Back to Journals » Therapeutics and Clinical Risk Management » Volume 15
Fosfomycin: the characteristics, activity, and use in critical care
Authors Hashemian SMR , Farhadi Z, Farhadi T
Received 24 December 2018
Accepted for publication 22 February 2019
Published 27 March 2019 Volume 2019:15 Pages 525—530
DOI https://doi.org/10.2147/TCRM.S199119
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Seyed MohammadReza Hashemian,1,2 Zinat Farhadi,3 Tayebeh Farhadi1
1Chronic Respiratory Diseases Research Center (CRDRC), National Research Institute of Tuberculosis and Lung Diseases (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran; 2Clinical Tuberculosis and Epidemiology Research Center, National Research Institute of Tuberculosis and Lung Disease (NRITLD), Shahid Beheshti University of Medical Sciences, Tehran, Iran; 3Department of Microbiology, Shiraz Branch, Islamic Azad University, Shiraz, Iran
Abstract: Fosfomycin (C3H7O4P) is a phosphonic acid derivative representing an epoxide class of antibiotics. The drug is a re-emerging bactericidal antibiotic with a wide range of actions against several Gram-positive and Gram-negative bacteria. Among the existing antibacterial agents, fosfomycin has the lowest molecular weight (138 Da), which is not structurally associated with other classes of antibiotics. In intensive care unit (ICU) patients, severe soft tissue infections (STIs) may lead to serious life-threatening problems, and therefore, appropriate antibiotic therapy and often intensive care management (ICM) coupled with surgical intervention are necessary. Fosfomycin is an antibiotic primarily utilized for the treatment of STIs in ICUs. Recently, fosfomycin has attracted renewed interest for the treatment of serious systemic infections caused by multidrug-resistant Enterobacteriaceae. In some countries, intravenous fosfomycin has been prescribed for various serious systemic infections, such as acute osteomyelitis, nosocomial lower respiratory tract infections, complicated urinary tract infections, bacterial meningitis, and bacteremia. Administration of intravenous fosfomycin can result in a sufficient concentration of the drug at different body regions. Dose modification is not required in hepatic deficiency because fosfomycin is not subjected to enterohepatic circulation.
Keywords: fosfomycin, soft tissue infections, intensive care management
Introduction
Fosfomycin (C3H7O4P) is a phosphonic acid derivative representing an epoxide class of antibiotics. Fosfomycin is a re-emerging bactericidal antibiotic with a wide range of actions against several Gram-positive and Gram-negative bacteria, including vancomycin-resistant enterococci, methicillin-resistant Staphylococcus aureus, and carbapenem-resistant Enterobacteriaceae. Some non-fermenters such as Acinetobacter are inherently resistant to fosfomycin, whereas Pseudomonas is susceptible to the antibiotics in vitro.1–3 In the past decade, fosfomycin was isolated from some strains of Streptomyces, but nowadays it is produced synthetically. Among the existing antibacterial agents, fosfomycin has the lowest molecular weight (138 Da), which is not structurally associated with other classes of antibiotics.4,5
In intensive care unit (ICU) patients, severe soft tissue infections (STIs) may lead to serious life-threatening problems, and therefore, appropriate antibiotic therapy6 and often intensive care management (ICM) coupled with surgical intervention are necessary. Fosfomycin is considered an antibiotic primarily appropriate for the treatment of STIs in ICUs.7
Some appropriate properties of fosfomycin in healthy volunteers (eg, identical concentrations of the drug in the soft tissues and plasma) have made the drug a frequently administered antibiotic in the treatment of patients with sepsis and/or STI especially in Europe.8 Nevertheless, in USA, the Food and Drug Administration (FDA) has approved fosfomycin only for the management of uncomplicated cystitis.
Recently, the use of fosfomycin has attracted renewed interest for the treatment of serious systemic infections caused by multidrug-resistant Enterobacteriaceae.3 In some countries, intravenous fosfomycin has been prescribed for various serious systemic infections, such as acute osteomyelitis, nosocomial lower respiratory tract infections, complicated urinary tract infections, bacterial meningitis, and bacteremia.3,9 Fosfomycin trometamol, an oral formulation of fosfomycin, is available in USA and in Europe (in additional to the parenteral disodium compound) as used for therapy of cystitis in one 3 g dose or multiple oral doses of 3 g administered every other day.
