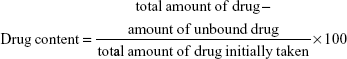Back to Journals » Drug Design, Development and Therapy » Volume 13
Formulation and evaluation of simvastatin polymeric nanoparticles loaded in hydrogel for optimum wound healing purpose
Authors Farghaly Aly U, Abou-Taleb HA, Abdellatif AH , Sameh Tolba N
Received 16 December 2018
Accepted for publication 20 February 2019
Published 10 May 2019 Volume 2019:13 Pages 1567—1580
DOI https://doi.org/10.2147/DDDT.S198413
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Usama Farghaly Aly,1 Heba A Abou-Taleb,2 Ahmed AH Abdellatif,3,4 Nahla Sameh Tolba2
1Department of Pharmaceutics, Faculty of Pharmacy, Minia University, EL-Minia, Egypt; 2Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Nahda University (NUB), Beni-Suef, Egypt; 3Department of Pharmaceutics, College of Pharmacy, Qassim University, Al Qassim, Kingdom of Saudi Arabia; 4Department of Pharmaceutics and Industrial Pharmacy, Al-Azhar University, Assiut, Egypt
Purpose: The aim of this study was to formulate a hydrogel loaded with polymeric nanoparticles (PoNPs) of simvastatin (SIM) for topical wound healing application.
Materials and methods: The SIM PoNPs were prepared by using the nanoprecipitation method to improve the drug solubility and skin permeation. Furthermore, drug content, solubility, particle size, surface charge, and transmission electron microscopy of the prepared PoNPs were evaluated. Then, the PoNPs were loaded on hydrogel, and physical characteristics, in vitro release, and ex vivo permeation of the hydrogel were evaluated. Finally, the prepared gel was applied on rat wounds, and a histopathological study was performed.
Results: The results showed that the drug content in the PoNPs was 86.4%. The PoNPs were spherical in shape with a smooth surface and a uniform size distribution. The particle size was 268.4±2.6, polydispersity index was ≤0.302, and zeta potential was -33±1.67 mV. The hydrogel loaded with SIM PoNPs was homogenous, and the pH was accepted and compatible with the skin. Moreover, the viscosity and spreadability assured its ease of application. The drug content was 97.25±0.02%. Furthermore, about 81% of SIM was released within 24 hours, while in the ex vivo permeation study 69.19% of SIM passed through the skin after 24 hours. Finally, the histopathological studies confirmed the efficacy of the SIM PoNPs-loaded hydrogel in wound healing due to the formation of the normal epithelial layer on day 11 after wound creation.
Conclusion: The hydrogel loaded with SIM PoNPs showed a good efficacy in accelerating the healing of the rat wound with complete epithelialization and minimal inflammatory cell infiltration.
Keywords: simvastatin, Carbopol® gel, ex vivo permeation, nanoprecipitaion method, wound healing
Introduction
Wound healing is viewed as one of the greatest clinical issues and is tricky for the patients and the public too. Wounds are susceptible to bacterial infections, which disturbs the healing process by inducing inflammation and tissue damage.1 Therefore, there is a need to find better wound care modalities to promote healing.2 Polymeric nanoparticles (PoNPs)-loaded gels such as hydrogel have widely been used as a carrier in drug delivery systems, especially in topical wound healing application. The hydrogels can be defined as three-dimensional cross-linked networks of water-soluble polymers. The density of the cross-links in the gel matrix can influence the porosity, which subsequently can influence drug loading into and release from the matrix.3 The self-sorting of different molecular building units into distinct coexisting nanoarchitectures with high aggregate numbers produces an orthogonal self-assembly, which can be used as a drug delivery gel with a postproduction tunable release rate.4 Gels with embedded vascular structures and multicompartment hydrogel can help in delivering many drugs and combination therapies.5
