Back to Journals » Clinical Ophthalmology » Volume 12
Foveal threshold and photoreceptor integrity for prediction of visual acuity after intravitreal aflibercept on age-related macular degeneration
Authors Sakai T, Okude S, Tsuneoka H
Received 6 November 2017
Accepted for publication 8 March 2018
Published 16 April 2018 Volume 2018:12 Pages 719—725
DOI https://doi.org/10.2147/OPTH.S156162
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Tsutomu Sakai, Sachiyo Okude, Hiroshi Tsuneoka
Department of Ophthalmology, Jikei University School of Medicine, Tokyo, Japan
Purpose: To determine whether baseline foveal threshold and photoreceptor integrity can predict best-corrected visual acuity (BCVA) at 12 months after intravitreal aflibercept (IVA) therapy in eyes with neovascular age-related macular degeneration (AMD).
Patients and methods: We evaluated 25 eyes of 25 patients with treatment-naïve neovascular AMD who received IVA once a month for 3 months, followed by once every 2 months for 8 months. BCVA, integrity of the external limiting membrane (ELM) or the ellipsoid zone (EZ) of the photoreceptors, and retinal sensitivity were determined before (baseline) and at 6 and 12 months after initial IVA. The average threshold foveal sensitivity and mean deviation within the central 10° were determined by Humphrey central 10-2 perimetry. Correlations between BCVA at 12 months and integrity of the ELM or EZ, foveal threshold, and mean deviation at each visit were determined.
Results: At 12 months, BCVA improved significantly from 0.20±0.23 to 0.10±0.22 logMAR (logarithm of the minimum angle of resolution) units, and foveal threshold and mean deviation improved significantly from 29.0±5.1 and -3.38±3.10 dB to 32.6±3.2 and -1.64±2.10 dB, respectively (P=0.0009 and P=0.0021). At baseline, both foveal threshold and integrity of the ELM were significantly correlated with BCVA at 12 months (P=0.0428 and P=0.0275).
Conclusion: These results indicate that both integrity of the ELM and foveal threshold at baseline can predict BCVA after treatment for neovascular AMD. There is a possibility that these parameters can predict the efficacy of IVA in each case.
Keywords: aflibercept, age-related macular degeneration, foveal threshold, foveal photoreceptor integrity
Introduction
Neovascular age-related macular degeneration (AMD) is the main cause of severe vision loss in many developed countries.1 Current therapy involves intravitreal administration of anti-vascular endothelial growth factor (VEGF) agents, with wide use of bevacizumab (Avastin; Genentech, South San Francisco, CA, USA) and ranibizumab (Lucentis; Genentech).2 Aflibercept (Eylea; Regeneron, Tarrytown, NY, USA) is another anti-VEGF agent with greater binding activity to VEGF than bevacizumab and ranibizumab.3 The VIEW1/2 studies showed that 3-monthly intravitreal injections of aflibercept (IVA) followed by bimonthly injections was not inferior to monthly intravitreal injections of ranibizumab (IVR) in maintaining vision in patients with treatment-naïve neovascular AMD over 96 weeks.4,5 In addition, a subgroup analysis revealed that central retinal thickness (CRT) on optical coherence tomography (OCT) decreased after IVA. Thus, IVA had a significant therapeutic effect functionally and morphologically in patients with neovascular AMD.
Visual acuity has commonly been used as an indicator of visual function; however, the visual acuity test uses high-contrast high spatial-frequency optotypes and evaluates only one aspect of visual function. Multimodal visual function tests are needed for assessment of therapeutic response in patients with neovascular AMD. The visual field provides information on visual function that is not evident from measurement of visual acuity alone. Some studies suggest that a 10° visual field measurement is appropriate for central macular function,6–8 and this visual field may be most important for patients in patients with moderate macular disease, especially AMD. This further supports the idea that visual field assessment, in addition to visual acuity, is needed to evaluate visual function in patients with AMD.
OCT enables evaluation of morphologic characteristics of the retina. Spectral-domain optical coherence tomography (SD-OCT) has better axial resolution than conventional time-domain OCT and is useful for evaluation of foveal structures of photoreceptors. Recent reports indicate that the integrity of foveal photoreceptors on SD-OCT is strongly correlated with visual function in different retinal diseases.9–17 Therefore, it is intriguing to determine if foveal photoreceptor integrity at baseline is correlated with final visual acuity after treatment in retinal diseases.
