Back to Journals » Drug Design, Development and Therapy » Volume 14
Formulation, Preparation and Evaluation of Nanostructured Lipid Carrier Containing Naringin and Coix Seed Oil for Anti-Tumor Application Based on “Unification of Medicines and Excipients”
Authors Zhu J, Huang Y, Zhang J, Feng Y, Shen L
Received 1 November 2019
Accepted for publication 18 March 2020
Published 16 April 2020 Volume 2020:14 Pages 1481—1491
DOI https://doi.org/10.2147/DDDT.S236997
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jianbo Sun
Jiayi Zhu,1 Yanling Huang,1 Jiquan Zhang,2 Yi Feng,2 Lan Shen1
1School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, People’s Republic of China; 2Engineering Research Center of Modern Preparation Technology of Traditional Chinese Medicine of Ministry of Education, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, People’s Republic of China
Correspondence: Lan Shen Tel +86 13916106844
Email [email protected]
Background: “Unification of medicines and excipients” is the special principle which means fatty oil with pharmacodynamic activity derived from traditional Chinese medicine are taken as liquid lipids in perparation for dual-drug delivery, which improve the treatment effect and reduce unnecessary excipients.
Purpose: The aim of this study was to prepare a nanostructured lipid carrier (NLC) with naringin (NG) containing coix seed oil (CSO) as liquid lipid based on the theory (NCNLC) in order to achieve synergistic antitumor activity against hepatocellular carcinoma.
Methods: We developed NCNLCs using ultrasonic melt-emulsification method. The antitumor effect in vivo/in vitro and drug release ability were compared to NLC prepared with conventional liquid lipids: neodecanoate triglycerides (NDNLC) and oleic acid (NONLC).
Results: Transmission electron microscopy showed that NCNLCs had a well-defined spherical shape, small size, and narrow polydispersity index. Importantly, the release of drugs from NDNLCs and NONLCs was slower than NCNLCs. In the cell study, the result showed a significantly greater antiproliferative effect towards HepG2 cells, and the half-maximal inhibitory concentration of NCNLCs was 3.24-fold, 1.70-fold and 1.52-fold lower to that of free drug, NDNLCs and NONLCs, respectively. Moreover, NCNLCs significantly induced HepG2 cells apoptosis by being 2.12-fold and 9.28-fold higher to that of NDNLCs and NONLCs, respectively. In the study of antitumor efficacy in vivo, the synergistic effect of NCNLCs formulation showed markedly enhanced antitumor efficacy in a xenograft model of liver cancer.
Conclusion: The advantages of “unification of medicines and excipients” in formulation characters, drug release and synergistic antitumor effect provide a new idea for the application of the fatty oil of traditional Chinese medicine in the nano-drug delivery for cancer therapy.
Keywords: unification of medicines and excipients, coix seed oil, nanostructured lipid carrier
Introduction
Hepatocellular carcinoma (HCC) is currently the third leading cause of malignancy-related mortalities worldwide.1 The two drugs that FDA has approved for treating advanced HCC, as a first line therapy, are sorafenib and lenvatinib.2,3 Nevertheless, the limitations of accessibility to these drugs in developing countries guarantee that HCC is still enormously restrict the clinical applications and underscore the need for new therapeutics.4,5 Compared to free drugs, nanoparticles have several advantages: passive targeted into the tumor site due to the enhanced permeability and retention (EPR) effect; reduced side effects, etc.6 Therefore, a number of strategies using nanoparticles such as Nano-structured lipid carrier (NLC), which is called the second generation lipid nanoparticles have been applied in the field of cancer therapy.7,8 By adding liquid lipids to solid lipids can delay drug leakage and increase drug entrapment efficiency and drug loading.9 The NLC is composed of a blend of the solid lipid and the liquid lipid leading to a crystal structure with more imperfections and therefore with more room for drug accommodation especially for hydrophobic drugs.10 Nowadays, fatty oil has an important application in many nanomaterials (NMs) of traditional Chinese medicine. Indeed, some active fatty oil enable effectively replace the liquid lipids to reduce unnecessary excipients and improve safety.11,12
