Back to Journals » Infection and Drug Resistance » Volume 14
Foscarnet Therapy for Pure Red Cell Aplasia Related to Human Parvovirus B19 Infection in Kidney Transplant Recipients: A Preliminary Exploration
Authors Yu Y, Bao R, Lyu J, Wu J, Chen J, Peng W
Received 26 May 2021
Accepted for publication 21 July 2021
Published 27 July 2021 Volume 2021:14 Pages 2911—2923
DOI https://doi.org/10.2147/IDR.S321936
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Yedong Yu,1 Ruijie Bao,1 Junhao Lyu,1– 4 Jianyong Wu,1– 4 Jianghua Chen,1– 4 Wenhan Peng1– 4
1Department of Kidney Disease Center, The First Affiliated Hospital Zhejiang University School of Medicine, Hangzhou, People’s Republic of China; 2Department of Kidney Disease Immunology Laboratory, State Administration of Traditional Chinese Medicine of China, Hangzhou, People’s Republic of China; 3Department of Key Laboratory of Multiple Organ Transplantation, Ministry of Health of China, Hangzhou, People’s Republic of China; 4Institute of Nephropathy, Zhejiang University, Hangzhou, People’s Republic of China
Correspondence: Wenhan Peng
Kidney Disease Center, The First Affiliated Hospital, College of Medicine, Zhejiang University, 79 Qingchun Road, Hangzhou, 310003, People’s Republic of China
Tel +86 571-87236871
Email [email protected]
Background: Parvovirus B19-associated pure red cell aplasia (PVB19-PRCA) is an uncommon but serious complication after kidney transplantation. Currently, intravenous immunoglobulin (IVIG) is preferred as the first-line treatment for PVB19-PRCA, but presents with disadvantages of disease recurrence and expensive cost. In this context, we propose that foscarnet therapy for kidney transplantation recipients (KTR) with PVB19-PRCA may be an alternative scenario. No related study has been reported, and we performed this study to assess the efficacy and safety of foscarnet for PVB19-associated PRCA in KTR.
Methods: We conducted a retrospective review of PVB19-PRCA in KTR at our center over 9-year period. The data on therapy and outcomes in all cases treated with foscarnet are detailed records and summarized.
Results: Among our 68 patients, PVB19-PRCA was confirmed in 50 based on inclusion/exclusion criteria. All patients presented with refractory anemia and low reticulocyte percentage (< 0.5%), the mean hemoglobin of patients was 79.8± 12.6g/L at the time of PVB19-PRCA was identified. The median serum genome copy number of parvovirus B19 at diagnosis was 9.6 log10 copies per milliliter. A total of 11 patients received foscarnet therapy, of 10 patients responded well to the treatment and maintained no recurrence. But 1 patient had a poor response to foscarnet therapy. Except for this patient, the mean hemoglobin level gradually increased from 68.5± 9.3 g/L to 73.2± 8.8 g/L, and the mean percentage of reticulocytes steadily increased from 0.1± 0.0% to 7.6± 2.9% after foscarnet therapy. The median serum genome copy number of parvovirus B19 decreased from 9.8 log10 to 6.1 log10 copies per milliliter. There was no significant difference (P=0.61, 0.60) in serum creatinine and glomerular filtration rate before and after foscarnet treatment. At the latest follow-up, the mean hemoglobin was 131.5± 12.5 g/L and the hemoglobin correction occurred in all patients.
Conclusion: Foscarnet therapy doesn’t seem to be worse than IVIG for PVB19-PRCA in KTR, and it can be an alternative option.
