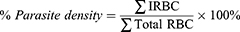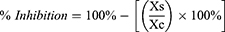Back to Journals » Infection and Drug Resistance » Volume 16
Fractions 14 and 36K of Metabolite Extract Streptomyces hygroscopicus subsp. Hygroscopicus Have Antimalarial Activities Against Plasmodium berghei in vitro
Authors Fitri LE , Endharti AT , Abidah HY , Khotimah ARH , Endrawati H
Received 10 December 2022
Accepted for publication 14 February 2023
Published 12 May 2023 Volume 2023:16 Pages 2973—2985
DOI https://doi.org/10.2147/IDR.S400538
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Loeki Enggar Fitri,1,2,* Agustina Tri Endharti,1 Hafshah Yasmina Abidah,3,4,* Alif Raudhah Husnul Khotimah,3,4,* Heni Endrawati1
1Department of Parasitology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia; 2Malaria Research Group, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia; 3Master Program in Biomedical Science, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia; 4Medical Doctor Profession Education, Faculty of Medical and Health Science, Maulana Malik Ibrahim State Islamic University, Malang, Indonesia
*These authors contributed equally to this work
Correspondence: Hafshah Yasmina Abidah, Master Program in Biomedical Science, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia, Tel +62 895 397 064 350, Fax +62 341 564755, Email [email protected]; [email protected]
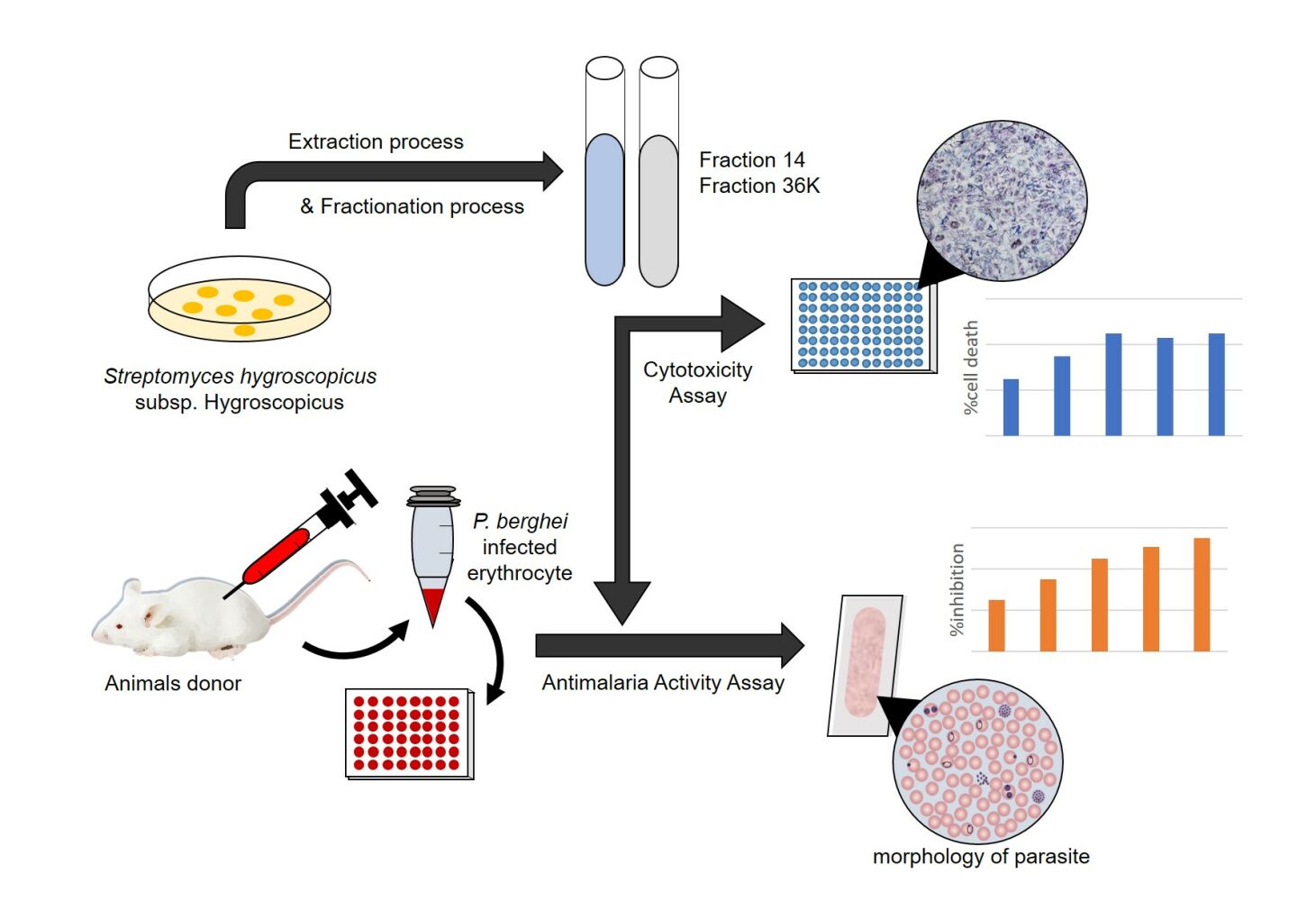
Purpose: The study was conducted to investigate the effectivity and the cytotoxicity of fractions 14 and 36K of metabolite extract of Streptomyces hygroscopicus subsp. Hygroscopicus as an antimalarial compounds against Plasmodium berghei in vitro.
Methods: Fractions 14 and 36K of metabolite extract of Streptomyces hygroscopicus subsp. Hygroscopicus produced by the fractionation process utilizing the Flash Column Chromatography (FCC) BUCHI Reveleris® PREP. Plasmodium berghei culture was used to assess the antimalarial activity of fractions 14 and 36K. Parasite densities and the ability of parasite growth were determined under microscopic. The cytotoxicity of the fractions was assessed using MTT assays on the MCF-7 cell line.
Results: Streptomyces hygroscopicus subsp. Hygroscopicus fractions 14 and 36K have antimalarial activity against Plasmodium berghei, with fraction 14 having the more potent activity. The percentage of Plasmodium berghei-infected erythrocytes was decreased as well as the increase of fraction concentration. Fraction 14 has the highest inhibition of parasite growth at a concentration of 156,25 μg/mL, with an inhibition percentage of 67.73% (R2 = 0.953, p = 0.000). IC50 of fractions 14 and 36K were found at 10.63 μg/mL and 135,91 μg/mL, respectively. The fractions caused morphological damage in almost all asexual stages of the parasite. Both fractions were not toxic against MCF-7, indicating that the fractions have a safe active metabolite.