Mechanism of action
Generally, antibiotics have the bactericidal and/or bacteriostatic activities and affect the vital functions essential for bacteria, including cell wall synthesis, protein translation, DNA duplication, RNA transcription, and/or cell membrane organization. Fosfomycin mechanism of action is unique; the drug irreversibly inhibits the initial phase of microbial cell wall synthesis.10 Chemical structure of fosfomycin is shown in Figure 1.
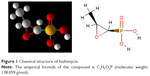  | Figure 1 Chemical structure of fosfomycin. |
Fosfomycin must enter into the bacterial cytoplasm for the bactericidal activity. To reach the target cell, fosfomycin uses the bacterial hexose monophosphate transport system (stimulated by glucose-6-phosphate [G6P]) and the bacterial L-a-glycerophosphate transport system (activated by glycerol-3-phosphate [G3P]). The chemical structure of fosfomycin mimics both G6P and G3P.11,12
In the bacterial cytoplasm, fosfomycin binds UDP-GlcNAc enolpyruvyl transferase (MurA) and inactivates a vital enzyme such as enolpyruvyl transferase (MurA) involved in the peptidoglycan biosynthesis.13 MurA catalyzes the first phase of the peptidoglycan biosynthesis.14 Fosfomycin inhibits the peptidoglycan biosynthesis via preventing the formation of UDP-GlcNac-3-O-enolpyruvate from UDP-GlcNAc and phosphoenolpyruvate (PEP), resulting in bacterial cell destruction.12 Fosfomycin acts as a PEP analog and competes with that.15 Furthermore, fosfomycin reduces penicillin-binding proteins (PBPs).10
Pharmacodynamic (PD) characteristics of fosfomycin
It is not clear whether fosfomycin shows a concentration-dependent or a time-dependent bactericidal function.16 In this regard, some studies have demonstrated that fosfomycin exhibits a concentration-dependent activity to destruct strains of Escherichia coli and Proteus mirabilis in vitro as well as strains of Streptococcus pneumoniae in vivo.17,18 However, other studies have indicated a time-dependent bactericidal action of fosfomycin to destroy strains of S. aureus in vitro.4,19
Depending on the applied concentration of fosfomycin, the drug may exhibit an extended post-antibiotic effect (PAE) (between 3.4 and 4.7 h) against strains of E. coli and P. mirabilis in vitro.17 However, a comparatively smaller PAE has been seen against S. aureus strains (0.5–1.4 h).20
Pharmacokinetic (PK) characteristics of fosfomycin
Fosfomycin disodium is a very hydrophilic agent. Approximately 3% of the drug is bound to serum proteins and permits favorable tissue availability. The low molecular weight warrants high diffusibility of the drug.3
After intravenous administration, blood content of fosfomycina shows a rapid disposition phase followed by a slow distribution phase.21 Following the administration of multiple doses, a cumulative effect is seen. Elimination half-life of fosfomycin disodium is 1.5–2 h.22–24 The Cmax calculated with the standard intravenous formulation of the drug ranges from 200 to 644 mg/L, which is 10–20 times greater than the oral dose.25,26 The volume of the drug distribution is 18–27 L at a steady state.11
Administration of intravenous fosfomycin can result in a sufficient concentration of the drug at different body regions, such as bone, muscle, lung, appendix, cerebrospinal fluid, gallbladder, common bile duct, and heart valves.11,25 Dose modification is not required in the hepatic deficiency because fosfomycin is not subjected to enterohepatic circulation.11 Approximately 93% of an administered dose undergoes the glomerular filtration in the kidney and is excreted unaltered in the urine.11 For serious systemic infections, fosfomycin disodium is utilized between 12 and 24 g as 2–4 divided doses. Reduction of daily dose of the drug is necessary for creatinine clearance of <40 mL/min. Addition of a dose of 2 g after each session has been suggested for patients subjected to intermittent hemodialysis. No dose adjustment is needed in continuous renal replacement therapy (CRRT).27