Keywords: human parvovirus B19, pure red cell aplasia, foscarnet therapy, kidney transplantation
Introduction
Human parvovirus B19 is a small non-enveloped single-stranded linear DNA virus of the family Parvoviridae.1 In 1975, Yvonne Cossart first discovered parvovirus particles, which were detected in the serum number 19 of panel B, hence the name.2 It has been proven that parvovirus B19 targets the erythroid precursor progenitors predominantly in the bone marrow, inducing apoptosis in infected target cell and resulting in acute and chronic anemia.3 Parvovirus B19 can have a variety of clinical manifestations under diverse situations. For immunocompromised population, the common phenomenon in the majority of patients is pure red cell aplasia. It is defined by the lack of mature erythroid erythroblasts, but with the presence of giant pronormoblasts in the bone marrow.4–6 Owing to the immunosuppression status, kidney transplant recipients were particularly prone to acquire parvovirus B19 infection. The persistence of refractory anemia with reticulocytopenia in KTR should be alerted for PVB19-PRCA, which posed a potential serious threat to the allograft and recipient survival.7 At present, there is no codified protocol of treatment in terms of PVB19-PRCA. In the literature review, intravenous immunoglobulin (IVIG) is found to be beneficial in kidney transplant recipients with PVB19-PRCA. Nevertheless, the recurrence rate of PRCA was 34%, and the one-year success rate was 47% following IVIG therapy.8 Due to the high cost of the IVIG, repeated relapses have increased the economic burden on individuals even in society, not to mention the side effects of IVIG. Foscarnet is an antivirus drug, which is mainly used to treat CMV, HSV and VZV viral infection in immunocompromised patients. As an analog of pyrophosphate, foscarnet can inhibit the DNA chains from extending further by reversibly and selectively blocking the binding site of the viral DNA polymerase enzyme.9 Based on these findings, we initially attempted to use foscarnet in KTR with PVB19-PRCA who were ineffective in multiple courses of IVIG therapy. To our satisfaction, we found that foscarnet therapy seems to be working well in these patients. Therefore, on this basis, we preliminarily explored the efficacy and safety of foscarnet therapy in kidney transplantation recipients with new-onset PVB19-PRCA.
Patients and Methods
Study Population
We retrospectively reviewed all cases of KTR with PVB19-PRCA between January 2011 and December 2019 in the center of kidney disease, the first affiliated hospital, College of Medicine, Zhejiang University. Recipients, who received kidney transplantation in combination with another organ, were ABO-incompatible kidney transplantation, and those blood specimens that could not be retained were excluded. We collected all relevant information on their demographics, clinical characteristics, treatment and outcomes. Then, we focused on analysis of the data on therapeutic scenario (previous therapy, duration and dose of foscarnet, adjustment in immunosuppression regimen, blood transfusion) and outcomes (changes in hemoglobin, virologic response, serum creatinine (Scr) and the estimated glomerular filtration rate (eGFR) at the start and end of foscarnet therapy, follow-up, death, cause of death, the latest hemoglobin and the parvovirus B19 copy number) in all cases treated with foscarnet. All data were collected from electronic medical record and kidney transplantation database at our center.
Definitions
KTR with PVB19-PRCA were included in our study according to the following criteria: (1) severe progressive anemia with no response to erythropoietin (the lowest hemoglobin <10g/dL), in the absence of active bleeding, iron and folate deficiency, neoplasms, autoimmune, chronical graft dysfunction and drug-induced PRCA after renal transplantation. (2) Reticulocytes < 0.5% (3) Standard qualitative or quantitative whole-blood polymerase chain reaction (PCR) positive of Parvovirus B19 (PVB19 DNA level was more than 6 log10 copies per milliliter). Human parvovirus B19 DNA was detected by commercial real-time PCR kit (LiferiverTM Shanghai ZJ Biotechnology Co, Ltd) in whole-blood. Due to the serological detection of immunocompromised patients was unreliable, no antibody of parvovirus B19 was detected in this study. In our study, KTR with good graft function refer to the estimated glomerular filtration rate (eGFR) of more than 45mL/min/1.73m2. The eGFR is estimated with the Chronic Kidney Disease Epidemiology Collaboration CKD-EPI formula. Correction of hemoglobin is defined as hemoglobin level more than 10g/dL or back to the level before PVB19-PRCA diagnosis.
Treatment
Recipients who received IVIG therapy were given IVIG 20g/day for 5–10 consecutive days and adjusted for immunosuppression. In case of nonresponse to the first IVIG course or recurrence, another course of IVIG (20g/day for 5–10 days) may be given. In situation of recurrence of IVIG therapy, we decided to switch from IVIG to foscarnet therapy after written informed consent was obtained from patients.
Recipients who received foscarnet therapy were given foscarnet 6g/day for 5–14 consecutive days and adjusted the immunosuppression. Recipients treated with foscarnet were given 1000 milliliters per day of adequate hydration and were monitored with daily serum creatinine and urine volume closely to prevent foscarnet-associated acute kidney injury.