According to the theory of “unification of medicines and excipients”, the curative effect of the preparation is not only determined by the activity of the active components, but also by selecting the liquid lipids with certain pharmacological activity.13 At present, coix seed oil (CSO) has been widely used as “unification of medicines and excipients” in microemulsion to treat HCC because of their anti-cancer effects and excellent excipient property.14,15 As for CSO, it has no adverse effects on heart, liver, kidney and hemopoietic system, which encourages patient compliance to improve.5 Furthermore, the anti-tumor effect of CSO involves inducing tumor apoptosis,16 inhibiting the formation of new tumor vessels, enhancing the immune function,17 modifying cytokine levels and reversing multidrug resistance.18 The application of Kanglaite (KLT) injection and soft capsule can be a powerful example. It is the first anti-HCC drug that its main ingredient is fatty oil from coix seed.16 For now, inspired by the“unification of medicines and excipients”, screening of CSO with the other synergistic drug, for example, flavonoids, encapsulating them into a nanoparticle (NP) system will be expected to render cancer cells more susceptible to apoptosis than administration of the two drugs separately.19
In this study, Naringin (NG) was determined as an anti-hepatoma model drug, a NG-loaded NLC containing CSO as liquid lipids (NCNLC) was developed and characterized. Importantly, the innovation of this study is that CSO replaces a conventional liquid lipids when creating a NLC, thereby improving drug loading efficiency and addressing the issue of creating a dual-drug NLC in a smart manner. In addition, the formulation evaluation and pharmacodynamics of the NCNLC over other conventional liquid lipids NLC were also evaluated with regard to cellular cytotoxicity and apoptosis, as well as the nude mice bearing HepG2 xenografts. Furthermore, the preliminary immunologic effects were also investigated.
Materials and Methods
Materials
According to the single-factor test and response surface optimization test, the extraction process of CSO was investigated by ultrasonic hydro-enzymatic extraction from Coix lacryma-jobi20 (Refractive index: 1.451, acid value: 0.58, iodine value: 103, saponification value: 171, relative density: 0.917; The contents of 1, 2- oleic acid −3- linoleate and trioleate were 1.43±0.11% and 1.72±0.09%, the characteristic map of CSO was established by HPLC-ELSD as well). HepG2 cells were purchased from Shanghai Branch of China Science Academy. NG was purchased from Shanghai Standard Technology Co., Ltd. (purity > 98.0%, Shanghai, People’s Republic of China). Glycerin monostearate, Tween-80, Oleic acid and neodecanoate triglycerides were obtained from Shanghai Ziyi Reagent Co., Ltd. (Klamar®, Shanghai, People’s Republic of China). Minimum essential medium, fetal bovine serum (FBS), penicillin-streptomycin solution and phosphate-buffered saline (PBS) were provided by Thermo Fisher Scientific Inc. (HyClone®, Waltham, MA, USA). Annexin V-PE assay kit were both provided by KeyGen Biotech Co., Ltd (Jiangsu, People’s Republic of China). The water used in this study was produced by Direct-Q 5UV Milli water purification system (Millipore, Billerica, MA). The other chemical reagents were of analytical grade, unless otherwise statement.
Animals
BALB-nu nude mice (6–8 weeks old, 18–22 g weight) were purchased from Slater Laboratory Animal Co., Ltd (Shanghai, China), and housed under standard laboratory conditions. All animal experiments had been approved and complied with the Ethics committee of Shanghai university of Chinese medicine and Animal Management Rules of the Ministry of Health of the People’s Republic of China. The animal use license number is: SYXK(HU)2014–0008.
In vitro Synergistic Studies of CSO Combined with NG
Five thousand of HepG2 cells were seeded in a well of 96-well plates for 24 h. The complete MEM medium was replaced with 100μL of different formulations, including NG (dissolved in isopropanol with 0.1g/L), CSO (dissolved in isopropanol with 0.05g/L, 0.1g/L, 0.2g/L, 0.5g/L, 0.8g/L, 1g/L, 1.5g/L) and different synergistic ratio of the two drugs combination formulation. After treatment for 24 h, the cells were stained with 10μL of the CCK-8 for 2h. The absorbance was detected at 450 nm using the microplate reader.19 The cell viability and Synergistic Q value were calculated by the following formulas, respectively. Cell proliferation inhibition rate (%) = (absorbance of control−absorbance of sample)/absorbance of control; Q=Eab/(Ea+Eb−Ea×Eb), Eab represents the inhibitory rates of different synergistic ratio in combination formulation, respectively; Ea and Eb represent the inhibitory rates of single component, respectively.