Conclusion: Fractions 14 and 36K of metabolite extract Streptomyces hygroscopicus subsp. Hygroscopicus contains non-toxic compounds that could damage the morphology and inhibit the growth of Plasmodium berghei in vitro.
Keywords: antimalarial, cytotoxicity, fraction 14 and 36K, Streptomyces hygroscopicus, Plasmodium berghei
Graphical Abstract:
Introduction
Malaria is an endemic disease that affects many developing nations worldwide. The World Malaria Report estimates that there were 241 million cases of malaria worldwide in 2020, causing 672,000 deaths.1 Limitations in malaria detection, treatment, and prevention, especially during this pandemic of coronavirus disease (COVID-19), are a major factor in many deaths brought on by the disease.2–4 Early diagnosis and appropriate therapy with effective antimalarial drugs are two key components in controlling and eliminating malaria.5 Currently, artemisinin-based combination therapy (ACT) is the first-line therapy advised by the WHO for P. falciparum parasite infection.1 However, the development and spread of artemisinin-resistant malaria have become a major problem hindering malaria control.6 The high morbidity and mortality caused by malaria, the lack of drugs that are safely administered to children and pregnant women, the limitation of a licensed vaccine, together with widespread artemisinin partial resistance, highlight the real problems of the urgency to find new antimalarial drugs.1,7–9
Numerous studies on biological control agents have been conducted over the past several years, and Actinomycetes are recognized as the biggest source of bioactive metabolites with antibacterial, antifungal, anticancer, antioxidant, antiparasitic, and anti-inflammatory properties.10,11 Streptomyces is the largest genus of Actinomycetes that has been identified as a promising microbe for the development of novel antimalarial therapeutics for Plasmodium infection.12–15 A previous study found that Streptomyces hygroscopicus subsp. Hygroscopicus possesses antimalarial effects, in silico, in vitro, and in vivo.13,15,16 S. hygroscopicus subsp. Hygroscopicus crude extract can alter the morphology of P. falciparum 3D7 in vitro studies.13 This extract can also decrease the density of P. falciparum 3D7 DNA and the proportion of P. falciparum 3D7 infected erythrocytes.13 The results of in silico, in vitro, and in vivo studies showed that there was an inhibition of Plasmodium growth in the sexual and asexual phases through inhibition of purine base formation, changing the Plasmodium epigenome through histone lysine hyperacetylation, and blocking the protein turn over the system of Plasmodium via the UPS route.16
In previous study, we extracted and fractionated secondary metabolites from the fermentation of S. hygroscopicus, then their profiles were identified by thin-layer chromatography (TLC) which produced 47 spots in TLC fractionation I and 60 spots in TLC fractionation II.17 The effectiveness of some fractions as antimalarial agents has been profiled and investigated. The active fractions 15 and 16 from the ethyl acetate extract of S. hygroscopicus subsp. Hygroscopicus have been shown to have antimalarial activity by inhibiting more than 50% of the growth of P. falciparum 3D7 via a mitochondrial membrane enzyme, Plasmodium L-malate: quinone oxidoreductase (PfMQO), and non-toxic to human cells.18 Furthermore, fractions numbers 41 and 44 extracts contain tryptanthrin, a weak alkaloid group molecule with antimalarial properties that reduce Plasmodium berghei density.15
Due to the urgency in antimalarial drug development, screen and identify of the antimalarial and cytotoxicity effect of other fractions of S. hygroscopicus subsp. Hygroscopicus are needed, therefore in this study, we explored two other fractions, there are fractions 14 and 36K. In previous research, we have shown that both fractions contain monoterpene, triterpene, and steroid chemicals (fraction I), as well as coumarins, scopoletin, and alkaloids (fraction II), which may have antimalarial activity.17 Monoterpene and triterpene compounds are included in the class of terpenoid compounds. Terpenoid compounds and their derivatives have a structure that allows them to cross the lipid bilayer of the erythrocyte membrane and prevent parasite cells from the synthesis of proteins.19 Terpenes may also compete with several enzymes that use isoprenic compounds as substrates, preventing the formation of isoprenoid compounds, such as the isoprenic chain of the benzoquinone ring of ubiquinones in the schizont stage.20,21
Previous TLC results found alkaloid compounds in the fraction 36K,17 this is in accordance with the discovery of tryptanthrin, a weak alkaloid compound in another fraction of S. hygroscopicus subsp. Hygroscopicus extract.15 Tryptanthrin blocks the binding of ligands and proteins during P. falciparum invasion because it has a high affinity for the target P. falciparum Aminopeptidase M1 (PfA-M1), Falcipain 2 Protease, and P. falciparum Chloroquine Resistance Transporter (PfCRT) proteins.15 Tryptanthrin’s antimalarial activity was based on its ability to disrupt the Plasmodium glycolysis enzyme pathway, impede the heme detoxification, and cause hematin crytallization through interactions with heme and hematin.22 Tryptanthrin also inhibits the maturation of adult-stage gametocytes.22 It is crucial for the transfer of exflagellation microgametocytes from humans to mosquitoes.23
The most popular approach for predicting the hazardous effects of novel drug is in vitro cytotoxicity testing.24 Cytotoxicity tests can be carried out on many different types of cells, including cancer cells. Cancer cells are unique cells because they have regressed to a stage that is much simpler, more primitive, in contrast to the normal parent, and divide constantly.25 Since most cancer cells are actively dividing as a result, they grow in cell culture more quickly than healthy cells do.26 Other studies have been using breast cancer cell MCF-7 in antimalarial toxicity test.27–32 In addition, cancer cell lines and normal cell lines exhibited relatively uniform toxicity results.24 So, the purpose of this study is to evaluate the antimalarial effectiveness of fractions 14 and 36K against Plasmodium berghei and their toxicity on MCF-7 cells as a novel prospective antimalarial drug candidate in vitro.
Materials and Methods
Research Design
This study uses a true experimental laboratory with a posttest-only controlled group design using in vitro method. This study was conducted to investigate the effectivity of fractions 14 and 36K of metabolite extract S. hygroscopicus subsp. Hygroscopicus as antimalarial against P. berghei and its cytotoxicity in vitro. In the antimalarial effectivity test, the fractions 14 and 36K of S. hygroscopicus subsp. Hygroscopicus, with five concentrations of 0.25, 1.25, 6.25, 31.25, 156.25 μg/mL, were administrated to P. berghei culture and then the densities and the growth of P. berghei were observed using a light microscope. For cytotoxicity test of the fractions 14 and 36K of S. hygroscopicus subsp. Hygroscopicus, we used MTT assay for five concentrations of 0.25, 2.5, 25, 250, 2500 μg/mL that were exposed to MCF-7. This study was conducted in accordance with the Declaration of Helsinki and has been approved by the Ethics Committee of the Faculty of Medicine Universitas Brawijaya with Ethic No. 13/EC/KEPK/01/2022.
