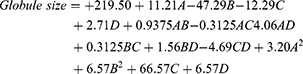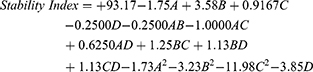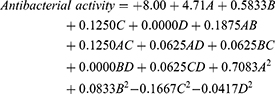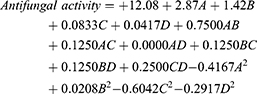Back to Journals » International Journal of Nanomedicine » Volume 16
Formulation, Optimization, and Evaluation of Oregano Oil Nanoemulsions for the Treatment of Infections Due to Oral Microbiota
Authors Hosny K , Asfour H, Rizg W , Alhakamy NA , Sindi A , Alkhalidi H, Abualsunun W, Bakhaidar R, Almehmady AM, Akeel S, Ali S , Alghaith A, Alshehri S , Khallaf R
Received 22 June 2021
Accepted for publication 31 July 2021
Published 13 August 2021 Volume 2021:16 Pages 5465—5478
DOI https://doi.org/10.2147/IJN.S325625
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Ebrahim Mostafavi
Khaled Hosny,1– 3 Hani Asfour,4 Waleed Rizg,1– 3 Nabil A Alhakamy,1– 3 Amal Sindi,5 Hala Alkhalidi,6 Walaa Abualsunun,1 Rana Bakhaidar,1 Alshaimaa M Almehmady,1 Sara Akeel,5 Sarah Ali,5 Adel Alghaith,7 Sultan Alshehri,7 Rasha Khallaf8
1Department of Pharmaceutics, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia; 2Center of Excellence for Drug Research and Pharmaceutical Industries, King Abdulaziz University, Jeddah, 21589, Saudi Arabia; 3Advanced Drug Delivery Research Group, Faculty of Pharmacy, King Abdulaziz University, Jeddah, 21589, Saudi Arabia; 4Department of Medical Microbiology and Parasitology, Faculty of Medicine, King Abdulaziz University, Jeddah, 21589, Saudi Arabia; 5Oral diagnostic sciences department, Faculty of Dentistry, King Abdulaziz University, Jeddah, Saudi Arabia; 6Department of Clinical pharmacy, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia; 7Department of Pharmaceutics, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; 8Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Beni-Suef University, Beni-Suef, 62511, Egypt
Correspondence: Khaled Hosny
Department of Pharmaceutics, Faculty of Pharmacy, King Abdulaziz University, Jeddah, Saudi Arabia
Tel +966 561682377
Email [email protected]; [email protected]
Introduction: Natural oil-based nanoemulsions (NEs) have been widely investigated in many diseases that affect the oral cavity. NEs are delivery systems that enhance the solubility of lipid therapeutics and improve their delivery to target sites; they are known as self-nanoemulsifying drug delivery systems (SNEDDSs). The current investigation’s aim was to produce an oregano essential oil-based nanoemulsion (OEO-SNEDD) that would have antibacterial and antifungal effects against oral microbiota and improve oral health.
Methods: Several OEO-SNEDDSs were developed using different percentages of OEO (10%, 14%, and 18%), percentages of a surfactant mixture Pluracare L64:Lauroglycol FCC (18%, 32%, and 36%), Smix ratios (1:2, 1:1, and 2:1), and hydrophilic-lipophilic balances (HLBs) of the surfactant mixture (8, 10, and 12) using the Box‒Behnken design. The optimized concentration of excipients was determined using a pseudoternary phase diagram to obtain the NEs. The formulations were evaluated for their droplet size, stability index, and antibacterial and antifungal activities.
Results: The NEs had a droplet size of 150 to 500 nm and stability index of 47% to 95%, and the produced formulation reached antibacterial and antifungal inhibition zones of up to 19 and 17 mm, respectively. The Box‒Behnken design was adopted to get the optimum formulation, which was 18% OEO, 36% Smix, 10.29 HLB of Smix, and a 1.25:1 Smix ratio. The optimized formulation had a lower ulcer index compared with various other formulations evaluated in rats.
Conclusion: This study illustrated that OEO-SNEDDSs can provide good protection against oral microbial infections.