Adverse effects
A number of adverse effects, including mild and self-limited gastrointestinal disorders (eg, nausea, abdominal pain, diarrhea, and dyspepsia), have also been reported following the oral administration of fosfomycin.27 Other side effects, including dizziness, headaches, vaginitis, respiratory infections, and microbial superinfections, may also occur. Laboratory changes involve alterations in the number of blood cells (eosinophils, neutrophils, red blood cells, and platelets) and increase in the liver enzymes and bilirubin but no change in the renal function.28
Following intravenous administration of fosfomycin, the potential adverse effects such as hypokalemia and sodium overload may occur. Each gram of intravenous fosfomycin consists of 0.32 g of sodium.29 Furthermore, fosfomycin may increase potassium renal excretion, resulting in hypokalemia. Other adverse effects, including infusion site reactions, heart failure, and hypertension (because of sodium overload), and increased alanine aminotransferase (ALT) may be developed by an intravenously administered fosfomycin dose.30 Therefore, administration of potassium supplements is deemed to be necessary in patients receiving fosfomycin, and their levels should be monitored regularly. In patients with heart failure, caution is also essential.27
Drug interaction
ICU patients are at a high risk for developing the resistant bacterial infections, and therefore, a combined antibacterial treatment is suggested for them.31,32 Fosfomycin has been reported to show a 100% synergistic effect after combining with other antibacterial drugs.33
Preventing the different stages of cell wall synthesis may lead to the synergistic effect of fosfomycin and β-lactam antibiotics; fosfomycin prevents the first stage of the cell wall synthesis procedure, whereas β-lactam antibiotics inhibit the final phase.34 The potency of fosfomycin to alter the function of PBPs may also induce the synergistic effect between fosfomycin and β-lactam antibiotics.35,36 The ciprofloxacin-mediated destruction of the bacterial outer membrane can enhance the penetration and action of fosfomycin and promote the synergistic effect between fosfomycin and ciprofloxacin.37 For treating Pseudomonas aeruginosa infections, synergy between fosfomycin and a wide range of other antibiotics, including cefepime, amikacin, aztreonam, meropenem, imipenem, ceftazidime, gentamicin, and ciprofloxacin, has been reported.38,39 Fosfomycin combined with amikacin or sulbactam has a synergistic effect to fight against Acinetobacter baumannii strains and may consider an efficient combination therapy for the treatment of A. baumannii infections.40,41 With respect to methicillin-resistant S. aureus, Enterococcus, Streptococcus, and Enterobacteriaceae species, fosfomycin also has synergistic effects when combined with other antibacterial agents.33,34 In addition to high antibacterial effectiveness, fosfomycin can decrease toxicity related to other drugs (eg, glycopeptides, aminoglycosides, and polymyxin B) as lower doses of these antibiotics can be administered.42–44
Fosfomycin resistance
Some mechanisms of fosfomycin resistance have been described.45 The chromosomal resistance is caused by mutations in the genes encoding the G6P transporter or the G3P transporter resulting in the reduced uptake of the drug by the pathogen.46,47 Another resistance mechanism is based on modifications in the targeted enzyme (Mur A) (point mutations at the binding site of the murA gene),48 which decreases the affinity of fosfomycin. Increased expression of the murA gene also results in clinical resistance to fosfomycin.49 The third mechanism of resistance is based on the inactivation of fosfomycin either by enzymatic cleavage of the epoxide ring or by phosphorylation of the phosphonate group. In the presence of the plasmid-mediated fosfomycin-modifying metalloenzymes (FosA, FosB, and FosX), the epoxide structure is cleaved.50 Some kinases including FomA and FomB cause phosphorylation of fosfomycin to the diphosphate and triphosphate states leading to fosfomycin degradation.51,52
