Back to Journals » Clinical Interventions in Aging » Volume 16
Free Flap Reconstruction of the Extremities in Patients Who are ≥65 Years Old: A Single-Center Retrospective 1-to-1 Matched Analysis
Authors Spindler N, Pieroh P , Spiegl U , Arakelyan S, Fakler JKM , Heyde CE, Langer S
Received 5 January 2021
Accepted for publication 26 February 2021
Published 18 March 2021 Volume 2021:16 Pages 497—503
DOI https://doi.org/10.2147/CIA.S300558
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Nandu Goswami
Nick Spindler, Philipp Pieroh, Ulrich Spiegl, Sergey Arakelyan, Johannes Karl Maria Fakler, Christoph-Eckhard Heyde, Stefan Langer
Department of Orthopedic Surgery, Traumatology and Plastic Surgery, University Hospital Leipzig, Leipzig, Germany
Correspondence: Nick Spindler
Department of Orthopedic Surgery, Traumatology and Plastic Surgery, University Hospital Leipzig, Liebigstrasse 20, Leipzig, 04103, Germany
Tel +49-341-9717140
Fax +49-341-9717139
Email [email protected]
Purpose: Demographic changes are leading to population aging, and free flap reconstructions for various indications are expected to become increasingly common among older patients. Therefore, this study evaluated free flap reconstruction of the extremities in older patients and compared the outcomes to those from younger patients who underwent similar procedures during the same period.
Patients and Methods: This single-center retrospective study used a case-control design to compare older and younger patients who underwent free flap reconstruction of soft tissue defects in the extremities. One-to-one matching was performed for older patients (≥ 65 years) and younger patients (≤ 64 years) according to indication, flap recipient site, and flap type. The parameters of interest were clinico-demographic characteristics, flap type, defect location, indication for free flap reconstruction, number of venous anastomoses, and postoperative complications (flap loss, infection, and wound healing disorders).
Results: The study included 48 older patients and 133 younger patients, with a mean follow-up of 12 months after discharge. The free flap reconstruction was performed at a mean interval of 19.8± 22.8 days (range: 0– 88 days). The 1:1 matching created 38 pairs of patients, which revealed no significant differences in the rates of flap necrosis and flap failure.
Conclusion: This study failed to detect a significant age-related difference in the flap necrosis rate after free flap reconstruction of extremity defects. Therefore, with careful perioperative management and patient selection, microsurgical free flap reconstruction is a feasible option for older patients.
Keywords: older patients, microsurgery, free flap, limb reconstruction
Introduction
Microsurgery was introduced during the 1970s, although these procedures were considered dangerous for older patients at that time. However, improvements in anesthetic procedures, shorter intervention times, and postoperative care have steadily improved patient outcomes.1–3 Furthermore, advances in surgical techniques and equipment have made microsurgical procedures a safe option with improved success rates.4,5 Nevertheless, while age by itself is not an absolute contraindication for microsurgery, older patients typically have co-morbidities and decreased functional capacities of vital organs.6–8 Thus, procedural complications are possible and potentially fatal for older patients.
Demographic changes with an unprecedented shift in the demographic framework are leading to population aging.9 A falling birth rate has also lead to the proportion of European people who are >65 years old, increasing from 15% to 22% over the last 30 years.10 Moreover, increasing life expectancy is observed in the industrialized parts of the world, which is related to economic growth, improving education, and advances in medical treatments.11 Therefore, free tissue transfers will likely become increasingly common for older patients, which highlights the need for reliable and safe microsurgical strategies in this patient population.12
The common indications for free tissue transfer in older patients include major tumor resection, extremity injuries with impending amputation, and severe infection. The increasing use of perforator flaps has helped minimize donor site morbidity and broadened the spectrum of suitable cases. Moreover, improvements in microsurgical equipment, magnification systems, and operative experience have led to a shift toward increasing the use of free flaps in older patients. However, there is insufficient information regarding the outcomes, complications, and risk factors in this patient population. Therefore, this study aimed to review our experience using free flaps for older patients with soft tissue defects in their extremities and compare these outcomes to those of younger patients who underwent similar procedures that were performed by the same surgical team during the same period.
Patients and Methods
Study Population
The retrospective single-center study protocol was approved by the Institutional Ethics Committee. The study has been registered at the German clinical trial register (DRKS0024004).