Preparation of NCNLCs/NONLCs/NDNLCs
The NCNLCs were prepared using the ultrasonic melt-emulsification method as reported before, with some modification. Briefly, the liquid lipids were composed of glycerin monostearate, CSO, and NG at a ratio of 5:5:1 and was melted by heating to approximately 60°C. The factor of preparation for single-factor investigation, and the horizontal coding of independent variables is shown in Table 1. Moreover, on the basis of the results, box-behnken experimental design scheme was adopted as shown in Table 2. The formulation and preparation are 75 mg of glycerol monostearate, 15 mg of naringin, 75 mg of CSO/neodecanoate triglycerides/oleic acid, 250 mg of tween-80, 10 mL deionized water, stirring 10 min to form colostrum, ultrasonic cell disruptor (power 100 w) ultrasonic dispersing 25 min, then putting to ice water, though the 0.22 μm microporous membrane filtration.
 |
Table 1 The Factors Level Table of Single Factor Test |
 |
Table 2 The Factors Level Table of Response Surface |
Characterization of NLCs
The surface morphologies of NCNLCs were observed by a Transmission Electron Microscope. Diluted NCNLCs were placed on a carbon-coated copper grid, negatively stained with 2% phosphotungstic acid, and then observed with TEM. The particle size, polydispersity index (PDI), and zeta potential NCNLCs were analyzed using dynamic light scattering with a Zetasizer n90 (Malvern Instruments, Malvern, England). The average particle size was expressed as volume mean diameter.
The drug encapsulation efficiency (DEE) and drug loading efficiency (DLC) were analyzed by sephadex microcolumn centrifugation,21 and UV spectrophotometry could be employed to determine the DEE and DLC at 280 nm.
DEE was calculated as follows: DEE (%) = weight of (total drug - free drug)/weight of total drug ×100
DLC was calculated as follows: DLC (%) = weight of (total drug - free drug)/weight of total drug and lipid carriers ×100
In vitro Drug Release Profiles
In vitro release of NCNLCs, NGNLCs, NONLCs were carried out using a modified dialysis method. Briefly, different formulations were added to dialysis bags (molecular weight cutoff 10 kDa) separately, followed by immersion in 100 mL of release medium (0.2% Tween 80 in PBS, pH 7.40 and 5.00, respectively) at 37°C with stirring at 100 rpm. At predetermined time intervals (0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 22, 26, 30, 36 hrs), 1 mL of sample was withdrawn, replaced with equal volume of fresh buffer solution with the same pH value.22 The amount of NG released was determined using UV spectrometer (UV-1800, Shimadzu, Japan) at 288 nm.
In vitro Cytotoxicity Studies
The cytotoxicity of NCNLCs against HepG2 cells was investigated using conventional CCK-8 method. The HepG2 cells were seeded into 96-well culture plates at a density of 1×104. Afterwards, the culture medium was removed, and 200 µL of the various concentrations of NCNLCs/NONLCs/NDNLCs, and the mixture of NG and CSO and a negative control (200 µL of fetal bovine serum-free culture medium) were incubated with the cells at 37°C for 24 hrs. The cells were stained with 10μL of the CCK-8 for 2h. The absorbance was detected at 450 nm using the microplate reader. The results were expressed as a percentage of the absorbance of the negative control. Half-maximal inhibitory concentration (IC50) values of samples were calculated.
In vitro Apoptosis Studies
The strong apoptosis inducing ability of CSO has been validated previously in HepG2 cells.23,24 To investigate the synergistic effect of NG and CSO, the cell apoptosis after treatment with various formulations was studied by flow cytometry using Annexin V-PE apoptosis detection kit. In brief, 5.0×105 HepG2 cells were seeded into 6-well plates per well for 24 h. Next, the CSO concentrations of various formulations including NCNLCs, NONLCs, NDNLCs, and free drugs of NG and CSO at the ratio of 1:5 were adjusted as 10 μg/L, respectively. After incubation for 24 h, the cells were trypsinized by ethylenediaminetetraacetic acid-free pancreatin, rinsed with ice-cold PBS thrice and resuspended in 500μL of binding buffer successively, which was followed by adding 5μL of Annexin V and PE. At the end of the staining for 30 min at 4°C, the apoptotic induction was evaluated immediately by flow cytometry.
