DPDS-NCS (10 μM, 20 μM, 40 μM, 80 μM) in a medium containing 10 mM Tris-HCl buffer at pH 7.4. The reaction was stopped by the addition of SDS (8.1%), and LPO products were measured by the addition of acetic acid/HCl buffer, pH 3.4 and 0.6% TBA, pH 6.0. The color reaction was developed by incubating tubes in boiling water for 60 minutes. TBA-reactive substance levels were measured by spectrophotometer at 532 nm using a standard curve of MDA.
ROS production
The substrate 2′-7′-dichlorofluorescein diacetate (DCFH-DA) was utilized to measure the intracellular formation of ROS, according to Myhre et al.17 The brain and liver supernatants (S1) were incubated with unloaded nanocapsules (vehicle) and DPDS-NCS (10 μM, 20 μM, 40 μM, 80 μM). DCFH-DA (1 mM) was added to the medium, and the incubation was continued for 60 minutes in the dark. The fluorescence was measured using 488 nm for excitation and 525 nm for emission. ROS levels were expressed as nanomoles of DCF per milligram of protein.
In vivo experiments
Lethal dose–response
Male adult Swiss albino mice were treated with an ip injection of saline (control), unloaded nanocapsules (vehicle), or DPDS-NCS (10 μmol/kg, 50 μmol/kg, 100 μmol/kg, 500 μmol/kg, and 1,000 μmol/kg). According to Nogueira et al, the death was observed for up to 72 hours to determine the lethal properties of the DPDS-NCS.9
Ex vivo experiments
Animal treatment
Ex vivo experiments were performed in mice treated with an ip injection of saline (control), unloaded nanocapsules (vehicle), or DPDS-NCS (10 μmol/kg, 50 μmol/kg, 100 μmol/kg, 500 μmol/kg, and 1,000 μmol/kg) after 72 hours of exposure. All mice were sacrificed by cervical dislocation. The brains and livers were removed, weighed, dissected, and kept on ice until the time of assay. A portion of both tissues were homogenized in 10 mM Tris-HCl and centrifuged for 10 minutes at 2,000 rpm. The supernatant fraction (S2) was collected immediately for the ex vivo analysis. The remaining portions of brain and liver were used for the measurement of reduced (GSH) and oxidized (GSSG) glutathione levels.
Thiobarbituric acid-reactive substance levels
Aliquots (200 μL) of brain and liver supernatants (S2) were mixed with 500 μL TBA (0.6%), 200 μL SDS (8.1%), and 500 μL acetic acid (pH 3.4). The color reaction was developed by incubating tubes in boiling water for 60 minutes.16 TBA-reactive substance levels were measured at 532 nm using a standard curve of MDA, and the results were expressed as nanomoles of MDA per milligram of protein.
Fe(II) or SNP-induced lipid peroxidation
The end products of LPO were determined in tissue samples as previously described by Ohkawa et al.16 Aliquots of brain and liver supernatants (100 μL of S2) were incubated for 60 minutes with freshly prepared Fe(II) (10 μM) or SNP (5 μM). The assays were carried out as described in section in vitro experiments, except that the compounds were not added to the reaction medium.
Measurement of intracellular reactive oxygen species production
Aliquots (20 μL) of brain and liver supernatants (S2) were added to a medium containing Tris-HCl buffer (10 mM; pH 7.4) and DCFH-DA (1 mM). The assay was conducted as described earlier to the in vitro experiments, except that the compounds were not added to the reaction medium.17
Measurement of reduced (GSH) and oxidized (GSSG) glutathione levels
For the measurement of GSH and GSSG levels, we used a method previously described by Hissin and Hilf.18 Briefly, 250 mg of brain and liver were homogenized in 3.75 mL phosphate EDTA buffer (pH 8) plus 1 mL H3PO4 (25%). Homogenates were centrifuged at 4°C at 130,000× g for 30 minutes, and the supernatants (S3) were separated in two different aliquots of 500 μL each for measurement of GSH and GSSG. For GSH determination, 100 μL of the supernatant (S3) was diluted in 1.8 mL of phosphate buffer and 100 μL of OPT (1 μg/μL). The mixtures were incubated at room temperature for 15 minutes, and their fluorescent signals were recorded in the RF-5301PC Shimadzu spectrofluorometer (Shimadzu Corporation, Kyoto, Japan) at 420 nm of emission and 350 nm of excitation wavelengths. For the measurement of GSSG levels, a 250 μL of the supernatant (S3) was incubated at room temperature with 100 μL of N-ethylmaleimide (0.04 M) for 30 minutes at room temperature, and after that 140 μL of the mixture was added to 1,760 mL of NaOH (0.1 N) buffer, following the addition of 100 μL OPT and incubated for 15 minutes, using the above-outlined procedure for GSH assay.