Simvastatin (SIM) is an inhibitor of 3-hydroxy-3-methyl-glutaryl-CoA reductase.6 It is available as tablets taken for decreasing the blood cholesterol.7 Recently, SIM has been shown to have a unique activity, other than its antihyperlipidemic activity. It could also promote the wound healing process.2 It can increase the synthesis and release of vascular endothelial growth factor at the wound site, which is a serious step for the production of new blood vessels. SIM can also improve the epithelialization and revive the normal skin epidermal barrier by reducing isoprenylation downstream targets of mevalonate and farnesyl pyrophosphate (FPP). A decrease in FPP level can stimulate keratinocyte migration in vitro and epithelialization and wound closure in an ex vivo human culture wound healing model.8 The observations and the animal studies proved the effective role of SIM in accelerating the wound healing.2 Thus, it can offer a solution to the problem of interest to a wide range of patients. SIM has also been shown to have antimicrobial activity, it can suppress Gram-positive bacteria, and thus, it can be used as an alternative to some of the conventionally known antibiotics.9 SIM is water-insoluble and has low bioavailability.10 Therefore, it was formulated as a nanoparticle to improve its solubility and skin permeation.11 Nanoprecipitation method is an extensively applicable method for the preparation of PoNPs. It presents several advantages as it is a direct technique, is rapid, is easy to make besides being appropriate for most of the poorly soluble drugs.12 In nanoprecipitation method, an organic solvent such as acetone, acetonitrile, methylene chloride, methanol, or ethyl acetate was used to dissolve the drug. Then, the organic solvent was evaporated by pressure reduction or via constant stirring.13 Several factors may influence the particle size such as stabilizer type, its concentration, and homogenizer speed. Ultrasonication or high-speed homogenization may be used to yield a small particle size.14 Nanoprecipitation technique mainly produces small nanoparticles with a narrow distribution.15
This research intended to formulate the SIM as a PoNP to advance its solubility and skin permeation to be topically used on skin wounds. The topical application of the antimicrobial agent is a valued mean for curing skin and soft tissue infection as it has many advantages compared with systemic therapy.16
Materials
SIM was purchased from Sigma-Aldrich (Taufkirchen, Germany). Tween-80 was purchased from Merck Schuchardt OHG (Hohenbrunn, Germany); sodium deoxycholate and methyl cellulose from Sigma-Aldrich (St Louis, MO, USA); and polyvinylpyrrolidone K-90, polyethylene glycol 4000 (PEG 4000), and cellulose acetate membrane (dialysis membrane with a molecular weight cutoff of 12,000–14,000, Fisherbrand) from Fisher Scientific (Leicestershire, UK). All other chemicals and analytical reagents were of analytical grades and used as received.
Methodology
Preparation of PoNPs
The formulated nanoparticles were prepared by the nanoprecipitation method.14 Briefly, SIM was dissolved in an organic phase at a room temperature. This organic phase was added into 30 mL aqueous phase containing a water-soluble polymer as shown in Table 1. The organic phase was added using a syringe positioned with the needle directly into the beaker at a rate of 10 mL every 2 minutes and subsequently stirred on a magnetic stirrer to allow the volatile solvent to be evaporated.
Formulae 1, 2, 3, 10, 11, and 12 contained 1 mg methyl cellulose (equivalent to 80.82 μM) as a stabilizer, while the stabilizer in formulae 4, 5, 6, 13, 14, and 15 was a mixture of 1 mg stearic acid (equivalent to 117.17 μM) and 1 mg sodium deoxycholate (equivalent to 80.41 μM). The rest of the formulae contained 10 mL glycerol (equivalent to 6.11 M) as a cosolvent.
The prepared formulae were then purified by centrifugation to separate the formulated PoNPs from any large-sized nonreacting molecules. Then, they were centrifuged at 5,000 rpm for 5 minutes.