The purpose of this study was to determine whether foveal threshold and photoreceptor integrity can predict best-corrected visual acuity (BCVA) at 12 months after periodic IVA in eyes with treatment-naïve neovascular AMD.
Materials and methods
The study was performed as a prospective, nonrandomized, exploratory, interventional study in an institutional setting. The study design was approved by the Institutional Review Board/Ethics Committee at Jikei University, and adhered to the tenets of the Declaration of Helsinki and guidelines of the Japanese Ministry of Health, Labor, and Welfare. The protocol was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials registry prior to the start of the study (UMIN000012126). Each patient gave written informed consent for participation in the study.
Subjects
The subjects were 25 consecutive patients with untreated active subfoveal choroidal neovascularization (CNV) or juxtafoveal lesions (1–200 micron from the foveal center) with leakage affecting the fovea, secondary to AMD. All patients were Japanese and their median age was 70.0 years (range, 54–81 years). The inclusion criteria were ≥50 years of age with BCVA of 0.1 to 1.0 (20/200–20/20 Snellen equivalent) and CNV covering at least 50% of the total lesion area. Active CNV was confirmed by observation of leakage in fluorescein angiography (FA). A diagnosis of polypoidal choroidal vasculopathy (PCV) was established based on indocyanine green angiography (ICGA) findings of a network of vessels with a complex of branching vessels and multiple, terminal, reddish-orange, aneurysmal or polypoidal lesions. Patients were excluded if they had received treatment such as laser photocoagulation, submacular surgery, and IVR; and if they had glaucoma, diabetic retinopathy, or retinal vascular occlusion. Eyes with significant lens opacity (cataract grade ≥2 in the Lens Opacities Classification System) were also excluded from the study.
All patients received a fixed regimen of IVA over 12 months. A loading dose of three consecutive monthly injections was followed by a retreatment regimen every 8 weeks. A complete clinical examination with slit-lamp biomicroscopy, fundus color (VISCUM NM ProNM; Carl Zeiss Meditec, Dublin, CA, USA), FA, ICGA with confocal scanning laser ophthalmoscopy (Heidelberg Retina Angiograph 2; Heidelberg Engineering, Heidelberg, Germany), and macular OCT using a Cirrus HD-OCT system (Carl Zeiss Meditec) was performed at baseline and after 6 and 12 months. Lesion size (mm2) detected with FA was measured at 10 minutes and automatically calculated by the HRA-embedded software.12
BCVA was measured with a Japanese standard decimal visual acuity chart and the mean BCVA was calculated using the logarithm of the minimum angle of resolution (logMAR) scale. SD-OCT scans were recorded using the HD 5-line raster scan protocol (horizontal scan of 6 mm). OCT scans were reevaluated at 6 and 12 months after initial IVA.
Optical coherence tomography
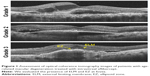
We measured the CRT, which was defined as the distance between the vitreoretinal surface and the inner surface of retinal pigment epithelium. The integrity of the external limiting membrane (ELM) and ellipsoid zone (EZ) in the horizontal OCT images at the fovea was classified into three grades: Grade 1, absent; Grade 2, abnormal or difficult to identify; and Grade 3, present or continuous ELM and EZ (Figure 1). Both structures of ELM and EZ were evaluated separately. All grading were done by one observer (TS).18
Static automated perimetry
Static automated perimetry (SAP) in the central 10° visual field was performed with a Humphrey field analyzer 750 (Zeiss-Humphrey, Dublin, CA, USA) using the standard protocol. The age-adjusted correction, as per standard perimetric procedure, was placed in the lens holder for each eye and the foveal threshold tested first, followed by the complete 10-2 program. The SAP stimuli were circular luminance increments and the test conditions were as follows: Goldmann size III stimulus (0.43°), 31.5 apostilb background, and 200 ms stimulus duration. Breaks were taken during the test upon request of the patient. The 10-2 program with the Swedish interactive threshold strategy was used for SAP. Before testing, all patients had undergone at least one perimetry examination. Using the perimeters, the mean deviation (MD) and pattern standard deviation were calculated. To examine the extent of visual field defects detected by perimetry, the percentage of significantly depressed points was determined within confidence limits of 0.5% in the total deviation probability plot. There were 69 test points in the visual fields in SAP. The tests were considered to be reliable if fixation losses and false-negatives and false-positives were <20%. Patients were reexamined after 6 and 12 months.