Fosfomycin in critically ill patients
In recent years, the multidrug resistance to the used antibiotics has increased. Therefore, there is a crucial need for the development of new antimicrobial candidates.53–61 Obtaining the appropriate concentrations of antibiotics at target sites is crucial to eliminate the relevant pathogens and clinical outcomes.62–64 Recent studies in patients with sepsis have shown that despite sufficient concentrations of antibiotics in plasma, their concentrations in the interstitial fluid of soft tissues may be inadequate. This may be due to deficiency in transcapillary transfer of antibiotics to target sites.65–69 Thus, most available antibiotics have a reduced tissue penetration in the septic patients. In this regard, the target site penetration of fosfomycin, an antibiotic mainly appropriate for the treatment of STIs in ICU patients, has been investigated. In nine patients with sepsis, the microbiologically active levels of fosfomycin were evaluated in the interstitial space fluid of skeletal muscle and correlated with the corresponding plasma concentrations.7 The results demonstrated that fosfomycin concentrations in plasma and muscle interstitium exceeded the minimum inhibitory concentrations (MICs) for various clinically important pathogens (Streptococcus pyogenes, S. aureus, and P. aeruginosa). Thus, fosfomycin shows a tissue PK profile, which suggests a substitute for other broad-spectrum antibiotics in critically ill patients undergoing STI.7
In a study on critically ill patients, the optimal dosage regimen of intravenous fosfomycin in combination with carbapenem and based on PK/PD targets was evaluated for the treatment of P. aeruginosa.70 The P. aeruginosa isolates were recovered from various clinical specimens. MICs of all the isolates were determined, and PK parameters were obtained. Monte Carlo simulation was performed to determine the percentage of target attainment (PTA) and cumulative fraction of response (CFR). The results indicated that the extended infusion of fosfomycin 16–24 g combined with prolonged carbapenem infusion could be utilized in non-MDR P. aeruginosa treatment.70
Gram-negative resistance is a crucial global crisis that has been illustrated by the rapid growth of carbapenem-resistant Enterobacteriaceae (CRE). For serious systemic infections induced by multidrug-resistant Enterobacteriaceae, the use of fosfomycin as an essential and beneficial option has been recently renewed. The new evidence on the hidden capacity of intravenous fosfomycin to destroy Gram-negative pathogens has been demonstrated elsewhere.3 Although several hopeful evidence are available for fosfomycin as the last antibacterial option to treat severe Gram-negative infections, more investigations are still necessary before using the intravenous fosfomycin.3
Along with harmonization of current breakpoints and according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Clinical and Laboratory Standards Institute (CLSI), fosfomycin has a high potency to treat serious systemic infections. However, breakpoints for Pseudomonas sp. need to be defined urgently. Dose of fosfomycin requires to be defined for serious infections where probably higher daily dosages (24 g/day) may be required to prevent the heteroresistant mutant selection. Well controlled and randomized studies comparing fosfomycin versus colistin and investigating mono and combination therapy are essential to identify optimal regimens of fosfomycin in critically ill populations with resistant Gram-negative infections. Until the abovementioned need gaps are clear, fosfomycin should not be used as a monotherapy option to treat severe systemic infections.3
Disclosure
The authors report no conflicts of interest in this work.