Keywords: oregano essential oil, nanoemulsion, Box‒Behnken design, ulcer index, required HLB, carvacrol, thymol, stability index
Introduction
Good oral health and hygiene are necessary for adequate total health and life quality.1 The World Health Organization in 2012 defined oral health as the state of being free from facial and oral pain, mouth infections, lesions, and other oral illnesses that hinder the individual’s ability to bite, chew, smile, or speak.2 Insufficient oral care can negatively affect an individual’s overall health. Several diseases have originate in the oral cavity, and some drugs can endanger the health of the oral cavity.3
The diversity in the composition of oral microbiomes may be ascribed to the continuous contact of the oral cavity with the external environment.4 The main components of the oral microbiome include several strains of bacteria (eg, Streptococcus gordonii, Porphyromonas gingivalis, Streptococcus mutans, and Streptococcus mitis), fungi (eg, Candida, Cladosporium, Aspergillus, and Cryptococcus), and protozoa (eg, Entamoeba gingivalis and Trichomonas tenax).5 These species can colonize oral cavity surfaces, causing microbial biofilms to form on the dorsal side of the tongue, buccal mucosa, teeth, and gingival surfaces.6 Several investigations have illustrated the association between disturbances in the oral microflora and the development of multiple diseases.7 For instance, poor oral health can lead to the development of certain oral, neck, and head cancers.8 The bacteria Staphylococcus aureus is one of the leading causes of several disorders resulting from either food poisoning or skin infections.9 Candida overgrowth may result in oral thrush, and its effects can become systemic, causing conditions such as Crohn’s disease and ulcerative colitis.10 Improper habits and diets can increase microbial activation and affect phage members of oral microflora.11
Oregano essential oil (OEO) is a volatile essential oil extracted from the Origanum vulgare plant. It is rich in phenolic and terpenoid compounds.12 Components such as thymol, carvacrol, and rosmarinic acid are abundant in the oil, and this might explain their considerable antioxidant, antimicrobial, and antiinflammatory activities.13 The antioxidant action of OEO is ascribed mainly to its content of carvacrol and thymol, in addition to rosmarinic acid, which is a powerful antioxidant.14 Such compounds exert their antioxidant effects primarily through free radical scavenging. Thymol and carvacrol are reported to possess antimicrobial effects on the integrity of the outer membranes of fungi and bacteria, leading to their inactivation.15 Carvacrol was reported to inhibit inflammation and ulcer formation in several experimental models. These investigations theorized that carvacrol interferes to a great degree with inflammatory mediators, thereby causing better healing of ulcers.16 Based on this information, OEO has been used topically for skin conditions such as acne and athlete’s foot, and it may be taken orally for some gastrointestinal disturbances. It has also been used to relieve toothaches and other types of oral pain.17
Nanoemulsions (NEs) contain oil globules dispersed in an aqueous vehicle and stabilized by a film of surfactants and cosurfactants that forms around them. These systems ideally have a droplet size of 10 to 100 nm.18 NEs have several traits related to the small size of their globules that make them more effective than conventional emulsions, including better visual transparency, better performance, and more physical stability. They also have (1) a reasonable capacity for drug targeting, (2) good properties for protecting drugs against hydrolysis and enzymatic actions, (3) the ability to enhance drug loading, and (4) the ability to provide better drug dissolution and bioavailability. They are also suitable for use in laboratory studies and industrial scales.19
Experimental designs have been increasingly used in developing, characterizing, and optimizing various drug delivery systems; this includes examining the effect of different independent variables on different dependent variables.20 Such a methodology has many advantages, such as (1) employing fewer experiments to get the optimized formulation, (2) having a better ability to detect problems, (3) identifying drug/excipient interactions, and (4) anticipating optimized formulation behavior and being able to improve it.21 Experimental designs are cost-effective because they usually yield the best design for the formulation.22 Therefore, the purpose of the current investigation was to prepare an optimized oregano oil as an NE and incorporate it in a mouthwash so that its antibacterial and antifungal activities could improve oral health and treat oral infections.
Materials and Methods
Materials
Oregano oil was gifted by Qingdao Sigma Chemical Co., Ltd., Shandong, China. Pluracare L64 (a triblock copolymer with an average molecular weight of approximately 2900 and consisting of propylene oxide and ethylene oxide [HLB = 15]) was purchased from BASF SE Chemical Company (Ludwigshafen, Germany). Lauroglycol FCC (propylene glycol monolaurate [HLB=5]) was acquired as a gift from Gattefosse (Saint-Priest, France). Glycofurol (ethoxylate of tetrahydrofurfuryl alcohol) was obtained from Sigma Aldrich (St. Louis, Missouri, USA). All other chemicals were of analytical grade or better.
Methods
Pseudo-Ternary Phase Diagram
The OEO-SNEDDSs were formed from oregano oil, a surfactant (Pluracare L64:Lauroglycol FCC in a ratio of 1:1), and a cosurfactant (Glycofurol). Various mass ratios (1:1, 2:1, 1:2, 3:1, and 1:3) of the surfactant and cosurfactant, respectively, were used for the Smix. Aqueous titration methodology was adopted to construct the pseudoternary phase diagrams of water, the oil, and the Smix. A mixture of Smix and oil was titrated with water. The NE region in the generated pseudoternary phase diagram was determined; this showed the maximum and minimum levels of oil and Smix that would be suitable for formulating the NE.
Optimization and Statistical Design of Oregano Oil-Based Nanoemulsions (OEO-SNEDDSs)
The pseudo-ternary phase diagram provided the levels of the independent variables to be used in the Box‒Behnken experimental design (BBD) to determine the effects of the studied factors over 30 runs. Factor A was the oregano oil percentage = 10%, 14%, or 18%; factor B was the Smix percentage = 28%, 32%, or 36%; factor C was the HLB of the surfactant mixture of Pluracare L64:Lauroglycol FCC = 8, 10, or 12; and factor D was the Smix ratio (1:2, 1:1, or 2:1). The dependent variables were the globule size (Y1), stability index (Y2), antibacterial activity (Y3), and antifungal activity (Y4). The formulations were optimized using the BBD. The composition of the formulations and the dependent variables and constraints that were chosen for optimization are shown in Table 1.
 |
Table 1 Independent and Dependent Variables’ Levels Along with the Required Constraints |
Preparation of OEO-SNEDDSs
A simple procedure for mixing the constituents was followed to obtain 1 g of each formulation. The three components (oil, Smix, and water) in the mixture totaled 100%.
Characterization of OEO-SNEDDSs
Emulsification Ability of OEO-SNEDDSs
The emulsification ability and clarity of the emulsion were determined visually for each OEO-SNEDDS.