To see if MD was associated with lesion size, we performed correlation analysis on the subject of improvement in MD and lesion size.
Statistical analyses
A Wilcoxon matched-pairs signed-rank test was used to compare characteristics at baseline and 12 months. Correlation analyses were performed by Spearman’s correlation coefficient test. The decimal BCVA obtained from the Landolt ring test was converted to a logMAR BCVA. A change in logMAR VA of ≥0.3 was taken to be significant. Comparisons were considered significant at P<0.05. All analyses were carried out using Intercooled Stata ver. 7.0 (Stata Corp., College Station, TX, USA).
Results
Thirty patients met the inclusion criteria. Five patients dropped out because of colon cancer or for an unknown reason, leaving 25 patients (10 with typical AMD and 15 with PCV) for analysis at baseline and after 6 and 12 months of a fixed regimen of IVA. Baseline and 12-month characteristics of the participants are shown in Table 1. The mean age of the patients was 70.0±7.0 years (range, 54–81 years). In FA, the lesion was subfoveal in 17 eyes (68.0%), juxtafoveal in five (20.0%), and extrafoveal in three (12.0%). BCVA was measured trimonthly during IVA treatment (Figure 2A) and improved significantly after 12 months compared with baseline in all patients (P=0.0001).
Data for lesion size, CRT, MD of perimetric sensitivity, and foveal threshold at baseline and after 6 and 12 months of IVA are shown in Figure 2B–E. Lesion size, CRT, and MD of perimetric sensitivity improved significantly after 12 months compared with baseline (P=0.0090, P<0.0001, and P=0.0021). As shown in Figure 3, improvement in MD at 12 months was correlated significantly with a decrease in mean lesion size for neovascular AMD (P=0.0101; R=0.5047). At baseline, the ELM and EZ had integrities of Grade 3 in 22 and two eyes, respectively. The integrity of the EZ improved after 12 months, with 16 eyes in Grade 3, but the number of eyes in Grade 3 for the ELM remained unchanged (Figure 4). BCVA, lesion size, CRT, and MD of perimetric sensitivity improved significantly at 6 and 12 months, but foveal threshold did not change significantly between 6 and 12 months (P=1.000). This indicates that foveal threshold attained its final level earlier than the other parameters. Baseline foveal threshold and integrity of the ELM were significantly correlated with BCVA at 12 months (P=0.0428 and P=0.0275; Figure 5A and B), but baseline integrity of the EZ did not show this correlation (P=0.1264; Table 2).
  | Figure 3 Scatterplots showing the relationship between change in lesion size over 12 months and change in MD for each treated eye. |
Regarding adverse events, neither retinal pigment epithelium (RPE) tear nor systemic complications were observed in any patients over the 12 months of IVA treatment.
Discussion
Our results showed that foveal threshold and integrity of the ELM at baseline were significantly correlated with BCVA at 12 months after periodic IVA. Correlations among these parameters were significant during 12 months of treatment. In addition, integrity of the EZ at 6 months after IVA was significantly associated with the final BCVA. Thus, both parameters can be used to assess the effectiveness of a particular therapy.
Recently, Oishi et al showed that the status of ELM, greatest linear dimension (GLD), and polypoidal lesions are predictive of visual outcome in 1-year IVA for AMD,19 with the finding that the presence of ELM was the most significant factor for VA at 12 months. Our study also showed that the status of ELM is a predictor of visual outcome in patients with AMD after treatment with IVA, although the morphologic assessments differed from those in Oishi et al. In contrast, integrity of the EZ at baseline was not a predictor of visual outcome. Reconstruction of photoreceptors may be consistent with recovery of the EZ, but functional improvement seemed to occur earlier than could be detected on imaging in this study. In addition, it is difficult to evaluate the integrity of the EZ because of masking by hemorrhage, fibrin, or edema, which prevents a quantitative evaluation. Thus, if the ELM is healthy, it seems that functional and morphologic improvements in AMD may be detected at any time after treatment with IVA.