Drug content
The prepared nanosuspension was centrifuged at 10,000 rpm for 15 minutes. Then, 5 mL of supernatant was diluted with 100 mL methanol, and the amount of unbound drug was measured by taking the absorbance of the diluted supernatant solution at λmax=238 nm using a double-beam ultraviolet (UV) spectrophotometer (Genesis 10 UV; Thermo Electron Corporation, Beverly, MA, USA) against methanol as a blank. Drug content was calculated according to Equation 1. The experiment was performed in triplicate for each batch, and the average was calculated.17
|
|
Solubility studies
The aqueous solubility of SIM was determined by using the shake-flask method,18 and the results were analyzed by ultraviolet absorbance at 238 nm using a spectrophotometer (Genesis 10 UV). The solubility studies were carried out for both the unprocessed pure drug and the optimized batch of SIM nanosuspension (F20). The results were analyzed in triplicate, and SDs were calculated.19
Estimation of particle size and surface charge
Dynamic light scattering (DLS) was used to determine the size, count rate, and surface charge of SIM PoNPs; samples were adjusted to 25°C and subjected to laser light with an incident laser beam of 633 nm at a scattering angle of 90° using the Malvern Zetasizer Nano 6.01 (Malvern Instruments GmbH, Herrenberg, Germany). The results were calculated from the average of three independent measurements.12
Transmission electron microscopy (TEM)
Before the operation of the TEM, the formulated PoNPs loaded with SIM were diluted to 0.01% w/w and then placed in an ultrasonic bath (Model 3510; Branson Ultrasonics, Danbury, CT, USA) to reduce particle aggregation. A drop of the diluted sample was negatively stained with 5 μL of 2% w/v uranyl acetate, and the mixture was stirred for about 2 minutes to enhance the contrast of the TEM images and then placed onto a holey carbon-coated 400-mesh copper grid; the excess suspension was adsorbed immediately using a filter paper. The morphology of the SIM-loaded PoNPs was characterized using TEM (JEOL 100CX; JEOL Inc., Peabody, MA, USA) with an accelerating voltage of 80 kV. The distribution of SIM PoNP dimensions was evaluated from TEM images by using ImageJ 1.45 k software (Rasband, W.S., ImageJ; U.S. National Institutes of Health, Bethesda, MD, USA).20
Preparation of hydrogel loaded with SIM PoNPs
The prepared SIM PoNPs were designed for the purpose of wound healing. Therefore, it was formulated into a gel to ease the application and to protect the prepared SIM PoNPs from the environment. Carbopol® 934 (1%) was soaked in 10 mL of water for 24 hours. Then, it was dispersed gently into the prepared PoNPs-loaded SIM suspension with constant stirring using a magnetic stirrer (Nickel Electro™ CH-1E; Nickel-Electro Ltd., Weston-super-Mare, UK) to prevent lump formation or any aggregation and to form a homogeneous dispersion. Finally, 0.4% of NaOH was added as a neutralizing agent to the gel to adjust the pH to achieve maximum thickening to Carbopol® polymers.21
Physical evaluations and characteristics
Gel formulations were visually inspected for appearance, color, washing, phase separation, and odor after the gels had been set in a container.22 The pH value of the produced PoNP gel was measured using a digital pH meter (3500 pH meter; Jenway, Stone, Staffordshire, UK) by the method previously described by Aly et al.23 The viscosities of the prepared formulations were measured in centipoise (cps). By using a digital viscometer (Model: DV-ERVDEV230), the viscosities were determined at different angular velocities at 25.0°C±0.1°C using Spindle No 4 (DV. Ultra, RVDV-111 U; AMETEK Brookfield, Middleboro, MA, USA). The spreadability of the prepared gel measured in g·cm/sec was determined according to the method described by Ubaid et al.21
Estimation of the drug content of SIM in the formulated hydrogel
The amount of drug in the gel was determined by dissolving a specified amount of the gel in 10 mL methanol, stirring using a magnetic stirrer (Clifton™) until SIM was completely dissolved, and then centrifuging at 4,000 rpm for 30 minutes. Clear supernatant was diluted with methanol and measured spectrophotometrically at λmax=238 nm.24
In vitro release rate of SIM from the prepared gel