In vivo Antitumor Efficacy
It has been found that various immunosuppressive factors, including interleukin 6 (IL-6) and interleukin 10 (IL-10) are frequently enriched in the tumor microenvironment and facilitate tumor immune evasion.25,26 In this experiment, the antitumor activity of NCNLCs was evaluated in BALB/c nude mice generated by subcutaneous injection of HepG2 cells (8 × 106per mouse) into the right axilla. The mice were randomly divided into five groups (n=6) on day 10 post-xenograft implantation, treated with NCNLCs, NDNLCs, NONLCs, KLT and 0.9% saline as a control 20 mg/kg once daily for 10 days by intraperitoneal injection. The tumor inhibition was calculated according to the formula:
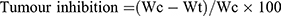
where Wt and Wc represent the mean tumor weight of the treated and control groups, respectively.
Preliminary immunologic effect of spleen was also investigated by weighing various tissues known to have an immune function. Body weight was recorded in each group as an indirect indicator of general toxicity. IL-6 and IL-10 levels were detected as a preliminary immunologic evaluation at the quantitative level by enzyme-linked immunosorbent assay (ELISA) kit at 37°C. All efforts were made to minimize the suffering of the animals and to reduce the number of animals used and the mice were humanely sacrificed post-experiment.
Results and Discussion
In vivo Synergistic Studies
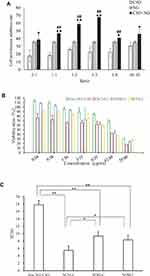
Importantly, both of CSO and NG had their certain dosage regimen in the clinical application.27 Therefore, it is essential to find the synergistic ratio of encapsulated NG to CSO. According to the synergistic studies of CSO combined with NG, with the cell inhibition rate result is shown in Figure 1A, the calculation of the Q value were 0.83, 0.98, 1.12, 1.35, 0.82, 0.83of the ratio 2:1, 1:1, 1:2, 1:5, 1:8, 1:10. When Q > 1.15, represents the two drugs have synergistic effect, 0.85 ≤ Q ≤ 1.15 represents additive effect, Q < 0.85 represents antagonistic effect.28 In all the groups with different proportions of administration, the cell proliferation on inhibition rates in the synergistic administration groups were all higher than that in the single administration group. Moreover, at the ratio of 1:5, Q = 1.35, which characterized as obvious synergy effect, and the rest of the synergistic proportion were just as additive effect. The preliminary findings suggested that the synergistic ratio of 1:5 displayed the most obvious HepG2 cellular inhibition among all the combination of NG and CSO in cytotoxicity. Finally, the group was divided into NG+CSO with a ratio of 1:5 as the best synergistic result.
Preparation and Characterization of NCNLCs
According to the experimental results, we obtained the following prescription and preparation method: an aqueous phase was prepared by dissolving Tween-80 250 mg, in double-distilled water and made up to 10 mL at 60°C. This aqueous surfactant solution was then heated with stirring to the same temperature as the lipid matrices. The oil phase was rapidly injected into the hot surfactant solution using a mechanical agitate (1000 rpm, 10 min). Next, the aqueous phase was sonicated using a probe sonicator together with the oil phase at 16.5% of the wattage employed in the ultrasound source for 25 mins. The produced emulsion was soaked in chilled distilled water for 20 mins, and then, using 0.22μm filter membrane for filtration. Single NG-loaded NLCs were prepared by the same method with the replacement of CSO to other conventional liquid lipids, oleic acid and neodecanoate triglycerides, named naringin-oleic acid NLC (NONLC) and naringin-neodecanoate triglycerides NLC (NDNLC). The produced formulations were stored at 4°C for further investigations.
Particle size and the PDI are important features of NLC, from which the stability of these NPs when drug-loaded can be predicted. Particle size can influence the distribution of nanocarriers, small particle size is an advantage for NLC because it decreases uptake by the liver, prolongs circulation time in the blood, and improves bioavailability.29 NCNLCs displayed a small size around 50 nm, which was speculated to have the potential for deep tumor penetration.30,31 Moreover, when NCNLCs extravasated in the tumor tissues while prohibiting the carriers to exit the capillaries in normal tissues, an ideal targeting goal would be achieved.32–34 And the NG encapsulated in NCNLCs can be accumulated efficiently in tumor sites because of the enhanced permeability and retention effect (EPR).35 In addition, a narrow polydispersity index (0.29) indicated an acceptable preparation technology. More importantly, the zeta potential is a key factor in prediction and evaluation of the stability of a colloidal dispersion.36 The zeta potential of NCNLCs was distributed between −3.5 mV and −5.2 mV, a lightly negative charge, suggesting good stability and high cellular uptake, and also facilitating the delivery of NCNLCs to the negatively charged cancer cells.37 The DEE and DLC of NG encapsulated in NCNLCs were 91.77±0.48%, and 6.96±0.02%, separately (Figure 2C). In order to investigate the influence of CSO as liquid lipids, the morphology of NCNLCs was also determined in Figure 2A and B, displayed as spherical particles with around 50 nm, which was essentially in agreement with the results of photon correlation spectroscopy analysis. We conducted a preliminary stability evaluation of NCNLCs. After 30 days at 4°C, the result of Table 3 showed only a slight decrease of DEE of NG, but no significant decrease. Preliminary results show that NCNLC has good stability.