Droplet Size Assessment of OEO-SNEDDSs
The dynamic light-scattering technique was utilized to assess the globule size and polydispersity index (PDI) of the OEO-SNEDDSs (Zetatrac, Microtrac, Montgomeryville, Pennsylvania, USA). A 1:10 v/v dilution of the samples with distilled water was done, and three sets of measurements were conducted to determine the globule size of the OEO-SNEDDSs in 300 µL of the diluted samples.23
Determination of Stability Index of OEO-SNEDDSs
Different OEO-SNEDDSs formulations were subjected to a freeze‒thaw accelerated stability study using different temperature variations to affirm the thermodynamic stability. During the test, the particle size was determined for each mixture, and then the mixture was subjected to three consecutive freeze‒thaw cycles (freezing at −25°C for approximately 24 h and thawing at +25°C for another 24 h). The droplet size of each formulation was measured after these consecutive cycles.24 The stability index was calculated using the following equation:25
Stability index = ([Initial size – Change in size]/Initial size) X 100
Antimicrobiological Activity Assessment of OEO-SNEDDSs
In order to evaluate the antimicrobial action of the OEO-SNEDDSs, the common oral bacteria Streptococcus mutans was employed. The bacterial growth inhibition zones produced by the NEs were measured. Blood agar was used as a culturing medium for S. mutans ATCC 25175 (Microbiologics, St. Cloud, Minnesota, USA) to obtain pure cultures of S. mutans. The susceptibility of the cultured S. mutans to the NEs was assessed using the disc diffusion method. Round discs made from filter papers 6 mm in diameter were placed onto the surface of blood agar plates, and the plates were placed in a hot air oven for 2 h at 160°C for sterilization. The discs were then mixed with 50 µL of each of the formulations. Four discs per plate were used. The incubation of the plates was done at 37°C for 24 h, and the inhibition zones formed around the filter paper discs were measured in millimeters. The diameters of the inhibition zones for each disk were calculated as the average of three determinations.26
Assessment of Antifungal Activity of OEO-SNEDDSs
Sabouraud dextrose agar (SDA) was the medium used in the current antifungal study and Candida albicans was the model fungus. Two mL of sterile normal saline was used to prepare a suspension of C. albicans containing three to five colonies of that strain, and the mixture was vortexed. The turbidity of the suspension was adjusted using 0.5 meq of the McFarland standard. A culture of the microorganism was made by dipping a sterile swab into the suspension and applying it to the surface of the SDA plates. Sterile filter paper discs of 6 mm in diameter were impregnated with the fungal suspension and laid down and inoculated onto the surface of an SDA plate. The plates were put in an incubator at 25°±1°C. The growth inhibition zones formed around the discs were observed for 2 days. An average of three determinations of each inhibition zone’s diameter were calculated for every formulation.27
Optimization of OEO–SNEDDSs
Statistical parameters for the analysis of variance (ANOVA), such as the degrees of freedom, F-ratios, and p-values, for all of the factors, and their possible interactions were evaluated. The model that best fit the obtained data was chosen. P-values lower than 0.05 indicated the impact of the model. Additionally, the predicted R2 and adjusted R2 values, coefficient of variation percentage, and determination coefficients were applied to verify the model’s validity and fitness. The most suitable statistical solution was used to prepare an OEO-SNEDDS that would have the smallest globule size, maximum stability index, and best antibacterial and antifungal activity. The optimized formulation was prepared and evaluated for its ulcer index values.
Ulcer Index Determination of OEO-SNEDDSs
Animals
The protocol for this work received prior approval from the Local Institutional Animal Ethics Committee of Beni-Suef University (Approval No. 323-4-21). The research was performed in accordance with the guidelines that ensure that the care and use of laboratory animals conforms to the Guide for the Care and Use of Laboratory Animals (NIH publication #85-23, revised in 2011.
Male albino rats weighing 160 to 240 g were used for this investigation. The animals were divided into four groups of six animals each. One group was treated with the optimized oregano oil NE, one group with oregano oil alone, one group with a commercially available mouthwash containing oregano oil, and one group with normal saline (negative control group). The ulcer index was calculated for each group.
Organisms and Inoculum Preparation
Three to five colonies of the standard strain of C. albicans were selected, suspended in saline (2 mL), and vortexed. A final concentration of 3×108 CFU/mL of the microorganism colonies was obtained by centrifuging a C. albicans suspension of 2500 g, washing the cells three times with phosphate buffered saline (PBS), and adjusting the suspension to get the required concentration.
Oral Candidiasis in the Rat
The oral candidiasis model in immunosuppressed rats was employed in this investigation according to a previously published study.28 The oral infection was induced using 1 mL of C. albicans suspension containing 3.108 viable cells of the microorganism. The infection process was done at defined intervals with 48 h between on days 7, 5, and 3 with 0.1 mL of saline suspension containing 3.108 viable cells of C. albicans. The infection was induced by rolling an infected cotton swab twice all over the oral cavity. The fungal infection caused ulcerative activity in the mouth mucosa with a score of 0 to 5. In each animal group, one of the tested formulations was administered for 3 days, and at the end of the 3 days, the ulcer indexes were calculated for every rat group. The ulcer determination scale was employed in the following manner: 0 (epithelial cell lining with normal color), 1 (red-colored epithelial cell lining), 2 (ulceration patches < 1 mm), 3 (ulcers > 1 mm but < 2 mm with no hemorrhagic lines), 4 (ulcers > 1 mm but < 2 mm and with hemorrhagic lines), and 5 (ulcers > 2 mm). The results were recorded. To determine the significance of the formulations on the ulcer index, a one-way ANOVA test was done using GraphPad Instat version 04+ software.