In vitro release of SIM from the gel was carried out on modified Franz diffusion cell, and the temperature was maintained at 37°C±1°C. The formulation was placed into the donor chamber. Dissolution test was carried out in two different media: phosphate buffer (pH 7.4), which simulates the natural wound environment (pH =7.15–8.90),6,25 and an aqueous medium that consists of a mixture of 78% deionized water, 20% methanol, and 2% Tween-80 with pH adjusted to 5.5, which is similar to the pH of the skin. At specific time intervals (namely 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 hours), aliquots of 1 mL were withdrawn and immediately restored with the same volume of fresh dissolution media. The amount of the drug released was determined by measuring the absorbance at 238 nm using a double-beam UV–visible (Vis) spectrophotometer (V-530; Jasco Corporation, Tokyo, Japan). The dissolution test was also applied to raw SIM (an equivalent amount of the prepared formulae) dispersed in the plain Carbopol® gel using the aqueous media to study the effect of nanosizing on the drug release rate.26
Ex vivo permeation study
The permeability of SIM from the gel was studied across the abdominal skin of white male Wistar rats (Rattus norvegicus). The rat’s abdominal hair was shaved before the rat was sacrificed. The permeability experiments were initiated within 2–3 hours after the sacrifice. The skin was cleaned using Dulbecco’s PBS (pH =7.4). The test was carried out on the modified Franz cell as follows: The skin was fixed between the donor and the receptor chamber so that stratum corneum was placed upward. The receptor chamber was filled with 5 mL aqueous media previously mentioned in the release test. Samples of 1 mL were collected at predetermined time intervals (namely 0.5, 1, 2, 3, 4, 6, 8, 12, and 24 hours), and an equivalent amount of fresh dissolution fluid equilibrated at the same temperature was replaced. Samples were diluted suitably and analyzed at 238 nm for the cumulative amount of drug released across the skin using a UV–Vis spectrophotometer. Permeability studies were carried out in triplicate.27
The release kinetics were determined by linear regression analysis of the in vitro release and ex vivo permeation curves in various mathematical models.28 The mathematical model that best expressed the kinetic release profile was selected based on the highest coefficient of determination (R2).
Study on the wound healing activity of SIM hydrogel and levofloxacin hemihydrate (LEV) hydrogel and their mixtures in rats
Animals
White male Wistar rats (Rattus norvegicus), weighing 150±20 g, were housed in individual cages at a temperature- and humidity-controlled room with free access to tap water and diet. The guidelines followed for the welfare of the animals are described in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.29 It was also approved by the Research Ethics Committee for Animal Experimentation, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Minia University, Egypt (Project Code No 2017:024). The rats were housed and bred under standardized conditions in the preclinical animal house. They were kept in mesh-bottomed stainless steel cages (six per cage), fed a standard diet, and allowed free access to drinking water. The animals were acclimatized to the environment for 1 week before the commencement of the experiment. Also all conditions were maintained to minimize animal suffering.
The rats were anesthetized by intramuscular injection of 30 mg/kg of ketamine and intraperitoneal administration thiopental 20 mg/kg.30 The rats were kept under a stable anesthesia for 90 minutes for wound creation. The back of the rat was shaved to remove the hair from the dorsal area between the shoulder blades, and then, the surgical site was wiped three times with fresh sterile cotton tips saturated with alcohol to sterilize the surgical site. Full thickness, excisional skin wounds using 8-mm skin biopsy punches were made on the back of the rats, with two wounds created on each side of the spine of each rat, using a biopsy punch (size 8 mm). Each rat acted as its own healing control to minimize the interindividual variability as one wound received gel with the active ingredient and the other wound received plain Carbopol® gel.31 The rats were divided into three groups; each group consisted of 10 rats.
Gel formulation
Three gel formulations were studied: 1) SIM PoNPs loaded on hydrogel; 2) LEV hydrogel; and 3) mixture of SIM PoNPs and LEV hydrogel at a ratio of 1:1. LEV was chosen in this experiment as it is a broad-spectrum antimicrobial agent and effective in skin and soft tissue infection.32 LEV hemihydrate is highly water-soluble,33 and it can be easily dispersed into plain Carbopol® gel at a concentration of 1%. After surgery, topical gel formulations were applied daily on the respective wounds of each animal using cotton swabs. Each group received the medicated gel for only one wound, while the other wound received plain gel as a control. The treatment was continued for each animal till sacrifice. Data including wound area measurements were collected on days 0, 4, 7, and 11. The percent of wound contraction was then calculated according to the following equation:28
|
|
where n = number of days.