Patients were considered eligible if they had undergone free flap reconstruction of soft tissue defects in their extremities between May 2012 and October 2017 at a university department that specializes in orthopedic surgery, traumatology, and plastic surgery. Based on the average life expectancy in Germany (men: 78.5 years, women: 83.3 years) and the average state pension age (65.8 years), we defined “older patients” as being ≥65 years old.13 The inclusion criteria were patients who were >65 years old, underwent free tissue transfer for soft tissue defects in the extremities, and had a mean follow-up of 12 months after discharge from the hospital. A control group was created using younger patients (18–65 years old) who had undergone similar treatments that were performed by the same surgical team at the same center during the same time period. Exclusion criteria were incomplete medical records, reconstructive techniques other than free flap reconstruction (eg, pedicled flaps), and free flaps used in other areas as extremities.
The parameters of interest were clinico-demographic characteristics, flap type, defect location, cause of the soft-tissue defect, indication for free flap reconstruction, donor site, type and number of venous anastomoses, co-morbidities, and postoperative complications (eg, flap loss, infection, and wound healing disorders at the donor and recipient sites).
Statistical Analysis
The older and younger age groups were directly compared, and a 1:1 matching was subsequently performed according to indication, flap recipient site, flap type, and race (sex was considered random and was not used as a matching criterion). Unmatched patients were not considered in the matched analyses. Continuous variables were reported as mean ± standard deviation, and categorical variables were reported as number (percentage). Continuous variables were analyzed using Student’s t-test or the Mann–Whitney U-test, depending on the data distribution. All analyses were performed using GraphPad Prism (version 9; GraphPad Software, Inc.), and results were considered statistically significant at p-values of <0.05.
Results
The study included 48 older patients (≥65 years old) and 133 younger patients (<65 years old) who had a mean follow-up of 12 months after discharge from the hospital. The older group included 23 men (48%) and 25 women (52%) with a mean age at surgery of 75.8±5.9 years (range: 65–89 years). In this group, 28 patients (58%) had arterial hypertension, 13 patients (27%) had diabetes mellitus, and the mean body mass index was 27±4.62 kg/m2 (range: 18.83–37.24 kg/m2) (Table 1). The mean operation time was 4.53±0.96 h (range: 2.87–8.06 h), and simultaneous osteosynthesis was performed in 11 cases (23%). The free flap reconstruction was performed at a mean interval of 19.8±22.8 days (range: 0–88 days) after the defect was created. The flaps involved anterolateral thigh flaps (30 cases), latissimus dorsi flaps (11 cases), lateral arm flaps (3 cases), deep inferior epigastric perforator flaps (2 cases), a vastus lateralis flap (1 case), and a parascapular flap (1 case). The mean flap dimensions were 12.9±5.8 cm (range: 5–28 cm) by 8.7±4.1 cm (range: 5–20 cm), and the mean hospital stay was 30±21 days (range: 6–95 days). Full flap necrosis occurred in 9 cases; partial necrosis was observed at the wound edges of 2 flaps, and a donor-site hematoma needed to be evacuated in 1 case.
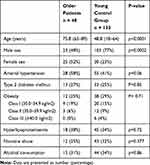 |
Table 1 Characteristics of the Older Patients and the Young Control Group |
The older and younger groups were compared according to flap type, donor site, recipient site, indication, number/type of anastomoses (arterial and venous branches), and postoperative complications. There was no significant difference regarding flap necrosis and failure between both groups (Table 2). Furthermore, there were no significant inter-group differences in terms of body mass index and duration of surgery; therefore, they could be excluded as potential risk factors. Flap failure did not appear to be associated with tumor-related indications or other indications for soft tissue reconstruction, or flap size.
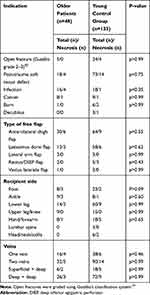 |
Table 2 Comparing the Necrosis Rate Among the Older Patients and the Young Control Group |
The 1:1 matching process generated 38 pairs of younger and older patients. Ten older patients were not included as they did not have a matching partner in the younger population (indication, defect location, and flap type did not match).
There was no significant inter-group difference in terms of flap loss. Furthermore, there were no significant inter-group differences in terms of type 2 diabetes, body mass index, hypertension, nicotine abuse, operation time, or hospital stay (Table 3).