δ-ALA-D activity
The enzymatic activity was assayed according to the method of Sassa by measuring the rate of porphobilinogen (PBG) formation.19 The incubation was initiated by adding 200 μL of brain and liver supernatants (S2). Brain and liver samples were incubated for 180 minutes and 30 minutes, respectively, at 39°C. The reaction was stopped by the addition of 10% trichloroacetic acid containing 0.05 mol/L HgCl2, and the PBG was measured with Ehrlich’s reagent, using the molar absorption coefficient of 6.1×104 for Ehrlich-PBG salt. The results were expressed as nmol PBG/h/mg protein.
Protein quantification
The protein concentration was estimated by the Bradford method using bovine serum albumin as the standard.20
Statistical analysis
All data are expressed as means ± standard error of the mean for each experimental group. Determination of statistical significance was performed by an one-way analysis of variance, followed by Newman–Keuls multiple range test when appropriate. Differences between groups were considered to be significant when P<0.05.
Results
In vitro results
Effect of DPDS-NCS on LPO induced by Fe(II) in the brains and livers of mice
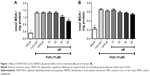
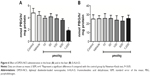
LPO in mice brain and liver homogenates was induced with Fe(II) (10 μM) (Figure 1), and the antioxidant effect of DPDS-NCS on these homogenates was investigated. The DPDS-NCS decreased LPO induced by Fe(II) at the highest concentration tested (80 μM) in the brain sample (Figure 1A); however, no antioxidant effects were observed in the liver sample (Figure 1B).
Scavenging of normal ROS production
Brains and liver samples were incubated with different concentrations of DPDS-NCS to test the scavenging of ROS (Figure 2). All concentrations of DPDS-NCS tested significantly decreased normal ROS production in the brain (Figure 2A); however, no effects on the concentration of ROS were observed in the liver sample (Figure 2B).
In vivo results
The administration of DPDS-NCS at any of the concentration tested (10 μmol/kg, 50 μmol/kg, 100 μmol/kg, 500 μmol/kg, and 1,000 μmol/kg) was not lethal after 72 hours of exposure (data not shown).
Ex vivo results
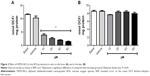
Thiobarbituric acid-reactive substance levels
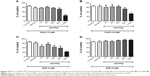
A significant reduction in brain MDA levels was observed with the administration of 1,000 μmol/kg DPDS-NCS as compared to the control group (Figure 3A); however, significant decrease in the formation of MDA was not observed in the liver (Figure 3B). In addition, when LPO was induced by Fe(II) (10 μM), the highest dose of DPDS-NCS led to significant antioxidant activity in both the brain and liver (Figure 4A and B). When LPO was induced with SNP (5 μM), the DPDS-NCS (1,000 μmol/kg) significantly reduced MDA formation in the brain (Figure 4C). The DPDS-NCS did not result in significant MDA reduction at any dose tested when LPO was induced with SNP in the liver (Figure 4D).
Quantification ROS production
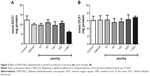
Figure 5A shows that significantly reduced ROS production in the brain by DPDS-NCS (1,000 μmol/kg); however, exposure to DPDS-NCS did not change normal ROS production in the liver (Figure 5B).
GSH and GSSG levels
All groups presented similar GSH and GSSG levels in the brain and liver, indicating that the DPDS-NCS did not interfere with these parameters after 72 hours of exposure (data not shown).
δ-ALA-D activity
The highest dose of the DPDS-NCS (1,000 μmol/kg) led to a significant decrease in brain δ-ALA-D activity (Figure 6A); however, none of the doses of DPDS-NCS tested changed the activity of δ-ALA-D in the liver (Figure 6B).
Discussion
In this study, we performed some antioxidant and toxicological assays to characterize DPDS-NCS in vitro, ex vivo, and in vivo properties. To the best of our knowledge, this is the first study that evaluated DPDS-NCS in the mentioned assays. In addition, DPDS was considering as a promising therapeutic compound of oxidative stress-induced tissue damage.21–23 However, DPDS has a narrow barrier between beneficial and the advent of toxicological effect, which is a limiting factor in their current usage.9,24
Additionally, DPDS-NCS was previously characterized as macroscopic homogeneous aspect, as well as submicronic sizes, low polydispersity indices, negative zeta potentials, and slightly acid or neutral pH values.13 Giordani et al also demonstrated that nanoencapsulation improves the DPDS-NCS pharmacokinetics when compared to the free DPDS.