BCVA, lesion size, and MD of perimetric sensitivity improved significantly with IVA for neovascular AMD over a 12-month period. Most of the increase in BCVA due to IVA occurred after the 3 monthly loading doses. We speculate that prompt inhibition of VEGF leads to improved BCVA by decreasing retinal fluid or serous pigment epithelium detachment (PED) immediately. The good effects of IVA on the retina and choroid may be attributed to strong blockade of VEGF-A, suppression of multiple cytokines in the VEGF family, or both.20 After the loading phase, there was a gradual progressive improvement in BCVA and SAP 10-2 MD. Larger CNV lesions and leakage size have been associated with vision loss in patients with neovascular AMD under anti-VEGF treatment.21,22 Therefore, the improvements in BCVA and SAP 10-2 MD might be associated with gradual reduction in the size of the CNV lesions, and we did find that lesions gradually decreased in size over 12 months. Measurements were only performed at baseline and after 6 and 12 months, but the improvement in SAP 10-2 MD may suggest that the effect of IVA on retinal fluid and CNV lesion size is sustainable under a fixed regimen and the improvements in BCVA and MD of perimetric sensitivity were well correlated.
MD of perimetric sensitivity improved concordantly with BCVA in our study. Luu et al suggested that visual field testing may be useful in assessment of the effectiveness of treatment in patients with AMD in clinical studies with appropriate exclusion criteria.9 A study using microperimetry showed that mean central retinal sensitivity improved progressively over 12 months of monthly anti-VEGF treatment.23–26 Comparison of SAP with microperimetry of the central visual field in eyes with AMD has not been performed, but Lima et al showed that macular sensitivity evaluated by microperimetry correlates significantly with SAP paracentral visual field defects.27 Thus, SAP appears to be a practical and useful test for assessment of visual function in a clinical study of the early-to-medium stages of AMD. MD of perimetric sensitivity seems to be a reliable marker for the effect of IVA.
This study has several strengths and limitations. The strengths include the prospective design, predetermined treatment protocol, and follow-up examinations. The treatment protocol is identical to that in the VIEW I/II studies. The limitations include the small sample size, lack of measurement of CRT by OCT, and lack of a fundus tracking system during visual field testing using the Humphrey field analyzer perimeter, which meant that unreliable responses due to poor fixation had to be discarded and the test had to be repeated to improve the reliability. However, most of the subjects had good vision (>20/40), and thus had no problem seeing the central fixation spot. Additionally, in a progression study, there is a need to account for worsening cataracts and potential involvement of fixation later in the disease course, which may affect interpretation of visual field results. However, due to the strict exclusion criteria, our findings are limited to AMD patients suitable for visual field testing, that is, those with foveal fixation and relatively clear ocular media.