Induction and Maintenance Immunosuppression
At our center, induction regimens include rabbit anti-thymocyte globulin (ATG, 1 mg/kg/day and a maximum of 5 doses) or interleukin-2 receptor antagonist (basiliximab, 20 mg, at days 0 and 4). All patients received methylprednisolone 10 mg/kg/day on day 0, 1 and 2. Dosage of prednisone was slowly reduced from 60 to 10 mg/d within one month after KT. Standard triple-drug maintenance immunosuppression consisted of prednisone, tacrolimus, and mycophenolate (EC-MPS or MMF).
Statistical Analysis
In this study, age, hemoglobin, reticulocytes percentage, serum creatinine, estimated glomerular filtration rate (eGFR), the doses of IVIG and foscarnet are described in the form of mean plus-minus standard deviation (mean±SD). The serum parvovirus B19 genome copy number and time of parvovirus B19 infection were described as median values with ranges. The copy number of parvovirus B19 genome is expressed by logarithm. Categorical variables are expressed as percentages. Reported P-values were two-sided, and P-values <0.05 were considered statistically significant. All analyses were performed using the GraphPad prism 8 software for Windows (Version 8.02).
Ethical Consideration
The study was approved by the first affiliated hospital of Zhejiang University Ethics Committee Board (Reference Number:20210212A), and all the involved activities were conformed the ethical guidelines of the Declaration of Helsinki. Kidney donations and transplants were also conducted in accordance with the Declaration of Istanbul. Before transplantations, all patients gave written informed consent to use their sample and data for medical research. Individual informed consent for this observational study was obtained before foscarnet therapy. Deceased donor kidneys in this study were procured from donation after the citizens’ death in accordance with the guidelines of the National Program for Deceased Organ Donation in China. There is a kinship in all living donor and the corresponding recipients. All donations were voluntary, unpaid and no organs were obtained from prisoners.
Result
Baseline Characteristics
From January 2011 to December 2019, we performed 3328 renal transplantations at our center. Over 9-year period, 68 patients were identified with human parvovirus B19 infection. According to the exclusion criteria, 10 recipients were excluded in this study and only 50 patients fulfilled the included criteria for PRCA (Figure 1). For the first-onset of PVB19-PRCA, 45 patients received IVIG therapy, 5 patients received foscarnet therapy, respectively. In the course of treatment, we switched from IVIG to foscarnet treatment in 6 patients who had 1 or more relapses after several courses of IVIG therapy. We further evaluated the therapeutic effect and safety of foscarnet therapy in a total of 11 patients.
 |
Figure 1 Final diagnosis of 68 patients for PVB19 infection between 2011 to 2019. Abbreviations: PVB19-PRCA, parvovirus B19-associated pure red cell aplasia; IVIG, intravenous immunoglobulin. |
For 50 included patients, the demographic, clinical characteristic, treatment and outcomes are described in Table 1. The mean age was 34.7±10.4 years, and 31 (62%) were living donor KTR; 41 patients received induction therapy, 34 (83%) with basiliximab and 7 (17%) with anti-human T lymphocyte rabbit immunoglobulin (ATG). All patients received triple immunosuppression (IS) regimens with prednisone, tacrolimus, and mycophenolate mofetil. The median time to PVB19-PRCA after KT was 34.5 days (ranging from 7 to 666 days). Forty-six patients developed PVB19 infection within 3 months after transplantation, but 1 patient had a late-onset (666 days after KT) infection.
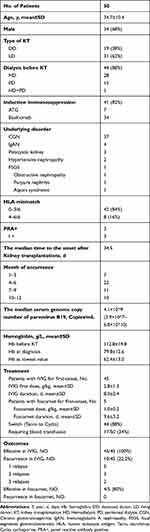 |
Table 1 Demographic, Clinical Characteristic, Treatment and Outcomes of 50 Patients Who Were Confirmed PVB19-PRCA |
Clinical Feature
All patients presented variable degrees of refractory anemia, and the mean hemoglobin of 50 patients was 112.8±14.8 g/L before KT, 79.8 g±12.6g/L at the time of PVB19-PRCA was identified. In the process of infection, the lowest value of hemoglobin was 62.4±13.0 g/L. All patients had a low reticulocyte percentage (<0.5%). Thirty-three recipients underwent a bone marrow examination, 19 (57.6%) demonstrated PRCA. The median serum genome copy number of parvovirus B19 at diagnosis was 9.6 log10 copies per milliliter (ranging from 7.6 log10 to 10.8 log10 per milliliter). No patients showed up with skin, joint, liver, cardiac or neurological involvement.