 |
Table 3 Particle Size, PDI, Zeta Potential, DEE and DLE of NCNLCs |
 |
Figure 2 Preparation characterization of NLCs. Notes: (A, B) TEM image and particle size distribution of NCNLCs. (C) In vitro NG release at pH 7.40 and pH 5.00. |
In vitro Release Behavior of NCNLCs, NONLCs, NDNLCs
In vitro NG release behaviors of NCNLCs, NONLCs, NDNLCs at pH 7.40 and 5.00 were performed using the dialysis technique and data are shown in Figure 2C. The results showed that there was a noteworthy burst drugs release during the first 8 h, followed by a sustained release in the subsequent 36 h from the NLC formulations, indicating that the drug release from NPs generally takes place by several mechanisms, including surface and bulk erosion, disintegration, diffusion and desorption.29 This sustained release of drug from NPs is due to homogenous entrapment of the drug. Moreover, the releases of drugs from NDNLCs and NONLCs were both slower than NCNLCs in pH 7.40 and 5.00, indicating high drug release while using CSO as liquid lipids in NCNLCs, which is due to the high solubility of the main ingredient, glyceride. Furthermore, the release of NG in the acidic condition was faster than that of neutral environment. This difference in release seen at different pH values were attributed to the stability of CSO under conditions of mild acidity. In addition, a pharmacokinetic model was used to describe the in vitro release of NG in NCNLCs, set the release rate as Q, the release time as T. The zero-order kinetics model (R = 0.89), first-order kinetics model (R = 0.95), HIGUCHI curve (R = 0.89), and RIGGER curve (R = 0.51) were used for simulation. The results showed that the first-order dynamic release model could best simulate the drug release in vitro (r > 0.95). At pH 7.40, the release equation is: ln(1-Q) = −0.039T - 0.148; At pH 5.00, the release equation is: ln(1-Q) = −0.031T - 0.179, which indicated that NCNLCs had a good sustained release effect because of the combination of solid oil and CSO. In general, the in vitro release profile shows that NLCs formulations have the capacity to release NG at a sustained rate, thus beneficial to free enough drug at tumor extracellular and endolysosomal site to achieve optimal anticancer efficacy.
In vitro Cytotoxicity Efficacy
The cytotoxicity of the NLCs formulations against HepG2 cells in vitro was evaluated using the CCK-8 assay. To demonstrate the advantage of a CSO-NG codelivery system in terms of enhancing cytotoxicity, a mixture of CSO and NG was also used as the other control group. As depicted in Figure 1B and C, free drug and NLC formulations decreased cell viability both in a dose-dependent manner. The half-maximal inhibitory concentrations (IC50) of the free drug, NCNLCs, NDNLCs and NONLCs formulations against HepG2 cells were calculated to be 17.766µg/mL, 5.475 µg/mL, 9.332 µg/mL and 8.319µg/mL, respectively. By comparison, the IC50 of NCNLCs was 3.24-fold, 1.70-fold and 1.52-fold lower relative to that of free drug, NDNLCs and NONLCs, respectively. The significant difference (P< 0.05) in antiproliferative activity between the NCNLCs and free drug, shown that the NLC formulations enhance the killing of HepG2 cells and the codelivery of two drugs was critical to exert a synergistic antitumor effect. The free drug was not able to guarantee that two components entered the cells at the same time, due to their respective differences in physicochemical properties. In addition, the significant difference in antiproliferative activity between the NCNLCs with NDNLCs and NONLCs, suggested that codelivery of CSO and NG was critical to exert a synergistic antitumor effect. Obviously, our results showed that combination with CSO significantly enhanced NCNLCs efficacy with higher cytotoxicity and exhibited synergistic effects for HepG2 cells. Besides, the cell viability of blank NLCs was over 85% at all studied concentrations, showing the good safety and biocompatibility of the carriers. This experiment confirms the advantage of a dual-drug codelivery system from the perspective of an anticancer effect in vitro.