References
Raz R. Fosfomycin: an old – new antibiotic. Clin Microbiol Infect. 2012;18:4–7. doi:10.1111/j.1469-0691.2011.03636.x | ||
Thaden JT, Pogue JM, Kaye KS. Role of newer and re-emerging older agents in the treatment of infections caused by carbapenem-resistant Enterobacteriaceae. Virulence. 2016;6:1–14. | ||
Saiprasad PV, Krishnaprasad K. Exploring the hidden potential of fosfomycin for the fight against severe gram-negative infections. Indian J Med Microbiol. 2016;34(4):416. doi:10.4103/0255-0857.195379 | ||
Grif K, Dierich MP, Pfaller K, Miglioli PA, Allerberger F. In vitro activity of fosfomycin in combination with various antistaphylococcal substances. J Antimicrob Chemother. 2001;48:209–217. | ||
Tessier F, Quentin C. In vitro activity of fosfomycin combined with ceftazidime, imipenem, amikacin, and ciprofloxacin against Pseudomonas aeruginosa. Clin Microbiol Infec. 1997;16:159–162. | ||
Dupont H, Mentec H, Sollet JP, Bleichner G. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 2001;27:355–362. doi:10.1007/s001340000640 | ||
Joukhadar C, Klein N, Dittrich P, et al. Target site penetration of fosfomycin in critically ill patients. J Antimicrob Chemother. 2003;51(5):1247–1252. doi:10.1093/jac/dkg187 | ||
Frossard M, Joukhadar C, Burgmann H, et al. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob Agents Chemother. 2000;44:2728–2732. | ||
Docobo-Pérez F, Drusano GL, Johnson A, et al. Pharmacodynamics of fosfomycin: insights into clinical use for antimicrobial resistance. Antimicrob Agents Chemother. 2015;59:5602–5610. doi:10.1128/AAC.00752-15 | ||
Dijkmans AC, Zacarías NV, Burggraaf J, et al. Fosfomycin: pharmacological, clinical and future perspectives. Antibiotics. 2017;6(4):24. doi:10.3390/antibiotics6040024 | ||
Popovic M, Steinort D, Pillai S, Joukhadar C. Fosfomycin: an old, new friend? Eur J Clin Microbiol Infect Dis. 2010;29:127–142. doi:10.1007/s10096-009-0833-2 | ||
Kahan FM, Kahan JS, Cassidy PJ, Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann N Y Acad Sci. 1974;235:364–386. doi:10.1111/nyas.1974.235.issue-1 | ||
Brown ED, Vivas EI, Walsh CT, Kolter R. MurA (MurZ), the enzyme that catalyzes the first committed step in peptidoglycan biosynthesis, is essential in Escherichia coli. J Bacteriol. 1995;177:4194–4197. doi:10.1128/jb.177.14.4194-4197.1995 | ||
Silver LL. Fosfomycin: mechanism and resistance. Cold Spring Harb Perspect Med. 2017;7:a025262. doi:10.1101/cshperspect.a025262 | ||
Kahan FM, Kahan JS, Cassidy PJ, Kropp H. The mechanism of action of fosfomycin (phosphonomycin). Ann NY Acad Sci. 1974;235:364–386. doi:10.1111/nyas.1974.235.issue-1 | ||
Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents. 2009;34(6):506–515. doi:10.1016/j.ijantimicag.2009.08.013 | ||
Mazzei T, Cassetta MI, Fallani S, Arrigucci S, Novelli A. Pharmacokinetic and pharmacodynamic aspects of antimicrobial agents for the treatment of uncomplicated urinary tract infections. Int J Antimicrob Agents. 2006;28:S35–S41. doi:10.1016/j.ijantimicag.2006.05.019 | ||
Ribes S, Taberner F, Domenech A, et al. Evaluation of fosfomycin alone and in combination with ceftriaxone or vancomycin in an experimental model of meningitis caused by two strains of cephalosporin-resistant Streptococcus pneumoniae J Antimicrob Chemother. 2006;57:931–936. doi:10.1093/jac/dkl047 | ||
Pfausler B, Spiss H, Dittrich P, Zeitlinger M, Schmutzhard E, Joukhadar C. Concentrations of fosfomycin in the cerebrospinal fluid of neurointensive care patients with ventriculostomyassociated ventriculitis. J Antimicrob Chemother. 2004;53:848–852. doi:10.1093/jac/dkh158 | ||