Treatment
Forty-five patients received IVIG therapy and 5 patients received foscarnet therapy for the first-onset of PVB19-PRCA. At the time of PRCA, the mean tacrolimus trough levels were 6.47 ng/mL, 6.55 ng/mL in IVIG group and foscarnet group, respectively. And the median daily doses for MMF were 1000 mg, and for EC-MPS, it was 720 mg in both groups. Forty-four patients (88%) were switched from tacrolimus to cyclosporine. And the IS regimens were decreased in all patients upon diagnosis of the disease. Seventeen patients required blood transfusion with an average of 2.3 (range,1–6) packed red blood cells per patient. In summary, for the first course, 45 patients received IVIG at a mean of 2.8±1.3 g/kg for 8.0±2.4 days (ranging from 3 to 16 days) and 5 patients received foscarnet therapy at a mean of 1.0±0.2g/kg for 9.6±3.2 days (ranging from 7 to 14 days).
Outcome
Forty-five patients received IVIG for the first-onset of PVB19-PRCA, and they all had a good response to the treatment. However, 5 patients developed one relapse following the administration of IVIG therapy, 3 patients recurred two times, 2 patients recurred three times and 35 patients did not relapse. There were 6 recipients switched from IVIG to foscarnet therapy due to relapses of IVIG. Hence, a total of 11 patients were treated with foscarnet therapy, and detailed information is shown in Table 2. Ten patients responded well to the foscarnet treatment and maintained no recurrence. However, 1 patient had a poor response to foscarnet therapy. Eventually, following additional 2 courses of IVIG, the patient’s condition was well controlled. Except for this patient, the mean hemoglobin level gradually increased from 68.5±9.3 g/L to 73.2±8.8 g/L, and the lowest mean hemoglobin value was 56.5±8.2g/L during foscarnet therapy (Figure 2). The mean percentage of reticulocytes steadily increased from 0.1±0.0% to 7.6±2.9% (Figure 3). The median serum genome copy number of parvovirus B19 decreased from 9.8 log10 to 5.8 log10 copies per milliliter. 1 month and 3 months after foscarnet therapy, the mean hemoglobin was 112.5±8.8 g/L,125.2±9.0 g/L, respectively. Before the foscarnet therapy, Scr were at a mean of 122.9±29.5μmol/L, and the estimated glomerular filtration rate (eGFR) was at a mean of 57.7±11.4 mL/min/1.73m2 in 11 patients. Accordingly, the Scr were 133.5±35.9 μmol/l and the eGFR were 56.1±14.8 mL/min/1.73m2 respectively after the end of foscarnet therapy. There were no significant differences (P=0.61, 0.60) in serum creatinine and eGFR before and after foscarnet treatment. Foscarnet is well tolerated without the development of any severe side effects in all patients. During follow-up, unfortunately 1 patient died of pulmonary infection and meningitis 8 months after kidney transplantation but with good allograft function and other 10 patients remain alive. The median follow-up durations were 19.5 months (ranging from 13 to 114 months) in 10 recipients. Follow-up date for blood B19 DNA PCR was obtained for 8 patients and only one patient turned into negative within 10 months after transplantation. Seven patients had low-level-viremia and no clinical manifestations of parvovirus B19 infection during follow-up (Figure 4). At the latest follow-up, the mean hemoglobin was 131.5±12.5 g/L and the hemoglobin correction occured in all patients (Table 3).