Results and Discussion
NEs that have been broadly studied in many diseases appear to be favorable platforms for delivering drugs to the oral cavity. The excipients used in formulating such systems are considered safe materials by the US Food and Drug Administration. The previously mentioned, inherently good features of NEs promote antimicrobial activity against many microorganisms, including bacteria, viruses, and fungi.29–31 Several studies have proven the efficacy of NEs in preventing and curing diseases of the oral cavity.32
Pseudoternary Phase Diagram Development
Five ternary phase diagrams were established (Figure 1), in which five different ratios (1:1, 2:1, 3:1, 1:2, and 1:3) were used to develop the Smix. In the 1:1 Smix (see Figure 1A), the highest concentration of oregano oil that could be solubilized was 15% using 35% Smix and 50% water. However, increasing the concentration of the glycofurol cosurfactant within the 1:2 Smix (see Figure 1B) increased the NE area in which 20% of the oregano oil could become emulsified using 30% Smix and 50% water. A further increase of the Glycofurol cosurfactant in the 1:3 Smix (see Figure 1C) led to a decrease in the NE region so that only 10% of the oregano oil could become emulsified using 35% Smix and 55% water. Upon increasing the concentration of the surfactant in the 2:1 Smix (see Figure 1D), higher NE regions were noticed, and 18% of the oregano oil could become emulsified using 34% Smix and 48% water. A further increase in the surfactant level in the 3:1 Smix (see Figure 1E) decreased the NE region so that only 16% of the oregano oil could become emulsified using 28% Smix and 56% water. This NE region was still larger than the NE region formed at an Smix ratio of 1:1. Therefore, the three Smix ratio levels used in the experimental design were 1:2, 1:1, and 2:1.
 |
Figure 1 Pseudoternary phase diagram constructed at different Smix ratios. (A) Smix 1:1. (B) Smix 1:2. (C) Smix 1:3. (D) Smix 2:1. (E) Smix 3:1. |
Box‒Behnken Design Analysis
The BBD used Design Expert software to test the effect of the independent factors and their combinations on the estimated responses, statistical parameters, and model fitness and validity. The ANOVA test validated the selected model at a confidence level of approximately 95% (p < 0.05). The accuracy and precision of the obtained mathematical model were evaluated using checkpoint analysis for the dependent variables’ expectations. Surface responses and the desirability ramp of the required responses were developed depending on the optimal region where the optimized formulation could be acquired. Eventually, a statistical solution with acceptable desirability was attained, and the expected and experimental parameters for the optimal NE were compared. The three-dimensional (3D) surface and contour plots, as well as the main effect outline that explored the effect of the studied factors on the measured responses, are illustrated in Figures 2–5.
 |
Figure 2 Main effect (A), contour (B), and 3D surface (C) outlines illustrating the effects of different factors on response Y1 (droplet size) of various OEO-SNEDDSs. |
 |
Figure 3 Main effect (A), contour (B), and 3D surface (C) outlines illustrating the effects of different factors on response Y2 (stability index) of various OEO-SNEDDSs. |
 |
Figure 4 Main effect (A), contour (B), and 3D surface (C) outlines illustrating the effects of different factors on response Y3 (antibacterial activity) of various OEO-SNEDDSs. |
 |
Figure 5 Main effect (A), contour (B), and 3D surface (C) outlines illustrating the effects of different factors on response Y4 (antifungal activity) of various OEO-SNEDDSs. |
Formulation and Characterization of OEO-SNEDDSs
OEO-SNEDDSs Visual Inspection
Clear transparent dispersions with no noticeable aggregations or cracking were observed upon visual inspection of the OEO-SNEDDSs formulations, supporting the accuracy of the used concentration levels of the substances and the formation of the physically stable NEs.
Droplet Size
The physicochemical characterization of NEs should be carefully studied during the development of such systems, especially in topical drug delivery systems.33
Microemulsions and NEs can be differentiated by measuring their globule sizes. In the current investigation, the globule size of the NEs fluctuated from 150 to 500 nm, with a PDI of between 0.1 and 0.4; this can be considered an acceptable midrange that imparted reasonable homogeneity and a satisfactory size distribution (Table 2).
 |
Table 2 Box‒Behnken Design Responses of OEO-SNEDDSs |
The polynomial analysis of the NEs’ globule size values yielded a quadratic model. The design used in this study supported the efficiency of the model in evaluating the effect of the OEO% (A), Smix% (B), HLB of Smix (C), and Smix ratio (D) on the globule size of the NEs. The suggested mathematical model had predicted and adjusted R2 values of 0.9893 and 0.9688, respectively; they are remarkably similar values (Table 3). The equation resulting from the one-way ANOVA was as follows;
 |
Table 3 Regression Analysis Results for Responses Y1, Y2, Y3, and Y4 |
As observed, factor A had a significant synergistic effect on the globule size, with a p-value of less than 0.0001, but factor B exerted a significant antagonistic effect for the same response at the same significance level. Such outcomes could be due to a higher percentage of oil producing larger droplets. Furthermore, when the OEO percentage increased, the Smix ratio decreased accordingly, and this might have lowered the capacity of the surface agents to decrease the globule size, leading to the production of larger globules. Similarly, the increase in the Smix ratio was associated with an increase in the ability to downsize the OEO droplets and encourage their stabilization. Similar findings were repeatedly reported in the literature.34,35 Another interesting observation was the significant effect of factor C (HLB of Smix) and its quadratic term C2 on the droplet size of the NEs. As shown in Figure 2A, this meant that the peripheral levels of factor C had a positive effect on the globule size, whereas the middle level had a negative effect, causing the formation of smaller droplets. This result could have been because each lipid material must have a specific HLB value to reduce the NE droplet size. Several research groups have supported this theory.36–38 Based on that information, it could be concluded that the optimum HLB value for OEO used in an NE should be in the middle of the range in the experimental design. It was also noteworthy that factor D exerted an insignificant effect on the globule size parameter (p-value = 0.1290). Figure 2 shows the influence of the independent factors on response Y1; this is demonstrated by the 3D surface and contour plots, in addition to the main effect diagram.