Hamilton-Miller JM. In vitro activity of fosfomycin against ‘problem’ gram-positive cocci. Microbios. 1992;71:95–103. | ||
Duez JM, Mousson C, Siébor E, et al. Fosfomycin and its application in the treatment of multidrug-resistant Enterobacteriaceae infections. Clin Med Rev Ther. 2011;3:123–142. | ||
Frossard M, Joukhadar C, Erovic BM, et al. Distribution and antimicrobial activity of fosfomycin in the interstitial fluid of human soft tissues. Antimicrob Agents Chemother. 2000;44:2728–2732. | ||
Joukhadar C, Klein N, Dittrich P, et al. Target site penetration of fosfomycin in critically ill patients. J Antimicrob Chemother. 2003;51:1247–1252. doi:10.1093/jac/dkg187 | ||
Kwan KC, Wadke DA, Foltz EL. Pharmacokinetics of phosphonomycin in Man. I. Intravenous administration. J Pharm Sci. 1971;60:678–685. | ||
Roussos N, Karageorgopoulos DE, Samonis G, Falagas ME. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of fosfomycin for the treatment of patients with systemic infections. Int J Antimicrob Agents. 2009;34:506–515. doi:10.1016/j.ijantimicag.2009.08.013 | ||
Reffert JL, Smith WJ. Fosfomycin for the treatment of resistant gram-negative bacterial infections. Insights from the society of infectious diseases pharmacists. Pharmacotherapy. 2014;34:845–857. doi:10.1002/phar.1434 | ||
Falagas ME, Vouloumanou EK, Samonis G, Vardakas KZ. Fosfomycin. Clin Microbiol Rev. 2016;29:321–347. doi:10.1128/CMR.00068-15 | ||
Michalopoulos AS, Livaditis IG, Gougoutas V. The revival of fosfomycin. Int J Infect Dis. 2011;15:e732–e739. doi:10.1016/j.ijid.2011.07.007 | ||
Infectopharm Arzneimittel und Consilium GmbH. Fomicyt Package Insert. Heppenheim, Germany: Infectopharm; 2015. | ||
Florent A, Chichmanian RM, Cua E, Pulcini C. Adverse events associated with intravenous fosfomycin. Int J Antimicrob Agents. 2011;37:82–83. doi:10.1016/j.ijantimicag.2010.09.002 | ||
Dellit TH, Owens RC, McGowan JE, et al. Infectious diseases society of America and the society for healthcare epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44:159–177. doi:10.1086/510393 | ||
Safdar N, Handelsman J, Maki DG. Does combination antimicrobial therapy reduce mortality in gram-negative bacteraemia? A meta-analysis. Lancet Infect Dis. 2004;4:519–527. doi:10.1016/S1473-3099(04)01108-9 | ||
Kastoris AC, Rafailidis PI, Vouloumanou EK, GkegkesMatthew ID, Falagas E. Synergy of fosfomycin with other antibiotics for gram-positive and gram-negative bacteria. Eur J Clin Pharmacol. 2010;66:359–368. doi:10.1007/s00228-010-0794-5 | ||
Samonis G, Maraki S, Karageorgopoulos DE, Vouloumanou EK, Falagas ME. Synergy of fosfomycin with carbapenems, colistin, netilmicin, and tigecycline against multidrug-resistant Klebsiella pneumoniae, Escherichia coli, and Pseudomonas aeruginosa clinical isolates. Eur J Clin Microbiol Infect Dis. 2012;31:695–701. doi:10.1007/s10096-011-1360-5 | ||
Grossato A, Sartori R, Fontana R. Effect of non-b-lactam antibiotics on penicillin-binding protein synthesis of enterococcus hirae ATCC 9790. J Antimicrob Chemother. 1991;27:263–271. | ||
Totsuka K, Uchiyama T, Shimizu K, Kanno Y, Takata T, Yoshida T. In vitro combined effects of fosfomycin and b-lactam antibiotics against penicillin-resistant Streptococcus pneumoniae. J Infect Chemother. 1997;3:49–54. doi:10.1007/BF02489184 | ||
Yamada S, Hyo Y, Ohmori S, Ohuchi M. Role of ciprofloxacin in its synergistic effect with fosfomycin on drug-resistant strains of Pseudomonas aeruginosa. Chemotherapy. 2007;53:202–209. doi:10.1159/000098419 | ||