Preparation of Streptomyces hygroscopicus subsp. Hygroscopicus
Streptomyces hygroscopicus subsp. Hygroscopicus was collected from LIPI Microbial Collection, Cibinong, Indonesia. Then, S. hygroscopicus subsp. Hygroscopicus was subcultured in the Laboratory of Parasitology, Faculty of Medicine, Universitas Brawijaya using analytic International Streptomyces Project 4 (ISP4) culture media. One liter of medium ISP4 was composed of 10 g soluble starch, 1 g MgSO4.7H2O, 1g K2HPO4, 1g NaCl, 2 g (NH4)2SO4, 2 g CaCO3, 20 g agar, 1 g trace salt solution (0.1 g Fe2SO4.7H2O, ZnSO4.7H2O, MnCl2.4H2O 0.1 g, distilled water 1 mL), and one liter distilled water with pH 7.0 to 7.4.33 The solution was autoclaved for 15 min at 121°C, and then S. hygroscopicus subsp. Hygroscopicus isolates were inoculated and fermented on the medium at 28°C shaking incubator.34 The bacteria were characterized macroscopically for its colony morphology and microscopically for its specific morphology based on Gram staining.
Fermentation and Extraction of Streptomyces hygroscopicus subsp. Hygroscopicus
In our previous study,17,18 S. hygroscopicus subsp. Hygroscopicus inoculation was carried out using 1000 mL ISP4 liquid media with a pH 7.0–7.4 in aseptic conditions. Bacterial colonies were scraped and homogenized in ISP4 medium, in a ratio between media and inoculum 10:1 (v/v). The media was closed with cotton and aluminum foil to prevent the presence of air in the erlenmeyer, and then incubated in a shaking incubator at 28°C and 150 rpm for 5 days. After 5 days, fermented bacteria were centrifuged at 3000 rpm for 10 minutes to separate the cells from the debris and the supernatant was taken for the extraction process.34 The filtrate was mixed with ethyl acetate in a 1:1 comparison (v/v), followed by 1 hour shaking. Then, the filtrate was left for 1–2 hours in a separatory funnel to form two layers (solvent phase and water phase). The bacterial metabolite transported to the solvent in the ethyl acetate phase. The solvent phase containing secondary metabolites of S. hygroscopicus subsp. Hygroscopicus bacteria were then separated from the water phase and then the solvent phase (ethyl acetate) was evaporated using a rotary evaporator at a temperature of 40–50°C until the liquid turns into a paste.18
Fractionation of Streptomyces hygroscopicus subsp. Hygroscopicus Metabolite Extract
In our previous study,17 fractionation of metabolite extracts of S. hygroscopicus subsp. Hygroscopicus was conducted using the BUCHI Reveleris® PREP Purification System flash column with silica gel 60 eluted by n-hexane and ethyl acetate in a ratio of 1:1 (v/v). The process used UV detectors with 254, 365, and 366 nm wavelengths as well as an evaporative light scattering detector (ELSD) to acquire all compounds detected at this wavelength. A running process set in gradient mode at a flow rate of 5 mL per minute, then the collected samples were evaporated. All fractions were stored in 4°C for maintaining its stability.
Preparation of the Donor Animals
For animal donors, we used male albino Balb/c strain mice, 6–8 weeks of age with an average body weight of 20–25 grams, which was obtained from the Laboratory of Parasitology, Faculty of Medicine, Universitas Brawijaya. The mice were kept in 30×30 cm cages, and fed with sufficient quantities of a standard meal once a day per cage to avoid food competition among mice. Drinking boiled water was changed daily. For the welfare of laboratory animals, we follow Government Regulation of the Republic of Indonesia No.95 year 2012 concerning Veterinary Public Health and Animal Welfare.
P. berghei was obtained from Laboratory of Parasitology, Faculty of Medicine, Universitas Brawijaya. P. berghei-infected erythrocyte which was reserved in a liquid nitrogen tank at −135°C were thawed and centrifugated at 2000 rpm for 5 minutes. The pellet was washed twice with Roswell Park Memorial Institute 1640 (RPMI 1640) medium (Gibco, USA) supplemented with 20% FBS and 0.5% gelatin, then diluted as needed for inoculation to mice. All procedures related with isolation of P. berghei were conducted in an aseptic laminar airflow. Each mouse was injected intra-peritoneal with 200 μL P. berghei-infected erythrocyte. Observation of the degree of parasitemia in mice was carried out every 2 days using blood smears obtained from the tails of mice, with the assumption that there was a two-fold increase in parasitemia on the blood smear.35 After the degree of parasitemia reaches at least 5%, the infected erythrocytes is taken entirely from the blood through the heart of the mouse.36
Anti-Malaria Activity Assay
Fractions 14 and 36K of metabolite extract S. hygroscopicus subsp. Hygroscopicus, divided into concentrations of 0.25; 1.25; 6.25; 31.25; 156.25 μg/mL and were added into each well in 24-microplate containing 25 μL P. berghei infected-erythrocytes in 1 mL complete medium. The positive control group was exposed to artemisinin 0.01 mM and the negative control group was 1% DMSO. The culture was incubated for 48 hours at 32°C with 5% CO2. Then, the culture was taken and centrifuged. Pellets from the culture were used to make blood smears. Thin smears of blood from the mouse tails were made on the object glass, then fixed with methanol. The smears were stained with 20% Giemsa and its buffer in a comparison of 1:9, for 30 minutes, then washed with water and dried. The densities of parasite was observed with a light microscope at 1000x magnification with immersion oil. Parasite density was calculated based on the number of infected red blood cells (IRBC) for an average of 5000 erythrocytes. The percentage of parasite density (%) was calculated by the formula below:37
While inhibition of parasite growth is expressed as a percentage, and calculated using the formula below.37
Xs is the parasite density of sample and Xc is the parasite density of negative control. The inhibitory concentration 50 (IC50) was evaluated using probit analysis SPSS.37
Human Breast Cancer Cells (MCF-7) Cultured
Human breast cancer cells (MCF-7) is a commercial cell line that were obtained from the American Type Culture Collection (HTB-22-ATCC) (Bioresource Center, Manassas, VA, USA). MCF-7 cells were cultured in flasks containing RPMI 1640 medium (Gibco, USA) supplemented with 10% fetal bovine serum (FBS), 10 μL Amphotericin B, and 10 μL Penicillin-Streptomycin. The cells were incubated in a 5% CO2 incubator at 37°C and the media was changed every two days. MCF-7 cells were observed under an inverted microscope. When the cells reached a high density about 80%, the cells could experiment by passing through their logarithmic growth phase.37
Cytotoxic Assay