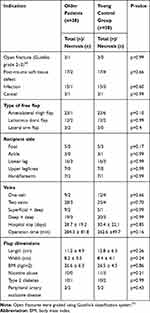 |
Table 3 Comparing the Necrosis Rate via 1:1 Matching of the Older Patients and the Young Control Group |
Discussion
This study revealed comparable flap necrosis outcomes after a direct comparison of our study groups and a 1:1 matching of older and younger patients who underwent similar procedures that were performed by the same surgical team during the same time period. Furthermore, the anastomosis number/type (superficial and/or deep veins) and surgical indication did not appear to be associated with the flap necrosis rate. Moreover, there was no significant difference between the older and younger groups in terms of operation time and hospital stay.
Although, some studies investigating the safety and viability of free flap reconstruction in the older patients have previously published, the majority of literature comprises small-volume studies mostly related to oncologic defects and reconstructions in the ear-nose-throat area.14–17 However, there are evident differences between elective defects due to tumor resection and post-traumatic or infectious limb reconstructions. There were controversies on study findings due to small sample size and heterogeneous indications for reconstruction.18
Although microsurgical free flap reconstruction has become the preferred treatment option for severe soft tissue defects, older patients frequently have co-morbidities and malnutrition, which can impair wound healing.19,20 In addition, changes in skin properties, immunosenescence, and tissue atrophy can increase the likelihood of infection among older patients.21 Thus, radical debridement and prompt coverage of the exposed bone and tendons are necessary. In most of these cases, the use of a local flap compromises the integrity of the extremity, whereas using a free flap can help minimize morbidity at the donor and recipient sites. However, microsurgical free flap reconstruction has historically been considered contraindicated in older patients because of the anesthetic load and prolonged operation time. Therefore, although these procedures have been widely accepted for the general population, their use is considered controversial for older patients.22–24 In addition, their decreased health and functional capacity may increase the risks of postoperative morbidity and mortality.4,5,25 Furthermore, a low stress reserve is associated with low cardiac and pulmonary functional reserves.5,26 These issues highlight the importance of careful patient selection and improved postoperative management when considering microsurgical free flap reconstruction for older patients.
The indications for free tissue transfer have expanded based on the development of relatively mild anesthesia procedures, increasingly standardized perioperative management, and improved microsurgical techniques. Twenty years prior, the reported standard operation time averaged 7.8 h in microsurgical interventions.24 Since then, operation time has subsequently shortened due to improved diagnostic procedures, better surgical equipment, and increasing microsurgical experience. In our study, the average time for microsurgical free flap reconstruction was 4.53 h, which included simultaneous osteosynthesis in 23% of the cases. Thus, shorter operation time may substantially reduce the patients’ risks that are related to anesthetic load.
Most reports regarding free tissue transfer in older patients have described tumor-related free flap reconstructions (mean flap size 20 cm2) in the ear-nose-throat area.15–17 These flaps are generally small, especially relative to our mean flap size of 92 cm2, which is related to the often substantial trauma patterns in the extremities. In this setting, patients often experience extensive soft tissue destruction, and a large flap with a long pedicle is needed to reach unaffected recipient vessels. The latissimus dorsi flap has been regularly used during limb-preserving reconstruction in cases with severe injuries that expose the bone or cases with a high risk of infection.27 In our study, the anterolateral thigh and latissimus dorsi flaps are the workhorses of our department, as they are used in 83% and 80% of older patients and younger patients, respectively. The latissimus dorsi flap is considered the gold standard for limb reconstruction.28,29 Although muscle and fasciocutaneous free flaps are currently thought to provide comparable long-term functional outcomes,30,31 the anterolateral thigh flap (as a fasciocutaneous flap) provides better cosmetic results.31 Thus, the anterolateral thigh flap is commonly selected in our department based on the low donor site morbidity and the possibility of secondary modifications, such as liposuction to address bulging flaps. Furthermore, these secondary procedures can be performed under tumescent anesthesia, which is a substantial benefit for older patients with multiple co-morbidities. This study failed to detect significant differences in outcomes according to flap type, donor location, or flap vessel diameter, supporting our belief that fasciocutaneous flaps are superior to muscle flaps for older patients.