Okazaki M, Suzuki K, Asano N, et al. Effectiveness of fosfomycin combined with other antimicrobial agents against multidrug-resistant Pseudomonas aeruginosa isolates using the efficacy time index assay. J Infect Chemother. 2002;8:37–42. doi:10.1007/s101560200004 | ||
Tessier F, Quentin C. In vitro activity of fosfomycin combined with ceftazidime, imipenem, amikacin, and ciprofloxacin against Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis. 1997;16:159–162. | ||
Martinez-Martinez L, Rodriguez G, Pascual A, Suárez AI, Perea EJ. In vitro activity of antimicrobial agent combinations against multiresistant Acinetobacter baumannii. J Antimicrob Chemother. 1996;38:1107–1108. doi:10.1093/jac/38.6.1107 | ||
Santimaleeworagun W, Wongpoowarak P, Chayakul P, Pattharachayakul S, Tansakul P, Garey KW. In vitro activity of colistin or sulbactam in combination with fosfomycin or imipenem against clinical isolates of carbapenem-resistant Acinetobacter baumannii producing OXA-23 carbapenemases. Southeast Asian J Trop Med Public Health. 2011;42:890–900. | ||
Inouye S, Watanabe T, Tsuruoka T, Kitasato I. An increase in the antimicrobial activity in vitro of fosfomycin under anaerobic conditions. J Antimicrob Chemother. 1989;24:657–666. doi:10.1093/jac/24.5.657 | ||
Yanagida C, Ito K, Komiya I, Horie T. Protective effect of fosfomycin on gentamicin-induced lipid peroxidation of rat renal tissue. Chem Biol Interact. 2004;148:139–147. doi:10.1016/j.cbi.2004.05.005 | ||
Nakamura T, Kokuryo T, Hashimoto Y, Inui KI. Effects of fosfomycin and imipenem-cilastatin on the nephrotoxicity of vancomycin and cisplatin in rats. J Pharm Pharmacol. 1999;51:227–232. | ||
Castaneda-Garcia A, Blazquez J, Rodriguez-Rojas A. Molecular mechanisms and clinical impact of acquired and intrinsic fosfomycin resistance. Antibiotics. 2013;2:217–236. doi:10.3390/antibiotics2020217 | ||
Tsuruoka T, Yamada Y. Charactertization of spontaneous fosfomycin (phosphonomycin)-resistant cells of Escherichia coli B in vitro. J Antibiot. 1975;28:906–911. doi:10.7164/antibiotics.28.906 | ||
Kadner RJ, Winkler HH. Isolation and characterization of mutations affecting the transport of hexose phosphates in Escherichia coli. J Bacteriol. 1973;113:895–900. | ||
Kim DH, Lees WJ, Kempsell KE, Lane WS, Duncan K, Walsh CT. Characterization of a Cys115 to Asp substitution in the Escherichia coli cell wall biosynthetic enzyme UDP-GlcNAc enolpyruvyl transferase (MurA) that confers resistance to inactivation by the antibiotic fosfomycin. Biochemistry. 1996;35:4923–4928. doi:10.1021/bi952937w | ||
Horii T, Kimura T, Sato K, Shibayama K, Ohta M. Emergence of fosfomycin-resistant isolates of shiga-like toxin-producing Escherichia coli O26. Antimicrob Agents Chemother. 1999;43:789–793. doi:10.1128/AAC.43.4.789 | ||
Rigsby RE, Fillgrove KL, Beihoffer LA, Armstrong RN. Fosfomycin resistance proteins: A nexus of glutathione transferases and epoxide hydrolases in a metalloenzyme superfamily. Methods Enzymol. 2005;401:367–379. | ||
Kobayashi S, Kuzuyama T, Seto H. Characterization of the fomA and fomB gene products from Streptomyces wedmorensis, which confer fosfomycin resistance on Escherichia coli. Antimicrob Agents Chemother. 2000;44:647–650. | ||
Kuzuyama T, Kobayashi S, O’Hara K, Hidaka T, Seto H. Fosfomycin monophosphate and fosfomycin diphosphate, two inactivated fosfomycin derivatives formed by gene products of fomA and fomB from a fosfomycin producing organism Streptomyces wedmorensis. J Antibiot. 1996;49:502–504. doi:10.7164/antibiotics.49.502 | ||