In conclusion, this study suggested that foveal threshold and photoreceptor integrity at baseline can predict visual function after IVA treatment in eyes with neovascular AMD. The findings were significantly correlated with BCVA at 12 months, and, therefore, foveal threshold and integrity of the ELM are useful parameters for assessment of the effectiveness of therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115(1):116–126. | ||
Jaffe GJ, Martin DF, Toth CA, et al; Comparison of Age-related Macular Degeneration Treatments Trials Research Group. Macular morphology and visual acuity in the comparison of age-related macular degeneration treatment trials. Ophthalmology. 2013;120(9):1860–1870. | ||
Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96(9):1157–1158. | ||
Heier JS, Brown DM, Chong V, et al; VIEW 1 and VIEW 2 Study Groups. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119(12):2537–2548. | ||
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121(1):193–201. | ||
Acton JH, Gibson JM, Cubbidge RP. Quantification of visual field loss in age-related macular degeneration. PLoS One. 2012;7(6):e39944. | ||
Abe K, Iijima H, Hirakawa H, Tsukahara Y, Toda Y. Visual acuity and 10 degrees automated static perimetry in eyes with retinitis pigmentosa. Jpn J Ophthalmol. 2002;46(5):581–585. | ||
Iijima H. Correlation between visual sensitivity loss and years affected for eyes with retinitis pigmentosa. Jpn J Ophthalmol. 2012;56(3):224–229. | ||
Luu CD, Dimitrov PN, Wu Z, et al. Static and flicker perimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54(5):3560–3568. | ||
Otani T, Yamaguchi Y, Kishi S. Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina. 2010;30(5):774–780. | ||
Murakami T, Nishijima K, Sakamoto A, Ota M, Horii T, Yoshimura N. Association of pathomorphology, photoreceptor status, and retinal thickness with visual acuity in diabetic retinopathy. Am J Ophthalmol. 2011;151(2):310–317. | ||
Shin HJ, Lee SH, Chung H, Kim HC. Association between photoreceptor integrity and visual outcome in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2012;250(1):61–70. | ||
Chhablani JK, Kim JS, Cheng L, Kozak I, Freeman W. External limiting membrane as a predictor of visual improvement in diabetic macular edema after pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2012;250(10):1415–1420. | ||
Shin HJ, Chung H, Kim HC. Association between integrity of foveal photoreceptor layer and visual outcome in retinal vein occlusion. Acta Ophthalmol. 2011;89(1):e35–e40. | ||
Kurashige Y, Tsujikawa A, Murakami T, et al. Changes in visual acuity and foveal photoreceptor integrity in eyes with chronic cystoid macular edema associated with retinal vein occlusion. Retina. 2012;32(4):792–798. | ||
Reibaldi M, Parravano M, Varano M, et al. Foveal microstructure and functional parameters in lamellar macular hole. Am J Ophthalmol. 2012;154(6):974.e1–980.e1. | ||
Wakabayashi T, Fujiwara M, Sakaguchi H, Kusaka S, Oshima Y. Foveal microstructure and visual acuity in surgically closed macular holes: spectral-domain optical coherence tomographic analysis. Ophthalmology. 2010;117(9):1815–1824. | ||
Aizawa S, Mitamura Y, Baba T, Hagiwara A, Ogata K, Yamamoto S. Correlation between visual function and photoreceptor inner/outersegment junctionin patients with retinitis pigmentosa. Eye (Lond). 2009;23(2):304–308. | ||
Oishi A, Tsujikawa A, Yamashiro K, et al. One-year result of aflibercept treatment on age-related macular degeneration and predictive factors for visual outcome. Am J Ophthalmol. 2015;159(5):853–860. | ||
Koizumi H, Kano M, Yamamoto A, et al. Short-term changes in choroidal thickness after aflibercept therapy for neovasular age-related macular degeneration. Am J Ophthalmol. 2015;159(4):627–633. | ||
Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B; MARINA and ANCHOR Study Groups. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology. 2011;118(3):523–530. | ||
Bloch SB, La Cour M, Sander B, et al. Predictors of 1-year visual outcome in neovascular age – related macular degeneration following intravitreal ranibizumab treatment. Acta Ophthalmol. 2013;91(1):42–47. | ||
Munk MR, Kiss C, Huf W, et al. One year follow-up of functional recovery in neovascular AMD during monthly anti-VEGF treatment. Am J Ophthalmol. 2013;156(4):633–643. | ||
Parravano M, Oddone F, Tedeschi M, et al. Retinal functional changes measured by microperimetry in neovasular age-related macular degeneration treated with ranibizumab: 24-month results. Retina. 2010;30(7):1017–1024. | ||
Cho HJ, Kim CG, Yoo SJ, et al. Retinal functional changes measured by microperimetry in neovasular age-related macular degeneration treated with ranibizumab. Am J Ophthalmol. 2013;155:118–126.e111. | ||
Sulzbacher F, Roberts P, Munk MR, et al; Vienna Eye Study Center. Relationship of retinal morphology and retinal sensitivity in the treatment of neovascular age-related macular degeneration using aflibercept. Invest Ophthalmol Vis Sci. 2014;56(2):1158–1167. | ||
Lima VC, Prata TS, De Moraes CG, et al. A comparison between microperimetry and standard achromatic perimetry of the central visual field in eyes with glaucomatous paracentral visual-field defects. Br J Ophthalmol. 2010;94(1):64–67. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.