In vitro Apoptosis Efficacy
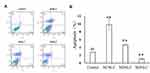
According to the design strategy of “unification of medicines and excipients”, a combination of CSO and NG was able to induce cell apoptosis. To validate the idea, we investigated the induction of apoptosis by the NCNLCs, NDNLCs and NDNLCs formulations using the Annexin V-PE apoptosis detection kit. The apoptosis results in Figure 3A were showed through quadrant analysis. Events in each of the four quadrants were as follows: lower-left, lower-right, upper-right and upper-left quadrant represented viable cells, early to midstage of apoptosis, late stages of apoptosis and dead cells, respectively. When the concentration of NG was set at 10 µg/mL, there was obvious induction of apoptosis with NLCs when compared with the negative control after treatment for 24 hrs, suggesting that NG and CSO were capable of apoptosis induction synergistically using such dual-drug formulation. More interestingly, owing to the synergistic effect of CSO and NG, the NCNLCs formulation exhibited the stronger capacity to induce HepG2 cells apoptosis relative to NDNLCs and NONLCs. According to Figure 3B, the NCNLCs displayed a 2.12-fold and 9.28-fold higher than that in the cell apoptosis compared with the NDNLCs and NONLCs, respectively. These findings indicated that NCNLCs enhance the anticancer activity of NG and CSO to induce HepG2 cells apoptosis by loading dual-drug onto active“unification of medicines and excipients” carrier NLCs. The theory was capable of apoptosis induction synergistically using such CSO component-based formulation.
In vivo Antitumor Efficacy
The in vivo anti-tumor efficiency of NLCs and KLT were evaluated in a xenograft model of HCC by measuring the body weight, tumor inhibition rate, spleen index, IL-6 and IL-10 levels. As shown in Figure 4A, the body weight of mice kept slight increasing during treatment period, indicating a low systemic side effect at a tested dose. As shown in Figure 4B, NCNLCs exhibited the highest inhibition rate of 70.09%, followed by KLT (54.78%), NDNLCs (39.89%) and NONLCs (35.98%). Compared to the saline treated control group, NCNLCs-treatment obviously suppressed tumor growth during treatment period. Moreover, NCNLCs was more effective in suppressing tumor growth in comparison with NDNLCs or NONLCs at an equivalent dose of NG, proving excellent anti-tumor efficacy of NCNLCs. The results showed the significantly anti-tumor effect of NG and CSO contained NLC compared with ordinary liquid lipids (P < 0.05), and suggested that the synergetic effect of the two drugs produced a better anticancer therapeutic effect.
As for the influence on the immune function, the weight to body ratio of the spleen was collected posttreatment to evaluate the influence of treatment on the important immune organs. The results shown in Figure 4C demonstrate that NLCs and KLT all caused a significant increase in the weight of the spleen compared with saline group (P < 0.05, P < 0.01). Moreover, the significantly higher spleen index of NCNLCs with other NLCs and KLT suggesting a superior effect on immune regulation. As shown in Figure 4D, NCNLCs up-regulated the expression IL-6 and IL-10 in the serum of tumor bearing mice. Moreover, significantly higher IL-6 and IL-10 levels were observed in comparison with saline group (P < 0.05) and NDNLCs, NONLCs and KLT (P < 0.05, P < 0.01), indicating enhanced efficiency of NCNLCs in immunity and inhibiting proliferation of tumor cells and inducing tumor cells apoptosis. This result confirmed the view that the NCNLCs formulation played a significant role for effective delivery of dual-drug of cancer immunotherapy.
Conclusions
We developed a NLC formulations using CSO as a functional liquid lipids on the theory of “unification of medicines and excipients”. The optimal preparation technology was determined at a ratio of 1:5:5 (NG/CSO/glycerin monostearate, w/w/w of crude drug). The characteristics of NCNLCs were investigated, and the drug-release was found to be increased when using CSO as liquid lipids. In the meantime, NCNLCs was more superior to suppress the proliferation of HepG2 cells and induce HepG2 cells apoptosis in vitro in comparison with free NG, NDNLCs and NONLCs. Furthermore, the synergistic anti-tumor efficacy was assessed in NCNLCs to xenograft tumor mice models. The NG + CSO combination up-regulated the expression IL-6 and IL-10 in the serum of tumor bearing mice, and the significantly higher spleen index of NCNLCs all suggesting the combination played a significant role for effective delivery of dual-drug of cancer immunotherapy. This system could achieve co-delivery of NG and CSO, exert the ability of the two drugs and get the significant better tumor inhibition efficiency. Hence, the advantages of CSO as liquid lipids and auxiliary anti-tumor ingredients in NLC are very important and represents a marked advance of “unification of medicines and excipients” in anti-tumor therapy. Overall, the CSO, a fatty oil derived from the traditional Chinese medicine has effectively replaced the conventional liquid lipids. This strategy is expected to be applied in NLCs to increase anti-tumor efficiency and reduce the potential risks of supplementary materials. Further studies will be conducted to investigate the mechanism of the NG + CSO combination regulates activation, intracellular transportation, subcellular localization and their underlying mechanism in regulating carcinogenesis by our group.