 |  |  |
Table 2 Demographic, Clinical Characteristics, Treatment and Outcomes of 11 Patients with PVB19-PRCA Treated with Foscarnet Therapy |
 |
Table 3 The Clinical Characteristics of 10 Patients with Foscarnet Therapy Effectively for PVB19-PRCA |
 |
Figure 2 The change trend of hemoglobin in 11 patients treated with foscarnet therapy. |
 |
Figure 3 The change trend of percentage of reticulocytes in 11 patients treated with foscarnet therapy. |
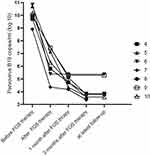 |
Figure 4 The change trend of serum genome copy number of parvovirus B19 in 7 patients effectively treated with foscarnet therapy. |
Discussion
We retrospectively reviewed the data for a series of 50 patients with PVB19-PRCA at our center over a 9-year period. From the literature, the total incidence of parvovirus B19 DNA positive in renal transplant patients is estimated at 10.3% and PVB19-PRCA occurred in only 3% renal transplantation recipient.10 Since parvovirus B19 DNA detections were routinely carried out in KTR after year of 2015, the overall incidence of PVB19-PRCA over 5-year period (year of 2015 to 2019) at our center was up to 2.3% (48/2113). Approximately 70% of PVB19-PRCA occurred within the first three months after kidney transplantations and the median time of parvovirus B19 infection was 1.25–2 months after KT.11–13 In our study, 46 (92%) recipients occurred PVB19-PRCA within first three months and the median time of diagnosis was 34.5 days, both of which were in good agreement with literature reports. Infections occur throughout the year, but especially in late winter to early summer.14 Interestingly, there are a large number of cases occur from April to June in our cases, which is also in accordance with the literature.
PVB19-PRCA can be diagnosed by the direct detection with a viral DNA PCR test or bone marrow examination, especially in recipients who presented progressive anemia, reticulocytopenia, and do not respond well to erythropoietin.15 In our study, all patients had typical clinical manifestations as well as the result of viral detection were positive. In PRCA, bone-marrow examination shows giant pronormoblasts or the absence of erythroid precursors. However, it was performed in 33 patients, only 19 cases demonstrated PRCA. One of the explanations for the low incidence of PRCA bone marrow evidence is probably the related to the operation time and quality of bone marrow materials.
From the literature reviewed, so far, the therapy options for PVB19-PRCA include any or all of the following: administration of IVIG, decreased immunosuppression regimen to improve recipient’s immune system, conservative therapy, including red-cell transfusion, erythropoietin or surveillance.14 IVIG containing antiparvovirus antibodies has been widely used and appears to be beneficial in a substantial number of kidney recipients with pure red cell aplasia related to human parvovirus B19 infection. However, the optimal duration and dosing regimen of IVIG therapy for parvovirus B19 infection have yet to be established. The effective dose of IVIG for most patients with up to 2.3±1.3g/kg per course. Unfortunately, the recurrence rate of IVIG in a heterogeneous population is up to 33%.8 In our cases, the mean dose of IVIG was 2.8±1.3g/kg in 45 patients for the first-onset of PVB19-PRCA. Among them, 10 of 45 (22.2%) patients had relapse with varying number of times and then were given IVIG again after recurrence, including 5 patients presented only 1 relapse and 5 patients developed 2 or more relapses. Furthermore, IVIG has potential toxicity and its cost is rather high, especially for patients with multiple relapses. The common side effects of IVIG treatment include fever, shivering, fatigue, headache, nausea, vomiting, myalgia, hypertension and renal failure.16,17 Based on this background, we attempted to practice foscarnet therapy as an exploratory treatment in 6 patients with a good allograft function (eGFR >45mL/min) but relapse after IVIG therapy. To our satisfaction, the outcomes were not bad. Therefore, the therapeutic effect and safety were observed in 5 patients treated with foscarnet for the first-onset of PVB19-PRCA. Although limited to 11 cases, to the best of our knowledge, this is the first reported series in the treatment of parvovirus B19 infection with foscarnet therapy. Nowadays, there are no specific antiviral drugs for the treatment of parvovirus B19 infection and the B19 vaccine project is not available.14
Foscarnet as a broad-spectrum and second-line antiviral drug, which is mainly used in the treatment of ganciclovir-resistant cytomegalovirus (CMV) infection in renal transplant recipient. As an analog of pyrophosphate, foscarnet can inhibit the DNA chains from extending further by reversibly and selectively blocking the binding site of the viral DNA polymerase enzyme. In our study of parvovirus as a single-stranded linear DNA virus, we make use of this feature to attempt the use of foscarnet therapy in KTR with PVB19-PRCA. The most important adverse effect of foscarnet is nephrotoxicity, but the renal function and pathological changes are reversible, as long as the fibrosis is not serious and the patient receives enough hydration.18 We selected patients with good allograft function (the eGFR >45mL/min/1.73m2) to be treated with foscarnet therapy and given 1000 mL per day adequate hydration. Iwami et al reported that a renal transplant patient with ganciclovir-resistant cytomegalovirus infection was treated successfully with foscarnet. Nevertheless, after a period of time, kidney allograft biopsy revealed crystal deposition in the tubules, suggesting tubulointerstitial damage perhaps caused by foscarnet. However, allograft damage recovered spontaneously after foscarnet discontinuation.19 This illustrates that foscarnet is relatively safe in renal recipients with good allograft function, but it is necessary to closely observe the changes of renal function.