Farhadi T, Hashemian SMR. Computer-aided design of amino acid-based therapeutics: A review. Drug Des Devel Ther. 2018;12:1239–1254. doi:10.2147/DDDT.S159767 | ||
Farhadi T, Fakharian A, Hashemian SM. Affinity improvement of a humanized antiviral antibody by structure-based computational design. Int J Pept Res Ther. 2017;25(1):181–186. | ||
Farhadi T, Ranjbar MM. Designing and modeling of complex DNA vaccine based on MOMP of Chlamydia trachomatis: an in silico approach. Netw Model Anal Health Inform Bioinforma. 2017;6(1). doi:10.1007/s13721-016-0142-5 | ||
Farhadi T, Ovchinnikov RS, Ranjbar MM. In silico designing of some agonists of toll-like receptor 5 as a novel vaccine adjuvant candidates. Netw Model Anal Health Inform Bioinforma. 2016;5(31). doi:10.1007/s13721-016-0138-1 | ||
Farhadi T, Nezafat N, Ghasemi Y. In silico phylogenetic analysis of Vibrio cholera isolates based on three housekeeping genes. Int J Comput Biol and Drug Des. 2015;8(1):62–74. doi:10.1504/IJCBDD.2015.068789 | ||
Hashemian SMR, Farhadi T, Ganjparvar M. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther. 2018;2018(12):1759–1767. doi:10.2147/DDDT.S164515 | ||
Farhadi T, Hashemian SMR. Constructing novel chimeric DNA vaccine against Salmonella enterica based on SopB and GroEL proteins: an in silico approach. J Pharm Invest. 2018;48(6):639–655. | ||
Farhadi T, Fakharian A, Ovchinnikov RS. Virtual screening for potential inhibitors of CTX-M-15 protein of Klebsiella pneumoniae. Interdiscip Sci: Comput Life Sci. 2018;10(4):694–703. | ||
Farhadi T. In silico designing of peptide inhibitors against pregnane X receptor: the novel candidates to control drug metabolism. Int J Pept Res Ther. 2018;24(3):409–420. | ||
Hyatt JM, McKinnon PS, Zimmer GS, Schentag JJ. The importance of pharmacokinetic/pharmacodynamics surrogate markers to outcome. Focus on antibacterial agents. Clin Pharmacokinet. 1995;28:143–160. doi:10.2165/00003088-199528020-00005 | ||
Schentag JJ, Nix DE, Adelman MH. Mathematical examination of dual individualization principles (I): relationships between AUC above MIC and area under the inhibitory curve for cefmenoxime, ciprofloxacin, and tobramycin. DICP: Ann Pharmacother. 1991;25:1050–1057. doi:10.1177/106002809102501003 | ||
Crokaert F. Pharmacodynamics, a tool for a better use of antibiotics? Intensive Care Med. 2001;27:340–343. doi:10.1007/s001340100865 | ||
Joukhadar C, Frossard M, Mayer BX, et al. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit Care Med. 2001;29:385–391. doi:10.1097/00003246-200102000-00030 | ||
Brunner M, Pernerstorfer T, Mayer BX, Eichler HG, Müller M, Surgery and intensive care procedures affect the target site distribution of piperacillin. Crit Care Med. 2000;28:1754–1759. doi:10.1097/00003246-200006000-00009. | ||
Joukhadar C, Klein N, Mayer BX, et al. Plasma and tissue pharmacokinetics of cefpirome in patients with sepsis. Crit Care Med. 2002;30:1478–1482. doi:10.1097/00003246-200207000-00013 | ||
De La Pena A, Dalla Costa T, Talton JD, et al. Penetration of cefaclor into the interstitial space fluid of skeletal muscle and lung tissue in rats. Pharm Res. 2001;18:1310–1314. | ||
Tegeder I, Schmidtko A, Bräutigam L, Kirschbaum A, Geisslinger G, Lötsch J. Tissue distribution of imipenem in critically ill patients. Clin Pharmacol Ther. 2002;71(5):325–333. doi:10.1067/mcp.2002.122526 | ||
Asuphon O, Montakantikul P, Houngsaitong J, Kiratisin P, Sonthisombat P. Optimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulation. Int J Infect Dis. 2016;50:23–29. doi:10.1016/j.ijid.2016.06.017 |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