The cytotoxic effect of fractions 14 and 36K of metabolite extract S. hygroscopicus subsp. Hygroscopicus on MCF-7 was measured using MTT assay. Ten thousand MCF-7 cells, calculated by hemocytometer, were added into each well in a 96-well microtitre plate and were cultured in RPMI medium supplemented with 10% fetal bovine serum (FBS) and 1% antibiotic (penicillin-G and streptomycin), then incubated at 37oC with 5% CO2 for 24h. Fraction 14 and 36K S. hygroscopicus subsp. Hygroscopicus cytotoxicity measurement were performed in vitro using five different concentrations (0.25; 2.5; 25; 250; 2500 μg/mL) that were prepared by serial dilution. 10 μL of fractions solution was added to each well. A negative control experiment was conducted using only MCF-7 cells and a medium control experiment was also set up. Triplicate experiments were performed. The microplate was incubated in a 5% CO2 humidified incubator at 37°C for 48 hours. One hundred microliters of MTT solution was added to each well then incubated for 4 hours. After 4 hours, the medium was decanted and dissolved in 100 μL of DMSO, before incubating the plate for thirty minutes. The MTT assay measures the purple-colored formazan resulting from the reduction of the yellow tetrazolium salt by cells with metabolically active. The absorbance of cytotoxicity was read using a microplate reader (Zenix-320) at the wavelength of 630 nm. The percentage cytotoxicity was calculated using the formula: (OD of control – OD of sample/OD of control) x 100%.38
Data Analysis
Morphology of parasite, after exposed to the fractions, was analyzed by comparing the treatment and control groups, descriptively. The inhibition of parasite growth and absorbance of cytotoxicity were analyzed with SPSS 25, using a one-way ANOVA test, posthoc Tukey’s test, Pearson correlation test, and linear regression test with p<0.05.
Result
Fractionation of Streptomyces hygroscopicus subsp. Hygroscopicus Metabolite Extract
According to the fractionation carried out in an earlier study,17,18 there were a total of 47 fractions from fractionation I (Sepacore® Flash Chromatography) and 60 fractions from fractionation II (Reveleris® PREP Purification System Chromatography), and in each tube, a different weight of fraction was produced during fractionation. The fractionation was done using column chromatography with hexane and ethyl acetate solvents to simplify the extract samples containing various components. Column chromatography with hexane and ethyl acetate solvents was used to fractionate the extract samples containing various components.
In our previous study,17 fractionation I showed spots on fractions 14 and 36K when exposed to UV light at 254 and 366 nm with retardation factors of 0.24 and 0.41, respectively. These spots, which showed gray or purple fluorescence (UV 254 nm) and blue fluorescence (UV 366 nm) in fraction 14 and dark gray spots (UV light 254 nm) in fraction 36K, were considered to be monoterpenes, triterpenes, or steroids. On fractionation II, due to black spots on UV 254 nm and blue stain when irradiated with UV 366 nm with retardation factor values of 0.24, it showed that fractions 36K have derivatives compound of coumarin, scopoletin, or alkaloids.
Parasite Morphology
Evaluation of the effects on the morphology of the intra-erythrocyte stages of the parasite was carried out by microscopic visualization of blood smears. Parasite morphology was observed after 48 hours of incubation as shown in Figure 1. At 0.25 μg/mL fraction 14 concentration, parasites showed irregular amoeboid-form (A1) and immature schizonts as the dominant stage (A2), whereas at the same dose of fraction 36K showed immature schizont with pigment (A3) and ruptured schizont with many merozoites released (A4) in almost all of the field of view. At 1.25 μg/mL fraction 14 concentration, parasites displayed mature trophozoite with irregular cytoplasm (B1, B2), similar to fraction 36K, which showed mature trophozoite with irregular cytoplasm (B3) and dominating matured schizont with thick cytoplasm contained a large number of merozoites (B4). At 6.25 μg/mL fraction 14 concentration, trophozoites with irregular and damaged cytoplasm (C1) and trophozoites in crisis form (C2) were seen. At fraction 36K, parasites displayed trophozoite with irregular shape and damage of the cytoplasm (C3) and schizont with shrinked cytoplasm (C4). At 31.25 μg/mL fraction 14 concentration, parasites showed trophozoite with big vacuole, the nucleus was pulled over to the edge of parasite cytoplasm, and begin to damage (D1), and schizont with shrinked cytoplasm (D2), while at fraction 36K showed young schizont failed to grow with enlargement of the erythrocyte, the presence of vacuole, and damage of the cytoplasm (D3) and trophozoite in crisis form (D4). At 156,25 μg/mL fraction 14 concentration, trophozoites with irregular and damaged cytoplasm (E1) and crisis form (E2) were seen. At similar dose of fraction 36K, parasite revealed ruptured schizont with a few merozoites and cytoplasm damage (E3), and crisis forms (E4) were found frequently. The negative control group showed growing schizont (F1) and mature trophozoite (F2). Many crisis forms of parasites were identified under positive control group (F3, 4).
Parasite Inhibition
The inhibitory effect of fraction intervention is measured using a light microscope to determine the percentage of erythrocytes infected with parasites. Figure 2A displays the inhibition effect of fraction 14 and 36K of S. hygroscopicus subsp. Hygroscopicus in significant (p<0.05) of parasite growth. In comparison to the negative control, each treatment group had a significant reduction in parasite-infected cells. The negative control group used in this study did not receive any intervention, had a parasite density percentage value of 4.75±0.14 and had zero percent parasite inhibition. The positive control group used Artemisinin 0.01 mM with a parasite density percentage value of 2.35±0.19 and a parasite growth inhibition percentage value of 50.61±4.10.
Fraction 14 showed the largest percentage of inhibition at concentration 156.25 µg/mL with a parasite inhibition percentage value of 67.73±3.80. This was followed by fraction 31.25 µg/mL, which showed a parasite inhibition percentage value of 63.20 ± 2.74. Both concentrations are higher than the positive control percentage of inhibition. Following treatment, a one-way ANOVA test of the percentage of parasite growth inhibition revealed a significant difference among the intervention groups with a value of p = 0.000 (p<0.05). The relationship between concentrations of fraction and the percentage of parasite growth inhibition was significant with P-value = 0.000 and r = 0.976 (Pearson correlation test), indicated a positive correlation; R2 = 0.953 from a linear regression analysis revealed that the administration of treatment was responsible for 95.3% of the parasite growth inhibition.