Skin sections, around 1 cm2, containing the wound areas were collected on days 0, 4, 7, and 11 after the wounds were created and fixed in 4% formaldehyde for histological study. The samples were frozen and transversely cut into 7-μm-thick sections from the middle part of the wounds, which were stained with H&E prior to examination using a light microscope. The samples were examined for epithelization and fibrosis and appearance of hair follicles.23 A quantitative estimate of wound healing was done by measuring the wound diameter. A histopathological study was also used.
Results and discussion
Stable SIM PoNPs were prepared using a new technique of stabilization. The optimum formula was stabilized using glycerol, which coated the SIM nanoparticles and increased its wettability. This step helped the particles to be easily inserted into the polymers. Moreover, the formula using glycerol enhanced the solubility of SIM up to 23.4-fold. Finally, the prepared formula showed highly improved wound healing capacity. A histopathological study confirmed the wound healing activity of SIM hydrogel, which gives our formulation the novelty. We are the only group who confirmed the wound healing activity of SIM in highly soluble PoNPs with a high percent of drug loading.
Drug content
The indirect method is suitable for determining the drug content of nanosuspensions when a high concentration of the free drug is present in the supernatant after centrifugation.34 The drug content of the SIM nanosuspension was found to be in the range of 31.7%–86.4% for the 24 prepared formulae as shown in Figure 1. The technique of SIM PoNP preparation mainly depended on dissolving the SIM in an organic solvent (acetone or methylene chloride); then, the organic solvent was mixed with an aqueous phase containing a water-soluble polymer.35 In general, SIM is poorly soluble in water26 and freely soluble in both acetone and methylene chloride,13 but acetone has a higher polarity index (5.1) and high water solubility (100% w/w) compared with methylene chloride, which has a lower polarity index (3.1) and low water solubility (1.6% w/w).36,37 The low drug content values of formulae prepared with methylene chloride as an organic solvent were mainly due to its low water solubility as it prevented the SIM from diffusing freely into the water-soluble polymer, while using acetone as an organic solvent allowed a higher drug entrapment due to its more hydrophilic nature, which helped the drug to be retained in the water-soluble polymer.7 In addition, a small amount of the drug may escape from the nanoparticles during diffusion from the organic phase to the aqueous phase.12 This may explain the low drug content of formulae F21 and F22.
  | Figure 1 Drug content of different SIM PoNP formulations. |
The following strategies were used to enhance drug content in the different formulae: increasing the amount of drug (SIM) used in formulae, changing the drug–polymer ratio, applying a slower rate of drug injection in the aqueous phase, and changing the mixing rate and time duration of the formulation process as it was observed that both particle size and drug entrapment decrease as the rate of mixing of the two phases increases. Formulae 19:24 were found to be higher in their drug content. Therefore, they were chosen to be subjected for the further evaluation studies.
Solubility studies
Solubility studies were undertaken to determine the effect of nanosizing and polymeric insertion of the SIM on its water solubility. SIM is poorly water-soluble. The untreated drug has a solubility of 1.485±0.43 μg/mL, which was in agreement with the literature,10 while the optimum formula of SIM PoNPs has a solubility of 34.74±1.1 μg/mL. This means that there was a ~23.4-fold increase in solubility. The increase in solubility may be due to the reduction in particle size to nanoscale and polymeric insertion. Furthermore, the glycerin increased the particle wettability and improved its solubility.38
Particle size and surface charge estimation
All the formulations were found to be in the nanometer size range. Figure 2 shows the mean diameter of some selected nanoparticle formulations. Formula F20 prepared by using acetone as an organic solvent, with a drug–polymer ratio of 1:3 and with glycerol, had a size of 268.4±2.6 nm with optimum drug content and was considered as the best optimized nanoparticle formulation, which was used for a further study.