Funding
This work was supported by the Scientific Research and Innovation Project of Shanghai University of Traditional Chinese Medicine [A1-182040224].
Disclosure
No potential conflict of interest was reported by the authors.
References
1. Jemal A, Bray F, MM C, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107
2. Dika IE, Abou-Alfa GK. Treatment options after sorafenib failure in patients with hepatocellular carcinoma. Clin Mol Hepatol. 2017;23(4):273–279. doi:10.3350/cmh.2017.0108
3. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi:10.1016/S0140-6736(18)30207-1
4. Heimbach JK, Kulik LM, Richard Finn MD, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi:10.1002/hep.29086
5. Wang D, Yang C, Wang Z, et al. Norcantharidin combined with coix seed oil synergistically induces apoptosis and inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation. Sci Rep. 2017;7(1):9373. doi:10.1038/s41598-017-09668-2
6. Ma P, Mumper RJ. Paclitaxel nano-delivery systems: a comprehensive review. J Nanomed Nanotechnol. 2013;4(2):1000164. doi:10.4172/2157-7439.1000164
7. Ai T, Shang W, Yan H, et al. Near infrared-emitting persistent luminescent nanoparticles for hepatocellular carcinoma imaging and luminescence-guided surgery. Biomaterials. 2018;167:216–225. doi:10.1016/j.biomaterials.2018.01.031
8. Bentz KC, Savin DA. Hollow polymer nanocapsules: synthesis, properties, and applications. Polym Chem. 2018;9:2059–2081. doi:10.1039/C8PY00142A
9. Kovacevic A, Savic S, Vuleta G, et al. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): effects on size, physical stability and particle matrix structure. Int J Pharm. 2011;406(1):163–172. doi:10.1016/j.ijpharm.2010.12.036
10. Weber S, Zimmer A, Pardeike J. Solid lipid nanoparticles (SLN) and Nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86:7–22. doi:10.1016/j.ejpb.2013.08.013
11. Min H, Shao FU, Xiao-Ling F. Screening of panax notoginsenoside water in oil microemulsion formulations and their evaluation in vitro and in vivo. Acta Pharmaceutica Sinica. 2007;42(7):780–786.
12. Okonogi S, Chaiyana W. Enhancement of anti-cholinesterase activity of Zingiber cassumunar essential oil using a microemulsion technique. Drug Discov Ther. 2012;6(5):249–255.
13. Zhang DK, Fu MC, Lin JZ, et al. Study on theory and application value of ‘unification of medicines and excipients’ in Chinese materia medica preparations. Chin Traditional Herbal Drugs. 2017;10:1921–1929.
14. Qu D, He J, Liu C, et al. Triterpene-loaded microemulsion using Coixlacyma-jobi seed extract as oil phase for enhanced antitumor efficacy: preparation and in vivo evaluation. Int J Nanomed. 2014;9(1):109–119.
15. Qu D, Sun W, Liu M, et al. Bitargeted microemulsions based on coix seed ingredients for enhanced hepatic tumor delivery and synergistic therapy. Int J Pharm. 2016;503(1–2):90–101. doi:10.1016/j.ijpharm.2016.03.001
16. Lu Y, Li CS, Dong Q. Chinese herb related molecules of cancer-cell-apoptosis: a minireview of progress between Kanglaite injection and related genes. J Exp Clin Cancer Res. 2008;27(1):31. doi:10.1186/1756-9966-27-31
17. Wang X, Ma C, Wang X. Clinical observation on effect of Kanglaite, in treating pulmonary carcinoma patients with Lung-Qi deficiency and immunologic inadequacy after pneumonectomy in treating pulmonary carcinoma patients with Lung-Qi deficiency and immunologic inadequacy after pneumonectomy. Chin J Integr Med. 1999;5(2):105–107.