Overall, we have got satisfying result of foscarnet therapy for PVB19-PRCA in KTR. In our study, clinical observation showed that the effective rate of IVIG reached 100% but the recurrence rate was 22.2% (10/45). In foscarnet therapy, the effective rate was 90.9% (10/11) and there was no recurrence. Although these results suggest that the foscarnet therapy had almost no relapse once it works, they remain inconclusive due to the low number of patients. It is regrettable that 1 patient had a poor response to foscarnet therapy and her percentage of reticulocytes was 0.2% after treatment. Except for this patient, we can observe in other 10 patients that there is a significant improvement in hemoglobin and no significant deterioration in allograft function after foscarnet therapy (Table 3), indicating that foscarnet is effective and relatively safe in these patients and can be used as an alternative therapy in addition to IVIG therapy. During follow-up, 10 patients did not show clinical or laboratory signs of PVB19 infection with a good outcome in terms of both renal function and graft survival except for 1 patient who died of pulmonary infection and meningitis. Parvovirus B19 DNA can be detected at low-grade levels in the peripheral blood of normal hosts within months or even years after primary infection and remission of symptoms.20–22 In our cases, only 1 patient was parvovirus B19 viremia negative, and the rest had ongoing viremia, which shows that our goal is to improve reticulocytes and hematocrit rather than generate a negative result of PVB19 DNA.
Several limitations of our study are worth declaring. First, the present study was limited by its small number cases and retrospective design. Another limitation was that there was no periodic detection of PVB19 quantitative PCR during follow-up to evaluate virologic behavior and the extermination of viremia after the improvement of hemoglobin and reticulocytes. However, PVB19 DNA PCR at low level are allowed as long as there is no obvious drop in reticulocyte count and hematocrit. Lastly, some patients received blood transfusion owing to low hemoglobin levels, which can influence the assessment of efficacy of foscarnet to some extent.
In conclusion, foscarnet therapy seems to be effective and safe in KTR with PVB19-PRCA. Furthermore, foscarnet does not appear to be worse than IVIG. Therefore, we proposed that foscarnet may be an alternative therapy for PVB19-PRCA in KTR with good allograft function in addition to IVIG therapy. Broader, prospective, multicenter studies are needed to better define the optimal schedule of foscarnet treatment for kidney transplant recipients.
Acknowledgments
The authors thank technician Yin Chen for the detection of parvovirus B19.
Funding
This study was funded by the National health and Family planning Commission of the People’s Republic of China (WKJ-ZJ-1924) and National Natural Science Foundation of China (81770752, 81970651).
Disclosure
The authors report no conflicts of interest for this work.
References
1. Waldman M, Kopp JB. Parvovirus B19 and the kidney. Clin J Am Soc Nephrol. 2007;2(Suppl 1):S47–56. doi:10.2215/CJN.01060307
2. Cossart YE, Field AM, Cant B, Widdows D. Parvovirus-like particles in human sera. Lancet. 1975;1(7898):72–73. doi:10.1016/s0140-6736(75)91074-0
3. Servant-Delmas A, Morinet F. Update of the human parvovirus B19 biology. Transfus Clin Biol. 2016;23(1):5–12. doi:10.1016/j.tracli.2015.11.006
4. Kurtzman GJ, Cohen B, Meyers P, Amunullah A, Young NS. Persistent B19 parvovirus infection as a cause of severe chronic anaemia in children with acute lymphocytic leukaemia. Lancet. 1988;2(8621):1159–1162. doi:10.1016/s0140-6736(88)90233-4
5. Frickhofen N, Chen ZJ, Young NS, Cohen BJ, Heimpel H, Abkowitz JL. Parvovirus B19 as a cause of acquired chronic pure red cell aplasia. Br J Haematol. 1994;87(4):818–824. doi:10.1111/j.1365-2141.1994.tb06743.x
6. Ammus SS, Yunis AA. Acquired pure red cell aplasia. Am J Hematol. 1987;24(3):311–326. doi:10.1002/ajh.2830240312
7. Alangaden GJ, Thyagarajan R, Gruber SA, et al. Infectious complications after kidney transplantation: current epidemiology and associated risk factors. Clin Transplant. 2006;20(4):401–409. doi:10.1111/j.1399-0012.2006.00519.x
8. Crabol Y, Terrier B, Rozenberg F, et al. Intravenous immunoglobulin therapy for pure red cell aplasia related to human parvovirus b19 infection: a retrospective study of 10 patients and review of the literature. Clin Infect Dis. 2013;56(7):968–977. doi:10.1093/cid/cis1046