Stability Index
The stability index is an important manifestation of the overall stability of an NE. The stability index (Y2) of the formulations in our study was estimated at 47% to 95%, as shown in Table 2. Based on the results, Y2 followed a quadratic mathematical model of polynomial analysis, as demonstrated by the adopted statistical design. The acquired model attained an experimental R2 value of 0.9390, which was in line with an expected R2 value of 0.8191, as seen in Table 3. The ANOVA of the collected data resulted in the equation below:
As per the equation, factor A had a significantly antagonistic influence on response Y2 (p-value < 0.0161), whereas factor B exerted a significantly synergistic action on the same response (p-value < 0.0001). Factors C and D had an insignificant effect on the stability index of the NEs (p-value = 0.1760 and 0.7040, respectively).
The negative effect of factor A, the percentage of OEO, on the stability index might have been because of the higher droplet size values associated with a higher level of OEO used, enhancing the globules’ creaming rate and consequently disrupting the stability of the NEs. Similarly, the positive effect of factor B, the percentage of Smix, on response Y2 could also have been due to its effect on the globules’ size. Because factor B decreased the droplets’ size, it would be expected that it would enhance the formulations’ stability; this is because it participates in the suspension of oil globules in the external phase for longer periods.39
Interestingly, the quadratic term for factor C (ie, C2) had a significantly negative effect on the stability of the NEs (p-value < 0.0001). As depicted in Figure 3A, this means that the peripheral values of factor C exerted a more prominent negative effect on Y2, whereas the middle values exerted a positive effect on the same response. The decrease in the stability index observed at the low or high HLB level of the used Smix can support the notion that each oil requires a specific HLB value. As previously assumed, the required HLB of OEO yields the smallest globule size; hence, at such an HLB, the surfactants’ blend would give the highest turbidity levels because it lowers the NEs’ creaming rate, increasing the NEs’ stability during the storage period. Comparable outcomes were found in the literature.36,39 The independent variables’ influence on the stability index was demonstrated by the 3D surface and contour plots and main effect outline as presented in Figure 3.
Antibacterial Activity of OEO-SNEDDSs
The antibacterial effect of the formulated NEs was detected for S. mutans by measuring the growth inhibition zones in petri dishes in which the NEs had been placed. As shown in Table 2, the diameter of these inhibition zones was 3.5 to 19 mm.
The BBD, which estimated the influence of the factors on the diameters of the bacterial inhibition zones, revealed that the antimicrobial activity against S. mutans (Y3) followed a quadratic statistical model of polynomial analysis. The obtained model had an adjusted R2 value of 0.9922, close to a predicted R2 value of 0.9769, as shown in Table 3. The collected data were analyzed by adopting the analysis of variance test and the equation below:
Factors A and B exerted a significantly positive effect on response Y3 (p-value < 0.0001), but factors C and D had an insignificant effect on the same response.
The bacterial phospholipid cell membrane is the most important focus of intervention for OEO constituents such as terpenoids.40 Carvacrol and thymol may decay the bacteria’s outer membrane and release its internal lipopolysaccharide compounds, increasing the permeation of adenosine triphosphate and, consequently, altering the all over passive cell permeation.41 Several reports have recorded the great ability of OEO to inhibit the growth of several bacterial strains.42
An increase in the Smix percentage increased the antibacterial activity of the NEs because it decreased the globule size. Smaller globules meant a broader surface area for interference with the bacterial cell membranes and the facilitation of the antimicrobial activity of the NEs. In addition, high Smix levels could be more effective in disrupting the lipid content of cell walls because of their surface active effect.43 The independent variables’ influence on antibacterial activity was demonstrated by 3D surface and contour plots and main effect outlines as shown in Figure 4.
Antifungal Activity of OEO-SNEDDSs
The antifungal effect of the formulated NEs was assessed against C. albicans by examining the inhibition zone in SDA petri dishes treated with the OEO-SNEDDSs. As shown in Table 2, the diameter of the inhibition zones of C. albicans ranged from 4 to 17 mm.
The BBD, which assessed the independent variables’ effect on the diameters of the inhibition zones showing fungal growth, revealed that the antifungal action against C. albicans (Y4) followed a quadratic model of polynomial equations. The proposed model had an adjusted R2 value of 0.9786, consistent with the predicted R2 value of 0.9396, as seen in Table 3. The one-way ANOVA was used to analyze the collected data to get the following equation:
Factors A and B exerted a significantly positive effect on response Y4 (p < 0.0001), but factors C and D had an insignificant effect on the same response.
The antifungal effect of the OEO phenolic compounds, such as thymol and carvacrol, might be ascribed to their ability to damage cellular enzymes, including those responsible for energy and the production of structural components.44 The OEO phenolic compounds are thought to denature enzymes or interfere with amino acids responsible for spore germination.45,46 Some research groups have illustrated the irreversible cellular damage exerted by OEO against Aspergillus parasiticus and Aspergillus flavus.47,48
An increase in the Smix percentage increased the antifungal activity of the OEO-SNEDDSs. Such an increase could be ascribed to a decrease in globule size, providing a larger surface area for contact with fungal cell membranes and thus facilitating the antifungal activity of the NEs. In addition, high Smix levels could also be more effective in disrupting the lipid content of cell walls through their surface active effect.49 The contour and 3D surface plots in Figure 5 demonstrate the factors’ effects on antifungal activity.