In this study, two veins were used for the anastomoses in 60% of the cases to reduce the load on the flap. In this context, increased blood flow to the flap may require a strategy to promote venous outflow and avoid secondary edema. Stranix et al have reported that, relative to single-vein flaps, two venous anastomoses in free flap reconstruction of the lower extremity, a four-fold reduction in complication rates is provided.30 However, we did not observe this level of reduction among our patients, and there were no significant differences between the older and younger groups in terms of the number or location of venous anastomoses (eg, superficial vs deep veins). Nevertheless, venous mismatch can lead to turbulent blood flow, causing platelet aggregation/activation and vessel stenosis. Therefore, the recipient vein is carefully selected to match the diameters as closely as possible. Similarly, supercharging via a superficial vein is used in cases with substantial diameter mismatch. Furthermore, we use a prophylactic perioperative and long-term low-dose anticoagulation treatment.
Approximately 20% of cancer patients experience venous thrombosis32,33 and have a 4–7-fold higher risk of venous thrombo-embolism than non-cancer patients.34,35 A clear consensus regarding the ideal anticoagulation protocol for free flap reconstruction is missing.36,37
In addition, literature is uncertain regarding the benefits and risks of anticoagulation therapy and its association with increased rates of hematoma and heparin-induced thrombocytopenia.38–42 Thus, in cooperation with our hematology department, we evaluated the risk factors for patients who are undergoing a free flap reconstruction and developed a standardized anticoagulation strategy. To avoid early stenosis at the anastomosis, all patients receive a single dose of heparin (2500 IU) before blood flow is restored to the flap. Furthermore, patients receive a 5-day postoperative course of intravenous low-dose heparin (400 IU/h), which is followed by a 6-week treatment using acetylsalicylic acid (100 mg/day). This strategy may be effective as comparisons of the cancer and non-cancer patients as well as the younger and older patients, revealed no significant differences in the flap-related outcomes, especially not due to venous thrombosis.
This study has several limitations that should be considered. First, the sample size was small, and the analyses might be underpowered; however, the sample size is comparable to those used in previous reports. Second, the retrospective study design is prone to various sources of bias. Third, we evaluated a heterogeneous sample of patients with multiple indications, different flap types, and procedures, which might have influenced the findings. However, this heterogeneity represents the emergence of free flap reconstruction in older patients in everyday life. Nevertheless, the microsurgical procedures and anastomoses were based on a standardized operative procedure. Therefore, large multi-center studies are needed to address these limitations. Additionally, it may be prudent to consider how nutritional status and chronic comorbidities can influence flap-related outcomes after microsurgery in older patients.
Conclusion
This study revealed that older and younger patients had comparable flap-related outcomes after microsurgical free flap reconstruction of soft tissue defects in their extremities. A standardized perioperative and long-term low-dose anticoagulation treatment should be administered to prevent anastomosis stenosis. Nevertheless, successful microsurgical free tissue transfer depends on various factors, including perioperative patient care, nutritional status, donor site, and appropriate preoperative investigations of the arterial and venous statuses. Therefore, meticulous surgical planning and expertise are needed to ensure successful outcomes in this patient population.
Data Sharing Statement
All study-related data are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
All procedures complied with the ethical standards of the University of Leipzig ethics committee (443/17-ek) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The participants provided written informed consent for participation in this study; copies are available for review by the editor of this journal.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This report was supported by the German Research Foundation and Leipzig University as part of an Open Access Publishing program.
Disclosure
Professor Christoph-Eckhard Heyde reports royalties from Medacta Int. All other authors declare that they have no competing interests.
References
1. Strom C, Rasmussen LS, Steinmetz J. Practical management of anaesthesia in the elderly. Drugs Aging. 2016;33(11):765–777. doi:10.1007/s40266-016-0413-y
2. Lim BG, Lee IO. Anesthetic management of geriatric patients. Korean J Anesthesiol. 2020;73(1):8–29. doi:10.4097/kja.19391
3. Murthy S, Hepner DL, Cooper Z, Bader AM, Neuman MD. Controversies in anaesthesia for noncardiac surgery in older adults. Br J Anaesth. 2015;115(Suppl2):ii15–ii25. doi:10.1093/bja/aev396
4. Coskunfirat OK, Chen HC, Spanio S, Tang YB. The safety of microvascular free tissue transfer in the elderly population. Plast Reconstr Surg. 2005;115(3):771–775. doi:10.1097/01.PRS.0000152424.91250.A5