Fraction 36K also showed the greatest percentage of inhibition at a concentration of 156.25 µg/mL, but it was still lower than that of positive control. A one-way ANOVA test of the percentage of parasite growth inhibition revealed a significant difference among the intervention groups with a value of p = 0.000 (p<0.05). Pearson correlation test showed P-value = 0.000 and r = 0.914 indicated a significant positive relationship between fraction concentrations and percentage of parasite growth inhibition. R2 = 0.835 from a linear regression analysis revealed that the administration of treatment was responsible for 83.5% of the parasite growth inhibition.
Inhibition Concentration 50 (IC50) of Fraction 14 and 36K
In this study, the parasite growth inhibition probit analysis was used to calculate the IC50, followed by a linear regression analysis using SPSS. The probit analysis of fraction 14 produced the equation y = 0.486x - 0.498 with R2 = 0.971. The IC50 for fraction 14 is calculated from this equation to be 10.63 µg/mL. While y = 0.570x - 1.217 and R2 = 0.743 were used to compute the I IC50 of fraction 36K. The IC50 for fraction 36K was determined by this equation to be at a concentration of 135,913 µg/mL (Figure 2B).
The Cytotoxicity of Fraction 14 and 36K
The cytotoxicity test was performed on MCF-7 cell lines with an MTT assay. Figure 3 depicts the cytotoxicity degree effect of S. hygroscopicus subsp. Hygroscopicus extract fractions 14 and 36K. All concentrations of fractions 14 and 36K had toxicity values that were lower than 50%. Because fractions 14 and 36K with the highest concentration of 2500 µg/mL have percentages of cell death 18.21% and 23.28% that did not reach 50% of cell death, the fraction LC50 cannot be determined in this study. Then, a result of p = 0.000 (p<0.05) for fraction 14 and p = 0.000 (p<0.05) for fraction 36K, the one-way ANOVA test of cytotoxicity of the degree of cell death revealed a significant difference between the intervention groups.
Discussion
The emergence of resistance to antimalarial drugs has become an urgent need to develop potent new antimalarial compounds. Streptomyces sp. is Gram-positive bacteria that have been known for its wide activity as an antimicrobial, antifungal, anticancer, and antiparasite, such as malaria.39 Therefore, this study was conducted to evaluate the effectivity of fractions 14 and 36K of metabolite extract S. hygroscopicus subsp. Hygroscopicus as antimalarial and its cytotoxicity. Fractionation was aimed to simplify the extract samples containing various components. From the previous study,17 49 samples of fractions were collected. Fraction numbers 14 and 36K were selected because previous studies have shown that the fraction contains secondary metabolite which has antimalarial activities.17 P berghei was used in this study since it is an appropriate parasite that is most commonly used because of its higher accessibility.40 P. berghei commonly was used in assessing the antiplasmodial activity of the new agent because all life cycle stages of the parasite are seen on smears due to the nonadherence of the species with endothelial cells.41 Studying in vitro is more rapid and accurate for observing parasite’s fragility due to antimalarial agents, without any disruption from the host factor.42
The antimalarial effectiveness of fractions 14 and 36K of metabolite extract S. hygroscopicus subsp. Hygroscopicus against P. berghei in vitro was investigated in this research. Both fractions disrupted the development of the parasite stage. This suggests that the antimalarial activity of the fraction is the result of the additive or synergistic interaction of various components in the fractions. This result was similar to a previous study that the metabolite extract of S. hygroscopicus can damage the parasite morphology. Schizonts and trophozoites failed to develop and appear morphologically damaged with loss of cytoplasmic content and pycnotic nuclei that call crisis form. Crisis forms were marked by the disappearance of the cytoplasm, the nucleus was pulled over to the edge of the parasite cytoplasm, and the chromatin was thick, compact, and dark.13,36
Parasite inhibition is the inhibitory effect caused by the treatment given. The Inhibitory Concentration 50 (IC50) is a concentration of S. hygroscopicus subsp. Hygroscopicus which is capable of causing 50% parasite inhibition. Based on the result of this study, fractions 14 and 36K of metabolite extract S. hygroscopicus subsp. Hygroscopicus can inhibit P. berghei in vitro. These results indicated that the higher the fraction concentration, the higher the percentage of inhibition of parasite will be obtained. Fraction 14 showed greater potency in inhibiting parasite than fraction 36, with the largest inhibition until 67% at concentration 156,25 μg/mL. This study showed that S. hygroscopicus have antimalarial properties against Plasmodium sp. A previous study about methanol extracts of the secondary metabolite of Streptomyces sp. against P. falciparum showed that a concentration of 100 µg/mL could suppress 0.66% average growth of P. falciparum with 95.20% average inhibition value.43 Another study showed that the highest effect percentage inhibition of secondary metabolite extract of S. hygroscopicus against P. falciparum-infected erythrocytes was 2.6 mg/mL and 13 mg/mL with 69.8% and 67.3% inhibition, respectively.13 Our previous study stated that other fractions namely fractions 15 and 16 of S. hygroscopicus metabolite showed inhibition of parasite growth more than 50% using lactate dehydrogenase (PfLDH) enzyme assay with a significant difference, against P. falciparum culture.18
In this study, the inhibitory concentration 50 (IC50) was obtained using a probit analysis of log concentrations converted from inhibition percentage in SPSS. IC50 value means the ability of the fractions 14 and 36K of metabolite extract of S. hygroscopicus subsp. Hygroscopicus in inhibiting the growth of P. berghei in erythrocytes in vitro by 50%. The greater the effectiveness of the inhibition on the growth of P. berghei, the smaller the IC50 value. The results of IC50 analysis of fraction 14 and 36K against P. berghei were 10.63 μg/mL and 135,91 μg/mL, respectively. Previous data classified antiplasmodial activity as high (IC50<5 μg/mL), promising (5< IC50<15 μg/mL), moderate (15< IC50<50 μg/mL), and inactive (IC50>50 μg/mL).44,45 Fraction 14 in this study showed promising activity, indicating that it contains the main active compound, while fraction 36K showed inactive activity. A previous study showed that the IC50 value of crude extract of Streptomyces sp. AB8 against P. falciparum 3D7 in vitro was 17.56 μg/mL.46 Another study stated that parasite inhibition of S. hygroscopicus subsp. Hygroscopicus strain i18 towards P. falciparum in vitro as measured using probit analysis was 11.07 μg/mL.37