Optimization of OEO-SNEDDSs
Based on the collected data, an optimized OEO-SNEDDS formulation was developed with the most useful properties. The software proposed various suggestions for several combinations of different levels of the factors. The optimum formulation contained 18% of factor A, 36% of factor B, 10.29 for factor C, and 1.25:1 for factor D. The optimum NE formulation had a droplet size of 192 nm, stability index of 90%, antibacterial inhibition zone of 14.5 mm, and antifungal inhibition zone of 17 mm, with a desirability of 0.855. Figure 6 illustrates the ramp of desirability for different levels of the factors and the expected results of the dependent variables of the optimized OEO-SNEDDS. Table 4 shows that the experimental and suggested values of the responses of the optimum NE formulation were in line without significant variations (p > 0.05), confirming the model’s validation and precision.
 |
Table 4 Actual and Experimental Values of the Optimized NE Formulation |
Checkpoint Analysis
The experimental and expected R2 values supported the predictive accuracy of the proposed regression models. Further, the ratios of the actual to expected values had a low percentage of error, and there were reasonable residuals between the predicted and experimental responses; this suggests a lack of curvature in the data and adequacy of the model (Table 5).
 |
Table 5 Composition of and Actual and Predicted Responses of the Optimal NE Formulation |
Ulcer Index Determination
N.B. Optimized oregano oil nanoemulsion (O1), oregano oil (O2), commercially available mouthwash containing oregano oil (O3), and normal saline (NS).
As shown in Table 6, the ulcer index estimations in the animal groups were variable. The group of rats that was treated with the optimized OEO-SNEDDS had the lowest ulcer index. The group that was treated with pure OEO had the highest ulcer index, comparable to the negative control group treated with normal saline. One-way ANOVA analysis proved that there was a significant improvement in the ulcer index with the use of the OEO-SNEDDS (Table 7).
 |
Table 6 Ulcer Index Values of Different Rat Groups (Mean ± SD, n = 6) |
 |
Table 7 Analysis of Variance of Ulcer Index Data in Different Rat Groups |
From the information shown, it can be deduced that OEO can greatly inhibit ulcer formation in the buccal mucosa when it is in an NE. Based on a literature review,16,50 we found that OEO can markedly decrease the levels of various inflammatory mediators, such as the interferon-inducible T-cell alpha chemoattractant (I-TAC), monokine induced by gamma interferon (MIG), vascular cell adhesion molecule 1 (VCAM-1), monocyte chemoattractant protein 1 (MCP-1), interferon gamma-induced protein 10 (IP-10), and intracellular cell adhesion molecule 1 (ICAM-1). In addition, some investigated tissue remodeling mediators, namely, the epidermal growth factor receptor (EGRF), collagen I, and collagen III, were also markedly diminished by OEO treatment.51
Notably, carvacrol has been reported to prevent carrageenan-induced inflammation, as well as tumor necrosis factor-alpha production, in a mouse model.52 The same compound was found to have antiulcer and antiinflammatory characteristics in a model of edema in rats. Such outcomes suggest that carvacrol might interfere with the production of inflammatory mediators and hence improve the healing of ulcers.53,54 As a result, OEO could be considered a good candidate for relieving the pain and inflammation associated with some oral and dental conditions. Similar results were reported in the literature for the ability of cinnamon oil-based NEs to exert good antifungal, antibacterial, and analgesic effects against oral microbiota. However, we feel that further investigations need to be performed in humans for confirming the protective effect of the formulation against oral microbiota.
Conclusion
OEO was successfully formulated as an NE with adequate properties. Optimum concentrations of OEO, Pluracare L64, Lauroglycol FCC, and Glycofurol mixtures were determined using a pseudoternary phase diagram to determine the most satisfactory NE regions that could produce the required drug delivery system. The produced NEs had a globule size ranging from 150 to 500 nm with a suitable homogeneous distribution and a stability index of 47% to 95%, indicating reasonable stability of the systems. Additionally, the formulations had antibacterial and antifungal inhibition zones of up to 19 mm and 17 mm, respectively. The BBD was adopted to get the optimum formulation, which was composed of 18% OEO, 36% Smix, 10.29 HLB of Smix, and a 1.25:1 Smix ratio. Finally, the optimized OEO-SNEDDS had the best ulcer index value compared with various formulations when used in rats. Collectively, this investigation illustrated that OEO-based NEs could provide good protection against oral diseases caused by microbial infections.
Acknowledgment
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia has funded this project, under grant no. (RG-18-166-42). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia has funded this project, under grant no. (RG-18-166-42).
Disclosure
The authors declare no conflict of interest.
References
1. Silva AE, Demarco FF, Feldens CA. Oral health-related quality of life and associated factors in Southern Brazilian elderly. Gerodontology. 2015;32(1):35–45. doi:10.1111/ger.12050
2. World Health Organization. Oral health fact sheet; 2012. Available from: https://www.who.int/news-room/fact-sheets/detail/oral-health.