5. Muravchik S. Anesthesia for the elderly. In: Miller R, editor. Anesthesia.
6. Simunovic F, Eisenhardt SU, Penna V, Thiele JR, Stark GB, Bannasch H. Microsurgical reconstruction of oncological scalp defects in the elderly. J Plast Reconstr Aesthet Surg. 2016;69(7):912–919. doi:10.1016/j.bjps.2016.03.021
7. Nicholas JA. Preoperative optimization and risk assessment. Clin Geriatr Med. 2014;30(2):207–208. doi:10.1016/j.cger.2014.01.003
8. Beck S, Buchi C, Lauber P, Grob D, Meier C. Perioperative Risikostratifizierung geriatrischer Patienten bei nicht kardialen Eingriffen [Perioperative risk assessment of geriatric patients undergoing noncardiac surgery]. Z Gerontol Geriatr. 2014;47(2):90–94. doi:10.1007/s00391-013-0589-2
9. Global Health Observatory Data Repository. Life expectancy. Data by country. Geneva, Switzerland: World Helath Statistics; 2015. Available from https://www.who.int/docs/default-source/gho-documents/world-health-statistic-reports/world-health-statistics-2015.pdf.
10. Population and budget development at federal and state level. Vol 1: statistical offices of the federation and the Länder; 2011. Available from https://www.destatis.de/DE/Themen/Querschnitt/Demografischer-Wandel/_inhalt.html.
11. Ezzati M, Friedman AB, Kulkarni SC, Murray CJL. The reversal of fortunes: trends in county mortality and cross-county mortality disparities in the United States. PLoS Med. 2008;5(4):e66. doi:10.1371/journal.pmed.0050066
12. Hennings R, Spiegl U, Fakler J, Ahrberg A. The AO triangular external fixator: a backup option in the treatment of ankle fractures in geriatric patients? Eur J Orthop Surg Traumatol. 2020. doi:10.1007/s00590-020-02740-0
13. für politische Bildung B. Zahlen und Fakten, die soziale situation in Deutschland; 2020. Available from https://www.bpb.de/nachschlagen/zahlen-und-fakten/soziale-situation-in-deutschland/61547/lebenserwartung.
14. Hammonds C, Jackson PC, Foster P, Wiper JD. Managing complex trauma injuries in the elderly: a case report of a free flap and circular frame in a 95-year old patient with an open IIIB tibial fracture. Eur J Plast Surg. 2018;41(4):475–478. doi:10.1007/s00238-018-1405-4
15. Bozec A, Poissonnet G, Chamorey E, et al. Radical ablative surgery and radial forearm free flap (RFFF) reconstruction for patients with oral or oropharyngeal cancer: postoperative outcomes and oncologic and functional results. Acta Otolaryngol. 2009;129(6):681–687. doi:10.1080/00016480802369260
16. Disa JJ, Pusic AL, Hidalgo DH, Cordeiro PG. Simplifying microvascular head and neck reconstruction: a rational approach to donor site selection. Ann Plast Surg. 2001;47(4):385–389. doi:10.1097/00000637-200110000-00004
17. Nao EE, Dassonville O, Chamorey E, et al. Head and neck free-flap reconstruction in the elderly. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128(2):47–51. doi:10.1016/j.anorl.2010.12.001
18. Üstün GG, Aksu AE, Uzun H, Bitik O. The systematic review and meta-analysis of free flap safety in the elderly patients. Microsurgery. 2017;37(5):442–450. doi:10.1002/micr.30156
19. Molnar JA, Vlad LG, Gumus T. Nutrition and chronic wounds: improving clinical outcomes. Plast Reconstr Surg. 2016;138(3):71S–81S. doi:10.1097/PRS.0000000000002676
20. Van de Kerkhof PC, Van Bergen B, Spruijt K, Kuiper JP. Age-related changes in wound healing. Clin Exp Dermatol. 1994;19(5):369–374. doi:10.1111/j.1365-2230.1994.tb02684.x
21. Anderson DJ, Kaye KS. Skin and soft tissue infections in older adults. Clin Geriatr Med. 2007;23(3):595–613. doi:10.1016/j.cger.2007.03.002
22. Koepple C, Kallenberger AK, Pollmann L, et al. Comparison of fasciocutaneous and muscle-based free flaps for soft tissue reconstruction of the upper extremity. Plast Reconstr Surg Glob Open. 2019;7(12):e2543. doi:10.1097/GOX.0000000000002543
23. Pederson WC, Grome L. Microsurgical reconstruction of the lower extremity. Semin Plast Surg. 2019;33(1):54–58. doi:10.1055/s-0039-1677878
24. Serletti JM, Higgins JP, Moran S, Orlando GS. Factors affecting outcome in free-tissue transfer in the elderly. Plast Reconstr Surg. 2000;106(1):66–70. doi:10.1097/00006534-200007000-00012
25. Variakojis R, Roizen M. Preoperative evaluation of the elderly. In: Leskey MC, editor. Geriatr Anaesthesiol. Baltimore: Williams & Williams; 1997:165–185.