In this regard, we evaluated the toxicity in mice, following exposure to DPDS-NCS, when administered intraperitoneally in mice. Accordingly, we showed that the administration of DPDS-NCS in doses fivefold higher than the LD50 of free DPDS were not toxic and did not cause seizures in mice, indicating that nanoencapsulation reduces the toxicity of DPDS.
Another important property of DPDS and its analogs is their antioxidant activity in vitro and ex vivo.21,25 In the most frequently cited mechanisms of organic selenium antioxidant activity, the selenium compound acts as substrate of the enzyme thioredoxin reductase or mimics glutathione peroxidase.5,26
Similarly, DPDS protected some tissues against the pro-oxidative effects of ROS and LPO.25,27,28 In addition, LPO and ROS were widely associated with several oxidative diseases and many types of antioxidants play an important protection role through the redox mechanism.29,30
In this regard, we observed a significant in vitro antioxidant activity in the brains of mice, including a reduction of MDA levels (Figure 1) and ROS production (Figure 2). Huang and Cols have shown significant in vitro free radical scavenger activity of nano red elemental selenium, despite its small particle size.31 Our findings corroborate with the findings of that study; although DPDS-NCS exhibit a small particle size, some DPDS in vitro antioxidant activity was maintained.
Also, the exposure of mice to DPDS-NCS reduced MDA levels (Figure 3) and ROS production (Figure 5) in the brain, demonstrating significant antioxidant activity following 72 hours of administration. In this way, we induced ex vivo LPO by Fe(II) to produce ROS and by SNP to generate RNS. We found that the highest dose of DPDS-NCS led to a significant reduction in the oxidant levels in the brain when induced by both pro-oxidants (Figure 4A and C). In addition, the highest DPDS-NCS dose also significantly decreased liver LPO when induced by Fe(II) (Figure 4B).
Another important DPDS characteristic is its ability to interact with and oxidize the sulfhydryl groups of proteins within biological systems. This is the most likely mechanism leading to the inhibition of δ-ALA-D.6 Several studies have shown that exposure to DPDS can induce toxicity by oxidizing cysteinyl residues for important enzymes.6,32 Thus, our findings demonstrate that the DPDS-NCS at the highest dose tested were able to inhibit δ-ALA-D in the brain, indicating that this dose maintained the enzyme inhibition 72 hours after administration of the DPDS-NCS (Figure 6A). However, we also verified that exposure to the DPDS-NCS did not inhibit the liver δ-ALA-D (Figure 6B).
Moreover, DPDS-NCS was absorbed faster than the free compound, which demonstrated that the metabolism of DPDS-NCS in the liver can occur earlier than 72 hours of exposure.13
In addition, our results established that DPDS-NCS in all tested doses did not present any significant effect on the GSH and GSSG levels in the brain and liver, indicating that even if the thiol groups of the compound are oxidized, normal levels can reestablished.
Conclusion
We developed in vitro, in vivo, and ex vivo assays to evaluate the antioxidant and the biological effects of DPDS-NCS in mice. The results of this study have demonstrated that antioxidant activity of DPDS-NCS can significantly influence the physico-chemical characteristics of the nanomaterial. Consequently, DPDS-NCS presented greater in vitro and ex vivo antioxidant effects in the brain than in the liver. Furthermore, the DPDS-NCS were not lethal to mice following 72 hours of exposure. Thus, DPDS exposure is safer after nanoencapsulation. In conclusion, further studies can be beneficial in evaluating the efficacy of DPDS-NCS as therapeutic option for the treatment against oxidative stress-associated diseases.
Acknowledgments
This work was carried out with funds from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq Universal #472669/2011-7 and #475896/2012-2), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES for providing a fellowship to STS) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (Programa de Apoio a Núcleos emergentes – PRONEM/FAPERGS #11/2029-1).
Disclosure
The authors report no conflicts of interest in this work.