18. Wu Y, Zhang J, Hong Y, et al. Effects of Kanglaite injection on serum miRNA-21 in patients with advanced lung cancer. Med Sci Monitor Int Med J Exp Clin Res. 2018;24:2901–2906.
19. Qu D, Guo M, Qin Y, et al. A multicomponent microemulsion using rational combination strategy improves lung cancer treatment through synergistic effects and deep tumor penetration. Drug Deliv. 2017;24(1):1179–1190. doi:10.1080/10717544.2017.1365394
20. Lin L, QIN L, LIU Ronglin, et al. Optimization of aqueous enzymatic extraction of adlay bran oil by response surface methodology. China Oils Fats. 2015;40(4):1–5.
21. Yang L, Shengyao T, Weiying G, et al. Determination of entrapment efficiency of oxaliplatin liposomes by sephadex microcolumn combined with HPLC. Chin J Modern Appl Pharm. 2014;31(1):69–77.
22. Sonali AP, Singh RP, Rajesh CV. et al. Transferrin receptor-targeted vitamin E TPGS micelles for brain cancer therapy: preparation, characterization and brain distribution in rats. Drug Deliv. 2016;23:1788–1798. doi:10.3109/10717544.2015.1094681
23. Changyuan W, Ting L, Zongping T. Study of coix seed extract in its effect on inducing proliferation,apoptosis and p53 expression in human hepatocellular carcinoma. J Guangxi Med Univ. 2001;8:793–795.
24. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi:10.1002/hep.24199
25. Zou WP. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nature Rev Cancer. 2005;5(4):263–274. doi:10.1038/nrc1586
26. Yan C, Ding Q, Yihua M, et al. Microemulsion-based synergistic dual-drug codelivery system for enhanced apoptosis of tumor cells. Int J Nanomed. 2015;10:1173–1187. doi:10.2147/IJN.S76742
27. Zhigang C, Haofeng LU, Jiye Z, et al. Anti-proliferative effects of combining Sorafenib and sodium cantharidinate in HepG2 liver cancer cells. Chin J Oncol Prev Treat. 2015;7:85–89.
28. Wang Y, Zhang H, Hao J, et al. Lung cancer combination therapy: co-delivery of paclitaxel and doxorubicin by nanostructured lipid carriers for synergistic effect. Drug Deliv. 2015;23(4):1398.
29. He Z, Sun J, Li L. Deep penetration of nanoparticulate drug delivery systems into tumors: challenges and solutions. Curr Med Chem. 2013;20(23):2881–2891. doi:10.2174/09298673113209990004
30. Wong C, Stylianopoulos T, Cui J, et al. Multistage nanoparticle delivery system for deep penetration into tumor tissue. Proc Natl Acad Sci U S A. 2011;108(6):2426–2431. doi:10.1073/pnas.1018382108
31. Zhang ZH, Wang XP, Ayman WY, et al. Studies on lactoferrin nanoparticles of gambogic acid for oral delivery. Drug Deliv. 2013;20(2):86–93. doi:10.3109/10717544.2013.766781
32. Zhang L, Jiang Y, Zheng Y, et al. Selective killing of Burkitt’s lymphoma cells by mBAFF-targeted delivery of PinX1. Leukemia. 2011;25:331–340. doi:10.1038/leu.2010.261
33. Ravar F, Saadat E, Gholami M, et al. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel, in-vitro characterization and in-vivo evaluation. J Control Release. 2016;229:10–22. doi:10.1016/j.jconrel.2016.03.012
34. Szczepanowicz K, Bzowska M, Kruk T, Karabasz A, Bereta J, Warszynski P. Pegylated polyelectrolyte nanoparticles containing paclitaxel as a promising candidate for drug carriers for passive targeting. Colloid Surface B. 2016;143:463–471. doi:10.1016/j.colsurfb.2016.03.064
35. Alavi M, Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Nephron Clin Pract. 2019;34(1):424–436.
36. Heshu R, Abdullah R, Wun HC, et al. Zerumbone-loaded nanostructured lipid carriers: preparation, characterization, and antileukemic effect. Int J Nanomed. 2013;8:2769–2781. doi:10.2147/IJN.S45313
37. Wang M, Qin LL, Li K, et al. The improvement of the anticancer effect of a novel compound benzoic acid, 2-hydroxy-,2-D-ribofuranosylhydrazide (BHR) loaded in solid lipid nanoparticles. AAPS PharmSciTech. 2012;13:1348–1354. doi:10.1208/s12249-012-9862-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.