For the development of novel drugs, it is crucial to test the cytotoxicity of new compounds. Preclinical cytotoxicity experiments on numerous biological systems demonstrate the hazardous effects of the items under study at species-, organ-, and dose-specific levels.47 In this study, the cytotoxicity test was conducted on MCF-7 cell line, which is one of the breast cancer cells. MCF-7 cell line has been used for examining the cytotoxic effect of antimalarial drugs in several previous studies.27–32 Cancer cells are more susceptible to toxic drugs than the majority of normal cells because they actively divide at considerably greater rates, and this is related to mitotic spindles and DNA replication in cancer cells.25,48 Other study showed relatively uniform toxicity results of cell lines and normal cell lines.24 As a result, this study attempted to carry out a cytotoxicity test experiment on MCF-7 cells, which have excellent qualities and are adherent cell types with the monolayer culture method, making them easier to examine than normal cells.49
The cytotoxicity of the MCF-7 cell line was examined using the MTT assay at five different concentrations with exponential increases. The MTT assay is a colorimetric testing system that measures the amount of reduction of the yellow MTT salt to violet-blue formazan by succinate tetrazolium reductase, which is included in the respiration chain in the mitochondria of living cells. The results are read by a microplate reader as the form of an optical density unit.50 According to Clarkson’s toxicity index,51 LC50 < 1000 µg/mL is toxic, while LC50 > 1000 µg/mL is non-toxic. Fractions 14 and 36K showed an LC50 value of < 1000 µg/mL. This suggests that both fractions 14 and 36K have a safe dosage range and are not toxic to the MCF-7 cell. This result was similar with previous study,18 stated that fraction 15 and 16 of S. hygroscopicus subsp. Hygroscopicus has been shown to have no hazardous effects on the colorectal cancer DLD-1 cell line.18
In earlier research, it was known that fractions 14 and 36K both have many compounds that may have an antimalarial activity that make a change in morphology and decrease the activity of Plasmodium growth. The compounds known in fractions 14 and 36K are monoterpene, triterpene, and steroid chemicals, as well as coumarins, scopoletin, and alkaloids.17 Terpenoid chemicals and their derivatives have a structure that can pass through the lipid bilayer of the erythrocyte membrane and inhibit protein synthesis in parasite cells.19 Erythrocyte membrane shape changes towards stomatocytes or echinocytes depending on their hydrogen bonding capabilities; both types of erythrocyte changes can invade and inhibit Plasmodium falciparum growth.19 Terpenes may prevent isoprenyl diphosphate synthases from condensing molecules of IPP and other isoprenic substrates into isoprene chains, preventing the parasite from producing polyisoprenoid biomolecules.21 Terpenes may also compete with a number of enzymes that utilize isoprenic compounds as substrates, preventing the production of isoprenoid compounds, such as the isoprenic chain of the benzoquinone ring of ubiquinones in the schizont stage.20,21
Another substance discovered in S. hygroscopicus metabolite extracts fraction 36K called coumarin is widely recognized for its antiplasmodial effects.52–56 According to other studies, daphetin or 7,8-dihydroxycoumarin as one of the coumarin compound can inhibit P. falciparum activity in vitro by preventing DNA synthesis.57 The reduction in DNA synthesis inhibits parasite replication, which causes the parasites to die from a lack of proteins that are essential for the parasite’s metabolic functions.52,57 Daphnetin revealed a suppression of DNA synthesis at the trophozoite stage during P. falciparum’s distinct development phases.57 One of the coumarin derivatives, scopoletin, is also present in fraction 36K.17 However, scopoletin (7-hydroxy,6-methoxycoumarin) does not appear to have any antiplasmodial activity in vitro due to the absence of a hydroxyl group at position C-8.58
Other compound in S. hygroscopicus metabolite extracts is alkaloid. On previous research, a weak alkaloid namely tryptanthrin has a strong affinity for the target proteins Plasmodium falciparum Aminopeptidase M1 (PfA-M1), Falcipain 2 Protease, and P. falciparum Chloroquine Resistance Transporter (PfCRT) has been demonstrated to be present in fractions 41 and 44 in both in vivo and in silico studies.15
Conclusion
In this study, it can be concluded that fractions 14 and 36K of metabolite extract of S. hygroscopicus subsp. Hygroscopicus were able to inhibit more than 50% growth of P. berghei in vitro and cause morphological damage to P. berghei. It was also proven that the two fractions were non-toxic to human cells in the cytotoxicity test. Although only fraction 14 showed promising activity as its antiplasmodial capacity classified as high (IC50<5 μg/mL).
Acknowledgments
The authors would like to thank to all members of the Malaria Research Group Faculty of Medicine Universitas Brawijaya especially to dr Nabila Erina Erwan, S. Ked., M. Biomed and dr Ajeng Maharani Putri, S. Ked., M. Biomed. The research funding was supported by Faculty of Medicine Universitas Brawijaya [grant number 2371/UN10.F08/PN/2022].
Disclosure
The authors report no conflicts of interest in this work.
References
1. WHO. World Malaria Report 2021. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
2. Orish VN, Akake K, Lokpo ISY, et al. Evaluating the impact of COVID-19 pandemic on complicated malaria admissions and outcomes in the paediatric ho teaching hospital of the Volta Region of Ghana. PLOS Global Public Health. 2022;2(9):e0000509. doi:10.1371/journal.pgph.0000509
3. Weiss DJ, Bertozzi-Villa A, Rumisha SF, et al. Indirect effects of the COVID-19 pandemic on malaria intervention coverage, morbidity, and mortality in Africa: a geospatial modelling analysis. Lancet Infect Dis. 2021;21(1):59. doi:10.1016/S1473-3099(20)30700-3
4. Zawawi A, Alghanmi M, Alsaady I, Gattan H, Zakai H, Couper K. The impact of COVID-19 pandemic on malaria elimination. Parasite Epidemiol Control. 2020;11:e00187. doi:10.1016/j.parepi.2020.e00187
5. Landier J, Parker DM, Thu AM, et al. The role of early detection and treatment in malaria elimination. Malar J. 2016;15(1):1–8. doi:10.1186/s12936-016-1399-y
6. Suresh N, Haldar K. Mechanisms of artemisinin resistance in Plasmodium falciparum malaria. Curr Opin Pharmacol. 2018;42:46. doi:10.1016/j.coph.2018.06.003
7. Rogerson SJ. Management of malaria in pregnancy. Indian J Med Res. 2017;146(3):328. doi:10.4103/ijmr.IJMR_1304_17
8. Ouattara A, Laurens MB. Vaccines against malaria. Clin Infect Dis. 2015;60(6):930. doi:10.1093/cid/ciu954
9. Thu AM, Phyo AP, Landier J, Parker DM, Nosten FH. Combating multidrug-resistant Plasmodium falciparum malaria. Febs J. 2017;284(16):2569. doi:10.1111/febs.14127
10. Selim MSM, Abdelhamid SA, Mohamed SS. Secondary metabolites and biodiversity of Actinomycetes. J Genet Eng Biotechnol. 2021;19(1). doi:10.1186/s43141-021-00156-9
11. Chen J, Xu L, Zhou Y, Han B. Natural products from Actinomycetes associated with marine organisms. Mar Drugs. 2021;19(11):629. doi:10.3390/md19110629
12. Ahmad SJ, Abdul Rahim MBH, Baharum SN, Baba MS, Zin NM. Discovery of antimalarial drugs from Streptomycetes metabolites using a metabolomic approach. J Trop Med. 2017;2017. doi:10.1155/2017/2189814
13. Fitri LE, Alkarimah A, Cahyono AW. Lady wahyudha ngatiril, Endharti agustina tri, Nugraha RYB. Effect of metabolite extract of Streptomyces hygroscopicus subsp. hygroscopicus on Plasmodium falciparum 3D7 in vitro. Iran J Parasitol. 2019;14(3):444–452.