26. Davenport HT. Anaesthesia for the geriatric patient. Can Anaesth Soc J. 1983;30(S3):S51–S55. doi:10.1007/BF03009980
27. Dabb RW, Davis RM, Dabb RW. Latissimus dorsi free flaps in the elderly: an alternative to below-knee amputation. Plast Reconstr Surg. 1984;73(4):633–640. doi:10.1097/00006534-198404000-00021
28. Steinau HU, Hebebrand D, Vogt P. Amputation alternatives preserving bipedal ambulation. Oper Tech Plastic Reconstruct Surg. 1997;4:199–208. doi:10.1016/S1071-0949(97)80026-1
29. Steinau HU. [Microvascular latissimus dorsi transfer. Clinical use including management of the site of tissue removal]. Der Chirurg. 1986;57(3):126–133. German.
30. Stranix JT, Lee ZH, Anzai L, et al. Optimizing venous outflow in reconstruction of Gustilo IIIB lower extremity traumas with soft tissue free flap coverage: are two veins better than one? Microsurgery. 2018;38(7):745–751.
31. Philandrianos C, Moullot P, Gay AM, et al. Soft tissue coverage in distal lower extremity open fractures: comparison of free anterolateral thigh and free latissimus dorsi flaps. J Reconstr Microsurg. 2018;34(2):121–129. doi:10.1055/s-0037-1607323
32. Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. doi:10.1182/blood-2013-04-460121
33. Blom JW, Vanderschoot JP, Oostindier MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4(3):529–535. doi:10.1111/j.1538-7836.2006.01804.x
34. Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American Society Of Clinical Oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33(6):654–656. doi:10.1200/JCO.2014.59.7351
35. Decousus H, Bertoletti L, Frappe P, et al. Recent findings in the epidemiology, diagnosis and treatment of superficial-vein thrombosis. Thromb Res. 2011;127(Suppl 3):S81–85. doi:10.1016/S0049-3848(11)70022-6
36. Askari M, Fisher C, Weniger FG, Bidic S, Lee WP. Anticoagulation therapy in microsurgery: a review. J Hand Surg Am. 2006;31(5):836–846. doi:10.1016/j.jhsa.2006.02.023
37. Xipoleas G, Levine E, Silver L, Koch RM, Taub PJ. A survey of microvascular protocols for lower-extremity free tissue transfer I: perioperative anticoagulation. Ann Plast Surg. 2007;59(3):311–315. doi:10.1097/SAP.0b013e31802fc217
38. Liu J, Shi Q, Yang S, Liu B, Guo B, Xu J. Does postoperative anticoagulation therapy lead to a higher success rate for microvascular free-tissue transfer in the head and neck? A systematic review and meta-analysis. J Reconstr Microsurg. 2018;34(2):87–94. doi:10.1055/s-0037-1606346
39. Senchenkov A, Lemaine V, Tran NV. Management of perioperative microvascular thrombotic complications - The use of multiagent anticoagulation algorithm in 395 consecutive free flaps. J Plast Reconstr Aesthet Surg. 2015;68(9):1293–1303. doi:10.1016/j.bjps.2015.05.011
40. Swartz JE, Aarts MC, Swart KM, et al. The value of postoperative anticoagulants to improve flap survival in the free radial forearm flap: a systematic review and retrospective multicentre analysis. Clin Otolaryngol. 2015;40(6):600–609. doi:10.1111/coa.12425
41. Chien W, Varvares MA, Hadlock T, Cheney M, Deschler DG. Effects of aspirin and low-dose heparin in head and neck reconstruction using microvascular free flaps. Laryngoscope. 2005;115(6):973–976. doi:10.1097/01.MLG.0000163539.97485.F4
42. Zaman SR, Rawlins JM. Heparin induced thrombocytopaenia (HIT) as a cause of free flap failure in lower limb trauma. J Plast Reconstr Aesthet Surg. 2014;67(6):884–886. doi:10.1016/j.bjps.2013.12.027
43. Paul HK, Seth SL. Gustilo-Anderson Classification. Clin Orthop Relat Res. 2012;470:3270–3274. doi:10.1007/s11999-012-2376-6
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