3. Rajan B, Ahmed J, Shenoy N, Denny C, Ongole R, Binnal A. Assessment of quality of life in patients with chronic oral mucosal diseases: a questionnaire-based study. Perm J. 2014;18(1):e123–7. doi:10.7812/TPP/13-095
4. Yilmaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology. 2008;154(10):2897–2903. doi:10.1099/mic.0.2008/021220-0
5. Bik EM, Long CD, Armitage GC, et al. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010;4(8):962–974. doi:10.1038/ismej.2010.30
6. Trim RD, Skinner MA, Farone MB, et al. Use of PCR to detect Entamoeba gingivalis in diseased gingival pockets and demonstrate its absence in healthy gingival sites. Parasitol Res. 2011;109(3):857–864. doi:10.1007/s00436-011-2312-9
7. Bahrani-Mougeot FK, Paster BJ, Coleman S, et al. Diverse and novel oral bacterial species in blood following dental procedures. J Clin Microbiol. 2008;46(6):2129–2132. doi:10.1128/JCM.02004-07
8. Mascitti M, Togni L, Troiano G, et al. Beyond head and neck cancer: the relationship between oral microbiota and tumour development in distant organs. Front Cell Infect Microbiol. 2019;26:232. doi:10.3389/fcimb.2019.00232
9. Van der Ploeg JR. Regulation of bacteriocin production in Streptococcus mutans by the quorum-sensing system required for development of genetic competence. J Bacteriol. 2005;187(12):3980–3989. doi:10.1128/JB.187.12.3980-3989.2005
10. Ghannoum MA, Jurevic RJ, Mukherjee PK, et al. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathogens. 2010;6(1):e1000713. doi:10.1371/journal.ppat.1000713
11. Sharma N, Bhatia S, Sodhi AS, Batra N. Oral microbiome and health. AIMS Microbiol. 2018;4(1):42–66. doi:10.3934/microbiol.2018.1.42
12. De Martino L, De, Feo V, Formisano C, Mignola E, Senatore F. Chemical composition and antimicrobial activity of the essential oils from three chemotypes of Origanum vulgare L. ssp. hirtum (Link) Ietswaart growing wild in Campania (Southern Italy). Molecules. 2009;14(8):2735–2746. doi:10.3390/molecules14082735
13. Daferera DJ, Ziogas BN, Polissiou MG. GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J Agric Food Chem. 2000;48(6):2576–2581. doi:10.1021/jf990835x
14. Rodriguez-Garcia BA, Silva-Espinoza LA, Ortega-Ramirez JM, et al. Oregano essential oil as an antimicrobial and antioxidant additive in food products. Crit Rev Food Sci Nutr. 2016;56(10):1717–1727. doi:10.1080/10408398.2013.800832
15. Ocak I, Ali Çelik M, Özel Z, Korcan E, Konuk M. Antifungal activity and chemical composition of essential oil of origanum hypericifolium. Int J Food Prop 2012;15(1):38–48. doi:10.1080/10942911003687249
16. Han X, Parker TL. Anti-inflammatory, tissue remodeling, immunomodulatory, and anticancer activities of oregano (Origanum vulgare) essential oil in a human skin disease model. Biochim Open. 2017;4:73–77. doi:10.1016/j.biopen.2017.02.005
17. Thosar N, Basak S, Bahadure RN, Rajurkar M. Antimicrobial efficacy of five essential oils against oral pathogens: an in vitro study. Eur J Dent. 2013;7(S 01):S071–S077. doi:10.4103/1305-7456.119078
18. Odriozola-Serrano I, Oms-Oliu G, Martín-Belloso O. Nanoemulsion-based delivery systems to improve functionality of lipophilic components. Front Nutr. 2014;1:24. doi:10.3389/fnut.2014.00024
19. Qian C, McClements D. Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting size. Food Hydrocoll. 2011;25(5):1000–1008. doi:10.1016/j.foodhyd.2010.09.017
20. Singh H, Kumar R, Ahuja N. Optimizing drug delivery systems using systematic “Design of Experiments.” Part I: fundamental aspects. Crit Rev Ther Drug Carrier Syst. 2005;22:27–106.
21. Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J Pharm Pharmacol. 2011;63(3):342–351. doi:10.1111/j.2042-7158.2010.01225.x
22. Huang J, Goolcharran C, Ghosh K. A quality by design approach to investigate tablet dissolution shift upon accelerated stability by multivariate methods. Eur J Pharm Biopharm. 2011;78(1):141–150. doi:10.1016/j.ejpb.2010.12.012
23. Hosny KM, Alhakamy NA, Sindi AM, Khallaf RA. Coconut oil nanoemulsion loaded with a statin hypolipidemic drug for management of burns: formulation and in vivo evaluation. Pharmaceutics. 2020;12(11):1061. doi:10.3390/pharmaceutics12111061
24. Ngan CL, Basri M, Tripathy M, Karjiban RA, Abdul-Malek E. Physicochemical characterization and thermodynamic studies of nanoemulsion-based transdermal delivery system for fullerene. Sci World J. 2014;2014:1–12. doi:10.1155/2014/219035
25. Hosny KM, Alhakamy NA, Almodhwahi MA, Kurakula M, Almehmady AM, Elgebaly SS. Self-nanoemulsifying system loaded with sildenafil citrate and incorporated within oral lyophilized flash tablets: preparation, optimization, and in vivo evaluation. Pharmaceutics. 2020;12(11):1124. doi:10.3390/pharmaceutics12111124
26. Moradian H, Bazargani A, Rafiee A, Nazarialam A. In vitro comparison of antimicrobial activity of aqueous decoction of coriandrum sativum, and dentol drop with chlorhexidine on Streptococcus mutans. Iran J Microbiol. 2013;5(3):239–243.
27. Beressa TB, Deyno S, Alele PE. Antifungal activity of the essential oil of Echinops kebericho Mesfin: an in vitro study. Evid Based Complement Alternat Med. 2020;3101324.
28. Martinez A, Regadera J, Jimenez E, Santos I, Gargallo-Viola D. Antifungal efficacy of GM237354, a sordarin derivative, in experimental oral candidiasis in immunosuppressed rats. Antimicrob Agents Chemother. 2001;45(4):1008–1013. doi:10.1128/AAC.45.4.1008-1013.2001
29. Hamouda T, Myc A, Donovan B, Shih AY, Reuter JD, Baker JR
30. Hamouda T, Hayes MM, Cao Z, et al. A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus species. J Infect Dis. 1999;180(6):1939–1949. doi:10.1086/315124
31. Karthikeyan R, Amaechi BT, Rawls HR, Lee VA. Antimicrobial activity of nanoemulsion on cariogenic Streptococcus mutans. Arch Oral Biol. 2011;56(5):437–445. doi:10.1016/j.archoralbio.2010.10.022
32. Narang JK, Narang RS. Emerging role of nanoemulsions in oral health management. Int J Pharm Investig. 2017;7(1):1–3. doi:10.4103/jphi.JPHI_32_16
33. Üstündaǧ Okur N, Apaydin Ş, Karabay Yavaşoǧlu NÜ, Yavaşoǧlu A, Karasulu HY. Evaluation of skin permeation and anti-inflammatory and analgesic effects of new naproxen microemulsion formulations. Int J Pharm. 2011;416:136–144.