14. Nugraha RYB, Faratisha IFD, Mardhiyyah K, et al. Antimalarial properties of isoquinoline derivative from Streptomyces hygroscopicus subsp. Hygroscopicus: an in silico approach. Biomed Res Int. 2020;2020:1–15. doi:10.1155/2020/6135696
15. Fitri LE, Putri AM, Erwan NE, Putri FF, Nugraha RYB. Antimalarial properties of Streptomyces hygroscopicus subsp hygroscopicus secondary metabolite active fractions: in silico and in vivo analysis. Int J Pharm Res. 2021;13(1):2553–2567.
16. Violita M, Widyastuti A, Astami CP, Yudhinata R, Nugraha B, Khasanah U. Exploration of Streptomyces hygroscopicus secondary metabolite compound as a development of antimalarial drug candidate. Phcog Commn. 2022;12(1):2–6. doi:10.5530/pc.2022.1.2
17. Ariel DG, Winarsih S, Putri FF, et al. Optimization of combination of N-Hexane solution and ethyl acetate on secondary metabolite compounds profile of Streptomyces hygroscopicus. J Kedokt Brawijaya. 2021;31(3):186–192. doi:10.21776/ub.jkb.2021.031.03.11
18. Cahyono AW, Fitri LE, Yudhinata R, Nugraha B, Aulia R. Non-toxic fractions of Streptomyces hygroscopicus Subsp. hygroscopicus metabolite suppressed the growth of Plasmodium falciparum in Vitro Possibly through L-malate: quinone Oxidoreductase (Pf MQO) mitochondrial enzyme inhibition. Sys Rev Pharm. 2020;11(10):524–531.
19. Ziegler HL, Franzyk H, Sairafianpour M, et al. Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: structure-activity relationships for betulinic acid analogues. Bioorg Med Chem. 2004;12(1):119–127. doi:10.1016/j.bmc.2003.10.010
20. Goulart HR, Kimura EA, Peres VJ, Couto AS, Duarte FAA, Katzin AM. Terpenes arrest parasite development and inhibit biosynthesis of isoprenoids in Plasmodium falciparum. Antimicrob Agents Chemother. 2004;48(7):2502. doi:10.1128/AAC.48.7.2502-2509.2004
21. Gabriel HB, Sussmann RA, Kimura EA, et al. Terpenes as potential antimalarial drugs. In: Terpenes and Terpenoids. IntechOpen;; 2018.
22. Onambele LA, Riepl H, Fischer R, Pradel G, Prokop A, Aminake MN. Synthesis and evaluation of the antiplasmodial activity of tryptanthrin derivatives. Int J Parasitol Drugs Drug Resist. 2015;5(2):48. doi:10.1016/j.ijpddr.2015.03.002
23. Delves MJ, Ruecker A, Straschil U, et al. Male and female Plasmodium falciparum mature gametocytes show different responses to antimalarial drugs. Antimicrob Agents Chemother. 2013;57(7):3268. doi:10.1128/AAC.00325-13
24. Espíndola MR, Varotti FD, Aguiar AC, Andrade SN, Rocha EM. In vitro assessment for cytotoxicity screening of new antimalarial candidates. Braz J Pharm Sci. 2022;58. doi:10.1590/s2175-97902022e18308
25. Ekwall B, Silano V, Zucco F. Chapter 7 - toxicity tests with mammalian cell cultures. In: Short-Term Toxicity Tests for Non-Genotoxic Effects. John Wiley \& Sons Ltd; 1990:75–98.
26. Cooper GM. The development and causes of cancer. In: The Cell: A Molecular Approach. Sinauer Associates; 2000.
27. Suberu JO, Romero-Canelón I, Sullivan N, Lapkin AA, Barker GC. Comparative cytotoxicity of artemisinin and cisplatin and their interactions with chlorogenic acids in MCF7 breast cancer cells. ChemMedChem. 2014;9(12):2791–2797. doi:10.1002/cmdc.201402285
28. Duarte D, Vale N. New trends for antimalarial drugs: synergism between antineoplastics and antimalarials on breast cancer cells. Biomolecules. 2020;10(12):1–20. doi:10.3390/biom10121623
29. Zin NNINM, Mohamad MN, Roslan K, et al. In vitro antimalarial and toxicological activities of Quercus infectoria (Olivier) gall extracts. Malays J Med Sci. 2020;27(4):36–50. doi:10.21315/mjms2020.27.4.4
30. Salempa P, Muharram M, Jumadi O, Pratiwi DE, Azis M, Amaliah N. Cytotoxicity test of methanol extract of belakang susu (Scindapsus pictus Hassk.) against MCF-7 breast cancer cells. Indones J Fundam Sci. 2022;8(1):38–42.
31. Goodarzi S, Nateghpour M, Asgharian P, et al. Antimalarial and cytotoxic activities of roots and fruits fractions of Astrodaucus persicus extract. Iran J Basic Med Sci. 2017;20(12):1318–1323. doi:10.22038/IJBMS.2017.9554
32. Sudha S, Masilamani SM. Characterization of cytotoxic compound from marine sediment derived actinomycete Streptomyces avidinii strain SU4. Asian Pac J Trop Biomed. 2012;2(10):770. doi:10.1016/S2221-1691(12)60227-5
33. Shepherd MD, Kharel MK, Bosserman MA, Rohr J. Laboratory maintenance of Streptomyces species. Curr Protoc Microbiol. 2010;Chapter 10:
34. Sharma H, Parihar L. Antifungal activity of extracts obtained from Actinomycetes. J Yeast Fungal Res. 2010;1(December):197–200.