References
Hesketh J. Nutrigenomics and selenium: gene expression patterns, physiological targets, and genetics. Annu Rev Nutr. 2008;28(1):157–177. | ||
Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–1268. | ||
Mugesh G, du Mont WW, Sies H. Chemistry of biologically important synthetic organoselenium compounds. Chem Rev. 2001;101(7):2125–2179. | ||
Nuttall KL. Evaluating selenium poisoning. Ann Clin Lab Sci. 2006;36(4):409–420. | ||
Nogueira CW, Zeni G, Rocha JBT. Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev. 2004;104(12):6255–6286. | ||
Nogueira CW, Rocha JBT. Diphenyl diselenide a janus-faced molecule. J Braz Chem Soc. 2010;21:2055–2071. | ||
Bhabak KP, Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc Chem Res. 2010;43(11):1408–1419. | ||
Rosa RM, Roesler R, Braga AL, Saffi J, Henriques JAP. Pharmacology and toxicology of diphenyl diselenide in several biological models. Braz J Med Biol Res. 2007;40:1287–1304. | ||
Nogueira CW, Meotti FC, Curte E, Pilissao C, Zeni G, Rocha JB. Investigations into the potential neurotoxicity induced by diselenides in mice and rats. Toxicology. 2003;183(1–3):29–37. | ||
Zhang JS, Gao XY, Zhang LD, Bao YP. Biological effects of a nano red elemental selenium. Biofactors. 2001;15(1):27–38. | ||
Kroll A, Pillukat MH, Hahn D, Schnekenburger J. Current in vitro methods in nanoparticle risk assessment: limitations and challenges. Eur J Pharm Biopharm. 2009;72(2):370–377. | ||
Caputo F, De Nicola M, Ghibelli L. Pharmacological potential of bioactive engineered nanomaterials. Biochem Pharmacol. 2014;92(1):112–130. | ||
Giordani CF, de Souza D, Dornelles L, et al. Diphenyl diselenide-loaded nanocapsules: preparation and biological distribution. Appl Biochem Biotechnol. 2014;172(2):755–766. | ||
Paulmier C. Selenium Reagents and Intermediates in Organic Synthesis. Pergamon: Pergamon press; 1986. | ||
Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nanocapsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55(1):R1–R4. | ||
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–358. | ||
Myhre O, Andersen JM, Aarnes H, Fonnum F. Evaluation of the probes 2′,7′-dichlorofluorescin diacetate, luminol, and lucigenin as indicators of reactive species formation. Biochem Pharmacol. 2003;65(10):1575–1582. | ||
Hissin PJ, Hilf R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal Biochem. 1976;74(1):214–226. | ||
Sassa S. Delta-aminolevulinic acid dehydratase assay. Enzyme. 1982;28(2–3):133–145. | ||
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. | ||
Stefanello ST, Flores da Rosa EJ, Dobrachinski F, et al. Effect of diselenide administration in thioacetamide-induced acute neurological and hepatic failure in mice. Toxicol Res. 2015;4(3):707–717. | ||
Brandão R, Acker CI, Leite MR, Barbosa NBV, Nogueira CW. Diphenyl diselenide protects against glycerol-induced renal damage in rats. J Appl Toxicol. 2009;29(7):612–618. | ||
Carvalho NR, da Rosa EF, da Silva MH, et al. New therapeutic approach: diphenyl diselenide reduces mitochondrial dysfunction in acetaminophen-induced acute liver failure. PLoS One. 2013;8(12):e81961. | ||
Rosa RM, Hoch NC, Furtado GV, Saffi J, Henriques JAP. DNA damage in tissues and organs of mice treated with diphenyl diselenide. Mutat Res. 2007;633(1):35–45. | ||
de Souza Prestes A, Stefanello ST, Salman SM, et al. Antioxidant activity of beta-selenoamines and their capacity to mimic different enzymes. Mol Cell Biochem. 2012;365(1–2):85–92. | ||
Nogueira C, Rocha JT. Toxicology and pharmacology of selenium: emphasis on synthetic organoselenium compounds. Arch Toxicol. 2011;85(11):1313–1359. | ||
Meotti FC, Stangherlin EC, Zeni G, Nogueira CW, Rocha JBT. Protective role of aryl and alkyl diselenides on lipid peroxidation. Environ Res. 2004;94(3):276–282. | ||
Posser T, Moretto MB, Dafre AL, et al. Antioxidant effect of diphenyl diselenide against sodium nitroprusside (SNP) induced lipid peroxidation in human platelets and erythrocyte membranes: an in vitro evaluation. Chem Biol Interact. 2006;164(1–2):126–135. | ||
Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med. 2010;49(4):503–515. | ||
Niki E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 2012;586(21):3767–3770. | ||
Huang B, Zhang J, Hou J, Chen C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radic Biol Med. 2003;35(7):805–813. | ||
Maciel EN, Bolzan RC, Braga AL, Rocha JBT. Diphenyl diselenide and diphenyl ditelluride differentially affect δ-aminolevulinate dehydratase from liver, kidney, and brain of mice. J Biochem Mol Toxicol. 2000;14(6):310–319. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.