34. Sakeena MHF, ElRashid SM, Munavvar AS, Azmin MN. Effect of oil and drug concentrations on droplet size of palm oil esters nanoemulsions. J Oleo Sci. 2011;60(4):155–158. doi:10.5650/jos.60.155
35. Okur ME, Ayla S, Yozgatlı V, et al. Evaluation of burn wound healing activity of novel fusidic acid loaded microemulsion based gel in male Wistar albino rats. Saudi Pharm J. 2020;28:338–348.
36. Orafidiya LO, Oladimeji FA. Determination of the required HLB values of some essential oils. Int J Pharm. 2002;237(1–2):241–249. doi:10.1016/S0378-5173(02)00051-0
37. Chong WT, Tan CP, Cheah YK, et al. Optimization of process parameters in preparation of tocotrienol-rich red palm oil-based nanoemulsion stabilized by Tween80-Span 80 using response surface methodology. PLoS One. 2018;13(8):e0202771. doi:10.1371/journal.pone.0202771
38. Wen-Chien L, Chiang B-H, Huang D-W, Po-Hsien L. Skin permeation of d-limonene-based nanoemulsions as a transdermal carrier prepared by ultrasonic emulsification. Ultrason Sonochem. 2014;21(2):826–832. doi:10.1016/j.ultsonch.2013.10.013
39. Boyd J, Parkinson C, Sherman P. Factors affecting emulsion stability and the HLB concept. J Colloid Interface Sci. 1972;41(2):359–370. doi:10.1016/0021-9797(72)90122-1
40. Gullapalli RP, Sheth BB. Influence of an optimized non-ionic emulsifier blend on properties of oil-in-water emulsions. Eur J Pharm Biopharm. 1999;48(3):233–238. doi:10.1016/S0939-6411(99)00048-X
41. Gumus T, Demirci A, Sagdic O, Arici M. Inhibition of heat resistant molds: aspergillus fumigatus and Paecilomyces variotii by some plant essential oils. Food Sci Biotechnol. 2010;19(5):1241–1244. doi:10.1007/s10068-010-0177-9
42. Guarda A, Rubilar JF, Miltz J, Galotto MJ. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011;146(2):144–150. doi:10.1016/j.ijfoodmicro.2011.02.011
43. Cosge B, Turker A, Ipek A, Gurbuz B, Arslan N. Chemical compositions and antibacterial activities of the essential oils from aerial parts and corollas of Origanum acutidens (Hand.-Mazz.) Ietswaart, an endemic species to turkey. Molecules. 2009;14(5):1702–1712. doi:10.3390/molecules14051702
44. Neumeister B, Woerndle S, Bartmann P. Effects of different surfactant preparations on bacterial growth in vitro. Biol Neonate. 1996;70(2):128–134. doi:10.1159/000244357
45. Kordali S, Cakir A, Ozer H, Cakmakci R, Kesdek M, Mete E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour Technol. 2008;99(18):8788–8795. doi:10.1016/j.biortech.2008.04.048
46. Bluma R, Amaiden MR, Daghero J, Etcheverry M. Control of Aspergillus section Flavi growth and aflatoxin accumulation by plant essential oils. J Appl Microbiol. 2008;105(1):203–214. doi:10.1111/j.1365-2672.2008.03741.x
47. Conner DE, Beuchat LR. Sensitivity of heat stressed yeast to essential oils of plant. Appl Environ Microbiol. 1984;47(2):229–233. doi:10.1128/aem.47.2.229-233.1984
48. Rasooli I, Owlia P. Chemoprevention by thyme oils of Aspergillus parasiticus growth and aflatoxin production. Phytochemistry. 2005;66(24):2851–2856. doi:10.1016/j.phytochem.2005.09.029
49. Helal GA, Sarhan MM, Abu Shahla AN, Abou El-Khair EK. Effect of Cymbopogon citratos L. essential oil on the growth, morphogenesis and aflatoxin production of Aspergillus flavus ML2-strains. J Basic Microbiol. 2007;47(1):5–15. doi:10.1002/jobm.200610137
50. Falk NA. Surfactants as antimicrobials: a brief overview of microbial interfacial chemistry and surfactant antimicrobial activity. J Surfactants Deterg. 2019;22:1119–1127.
51. Bimczok D, Rau H, Sewekow E, Janczyk P, Souffrant WB, Rothk€otter HJ. Influence of carvacrol on proliferation and survival of porcine lymphocytes and intestinal epithelial cells in vitro. Toxicol Vitro. 2008;22(3):652–658. doi:10.1016/j.tiv.2007.11.023
52. Marrelli M, Conforti F, Formisano C, et al. Composition, antibacterial, antioxidant and antiproliferative activities of essential oils from three Origanum species growing wild in Lebanon and Greece. Nat Prod Res. 2016;30(6):735–739. doi:10.1080/14786419.2015.1040993
53. Guimar~aes AG, Silva FV, Xavier MA, et al. Orofacial analgesic-like activity of carvacrol in rodents. Z Naturforsch. 2012;67:481–485.
54. Silva FV, Guimar~aes AG, Silva ERS, et al. Anti-inflammatory and antiulcer activities of carvacrol, a monoterpene present in the essential oil of oregano. J Med Food. 2012;15(11):984–991. doi:10.1089/jmf.2012.0102
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.