35. Li Q, Xie LH, Zhang J, Pybus BS. Identification and assessment of Plasmodium berghei merozoites and cell cycle by flow cytometry. Mil Med. 2021;186(Supplement_1):108–115. doi:10.1093/milmed/usaa272
36. Nugraha RYB, Alkarimah A. Metabolite extract of Streptomyces hygroscopicus hygroscopicus inhibit the growth of Plasmodium berghei through inhibition of ubiquitin – proteasome system. Trop Biomed. 2013;30(2):291–300.
37. Setyaningrum E, Arifiyanto A, Putri MH, Aeny T, Nukmal N. In vitro test for inhibition of Plasmodium falciparum 3D7 parasites using Streptomyces hygroscopicus subsp. hygroscopicus strain i18, isolated from a pineapple farm in lampung. J Pure Appl Microbiol. 2021;15(2):891–896. doi:10.22207/JPAM.15.2.45
38. Dadzie I, Avorgbedo SA, Appiah-opong R, Cudjoe O. Cytotoxic and antioxidant effects of antimalarial herbal mixtures. Int J Microbiol. 2020;2020:8–10. doi:10.1155/2020/8645691
39. Lima SMA, Melo JGS, Militão GCG, et al. Characterization of the biochemical, physiological, and medicinal properties of Streptomyces hygroscopicus ACTMS-9H isolated from the Amazon (Brazil). Appl Microbiol Biotechnol. 2017;101(2):711–723. doi:10.1007/s00253-016-7886-9
40. Kifle ZD, Adinew GM, Mengistie MG, et al. Evaluation of antimalarial activity of methanolic root extract of Myrica salicifolia A Rich (Myricaceae) against Plasmodium berghei – infected mice. J Evid Based Integr Med. 2020;25:1–12.
41. Nureye D, Sano M, Fekadu M, Duguma T, Tekalign E. Antiplasmodial activity of the crude extract and solvent fractions of stem barks of Gardenia ternifolia in Plasmodium berghei -infected mice. Evid Based Complement Alternat Med. 2021;2021:1–16. doi:10.1155/2021/9625169
42. Maji AK. Drug susceptibility testing methods of antimalarial agents. Trop Parasitol. 2018;8(2):70. doi:10.4103/2229-5070.248695
43. Sandy S, Harisma I, Sasto S. Inhibition of secondary metabolite extract of Streptomyces sp. on Plasmodium falciparum in vitro: a Study of soil sediment of papua’s hamadi mangrove forest. Indones J Med Health J. 2020;11:34–43.
44. Ogbeide OK, Dickson VO, Jebba RD, et al. Antiplasmodial and acute toxicity studies of fractions and cassane-type diterpenoids from the stem bark of Caesalpinia pulcherrima (L.) Sw. Trop J Nat Prod Res. 2018;2(4):179–184. doi:10.26538/tjnpr/v2i4.5
45. Lekana-douki JB, Lydie S, Liabagui O, et al. In vitro antiplasmodial activity of crude extracts of Tetrapleura tetraptera and Copaifera religiosa. BMC Res Notes. 2011;4(506):1–5. doi:10.1186/1756-0500-4-506
46. Arifiyanto A, Setyaningrum E, Nukmal N, Aeny TNUR. Short communication: in vitro antimicrobial and antimalarial screening of a crude extract of Streptomyces sp. AB8 isolated from Lapindo Mud Volcano Area, Sidoarjo, Indonesia. Biodiversitas. 2021;22(7):2817–2823. doi:10.13057/biodiv/d220731
47. Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2(2):74. doi:10.4103/0976-500X.81895
48. Lange SS, Takata KI, Wood RD. DNA polymerases and cancer. Nat Rev Cancer. 2011;11(2):96. doi:10.1038/nrc2998
49. Wang H, Qian J, Zhang Y, Xu W, Xiao J, Suo A. Growth of MCF-7 breast cancer cells and efficacy of anti-angiogenic agents in a hydroxyethyl chitosan/glycidyl methacrylate hydrogel. Cancer Cell Int. 2017;17(1). doi:10.1186/s12935-017-0424-8
50. Ghasemi M, Turnbull T, Sebastian S, Kempson I. The mtt assay: utility, limitations, pitfalls, and interpretation in bulk and single-cell analysis. Int J Mol Sci. 2021;22(23):12827. doi:10.3390/ijms222312827
51. Clarkson C, Maharaj VJ, Crouch NR, et al. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J Ethnopharmacol. 2004;92(2–3):177–191. doi:10.1016/j.jep.2004.02.011
52. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. From DNA to RNA. In: Molecular Biology of the Cell. Garland Science; 2002.
53. Tanjung M, Saputri RD, Fitriati FF, Tjahjandarie TS. Antimalarial and antioxidant activities of isoprenylated coumarins from the stem bark of Mesua borneensis L. J Biol Act Prod Nat. 2016;6(2):95–100.
54. Kaushik CP, Chahal M. Synthesis, antimalarial and antioxidant activity of coumarin appended 1,4-disubstituted 1,2,3-triazoles. Monatsh Chem. 2021;152(8):1001–1012. doi:10.1007/s00706-021-02821-8
55. Yadav N, Agarwal D, Kumar S, Dixit AK, Gupta RD, Awasthi SK. In vitro antiplasmodial efficacy of synthetic coumarin-triazole analogs. Eur J Med Chem. 2018;145:735–745. doi:10.1016/j.ejmech.2018.01.017
56. Moon HI, Lee JH, Lee YC, Kim KS. Antiplasmodial and cytotoxic activity of coumarin derivatives from dried roots of Angelica gigas Nakai in vitro. Immunopharmacol Immunotoxicol. 2011;33(4):663–666. doi:10.3109/08923973.2011.559248
57. Susidarti RA. In vitro antiplasmodial activity of coumarin 8-hydroxyisocapnolactone-2’,3’-diol isolated from micromelum minutum (G. Forst.) Wight & Arn. Indones J Pharm. 2014;25(1):44. doi:10.14499/indonesianjpharm25iss1pp44
58. Yang YZ, Ranz A, Pan HZ, Zhang ZN, Lin XB, Meshnick SR. Daphnetin: a novel antimalarial agent with in vitro and in vivo activity. Am J Trop Med Hyg. 1992;46(1):15–20. doi:10.4269/ajtmh.1992.46.15
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.