9. Garikapati S, Nguyen M. Foscarnet. StatPearls; 2021.
10. Thongprayoon C, Khoury NJ, Bathini T, et al. Epidemiology of parvovirus B19 and anemia among kidney transplant recipients: a meta-analysis. Urol Ann. 2020;12(3):241–247. doi:10.4103/UA.UA_89_19
11. Eid AJ, Brown RA, Patel R, Razonable RR. Parvovirus B19 infection after transplantation: a review of 98 cases. Clin Infect Dis. 2006;43(1):40–48. doi:10.1086/504812
12. An HPH, Diem HT, Cuong NT. Parvovirus B19-associated anemia in kidney transplant recipients: a single-center experience. Transplant Proc. 2019;51(8):2693–2696. doi:10.1016/j.transproceed.2019.03.076
13. Gallinella G, Manaresi E, Venturoli S, Grazi GL, Musiani M, Zerbini M. Occurrence and clinical role of active parvovirus B19 infection in transplant recipients. Eur J Clin Microbiol Infect Dis. 1999;18(11):811–813. doi:10.1007/s100960050406
14. Landry ML, Hayden RT, Wolk DM, Carroll KC, Tang Y-W. Parvovirus B19. Microbiol Spectr. 2016;4(3). doi:10.1128/microbiolspec.DMIH2-0008-2015
15. Eid AJ, Chen SF; AST Infectious Diseases Community of Practice. Human parvovirus B19 in solid organ transplantation. Am J Transplant. 2013;13(Suppl 4):201–205. doi:10.1111/ajt.12111
16. Plentz A, Wurdinger M, Kudlich M, Modrow S. Low-level DNAemia of parvovirus B19 (genotypes 1-3) in adult transplant recipients is not associated with anaemia. J Clin Virol. 2013;58(2):443–448. doi:10.1016/j.jcv.2013.07.007
17. Vo AA, Cam V, Toyoda M, et al. Safety and adverse events profiles of intravenous gammaglobulin products used for immunomodulation: a single-center experience. Clin J Am Soc Nephrol. 2006;1(4):844–852. doi:10.2215/CJN.01701105
18. Deray G, Martinez F, Katlama C, et al. Foscarnet nephrotoxicity: mechanism, incidence and prevention. Am J Nephrol. 1989;9(4):316–321. doi:10.1159/000167987
19. Iwami D, Ogawa Y, Fujita H, et al. Successful treatment with foscarnet for ganciclovir-resistant cytomegalovirus infection in a kidney transplant recipient: a case report. Nephrology (Carlton). 2016;21(Suppl 1):63–66. doi:10.1111/nep.12767
20. Kerr JR, Curran MD, Moore JE, Murphy PG. Parvovirus B19 infection–persistence and genetic variation. Scand J Infect Dis. 1995;27(6):551–557. doi:10.3109/00365549509047066
21. Cassinotti P, Schultze D, Schlageter P, Chevili S, Siegl G. Persistent human parvovirus B19 infection following an acute infection with meningitis in an immunocompetent patient. Eur J Clin Microbiol Infect Dis. 1993;12(9):701–704. doi:10.1007/BF02009384
22. Cassinotti P, Siegl G. Quantitative evidence for persistence of human parvovirus B19 DNA in an immunocompetent individual. Eur J Clin Microbiol Infect Dis. 2000;19(11):886–887. doi:10.1007/s100960000384
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