  | Figure 2 (A) Particle size and PDI of the prepared formulae. (B) Zeta potential for the prepared formulae. |
Polydispersity index (PDI) is the parameter for the determination of particle size distribution of the nanoparticles. Values >0.7 indicate that the samples have a very broad size distribution.39 Although F24 has the smallest particle size (105.7±3.98 nm), it was excluded due to its high PDI (1.00) and its zeta potential (−14 mV), which is a sign of instability of the particles.
The zeta potential of F20 was −33.0 mV, which is >−20 mV, the desired value;7 it is suggested to cause larger repulsive forces between particles that can prevent aggregation and ensure easy dispersion.
TEM
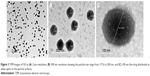
TEM was employed in studying the morphology of F20 after it was placed on a copper grid. The TEM analysis confirmed that the size of the nanoparticles was measured in nanometer, and all particles were found to be spherical in shape with PoNPs having a smooth surface and uniform size distribution.
The magnification of a single particle showed an internal cage-like structure where the drug molecules are dispersed uniformly throughout the polymer matrix (Figure 3). The drug appears as white spots on the surface as shown in Figure 3C.
The preparation procedure for TEM may affect the particle size. The particle size was in the range of 177.66–200.76 nm as shown in Figure 4, while the average size recorded with Zetasizer (DLS) was 268.4 nm, within a range of 210–320 nm, which was an indication of the similarity of the obtained results. Furthermore, the results obtained from the TEM also confirmed the uniformity of the formulated PoNPs. In addition, light scattering measured the hydrodynamic radii of the particles, which consist of the particles itself, in addition to the ionic and solvent layers associated with them in solution, under the measurement conditions. In addition, DLS is a dynamic measurement, extremely sensitive to the dispersion/aggregation behaviors of the particles in the solution.40,41
  | Figure 4 Size distribution of nanoparticles measured by TEM. |
Physical evaluations and characteristics of SIM hydrogel
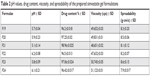
Most of the physical properties were pharmaceutically acceptable. The color was transparent to slight white without phase separation for all the prepared gels, and the appearance was translucent and smooth on application. All the prepared gels were homogeneous without any aggregation or rough particles and were washable. These characteristics are consistent with the ideal requirements for topical gel.42 The pH of the prepared gel formulations was in the range of 5.1–6.2 as shown in Table 2. This pH range is acceptable as it is not so much different from the physiological skin pH.43 In addition, it does not produce skin irritation and stabilizes the rheological behavior of the gel as Carbopol® hydrogel has an advantage that its rheological behavior does not change appreciably in the pH range of 5.0–8.0.44 The viscosity of the prepared gel formulations was in the range of 47,632–51,123 cps as shown in Table 2, which was close to the acceptable range of topical formulation used for wound healing.45 Spreadability was found to be in the range of 7.9±0.017 to 8.6±0.15 as shown in Table 2, indicating that the gel is easily spreadable by a small amount of shear.46
  | Table 2 pH values, drug content, viscosity, and spreadability of the prepared simvastatin gel formulations |
Estimation of the amount of SIM in Carbopol® gel (drug content)
Drug contents of the SIM nanosuspensions were in the range of 96.2%–98.96%, which is acceptable according to United States Pharmacopoeia47 as shown in Table 2.
In vitro release studies from the prepared gel
In the present study, the in vitro release was carried out at two different pH values. Aqueous media consist of a mixture of 78% deionized water, 20% methanol, and 2% Tween-80 with a pH similar to that of the skin (5.5) and phosphate buffer pH 7.4, which simulates the natural wound environment (pH =7.15–8.90).25
In phosphate buffer pH 7.4, SIM hydrogel showed a release rate in the range of 1%–3% after the first half an hour, increased to 24%–29% after 12 hours, and reached 36%–44% after 24 hours. In the aqueous media, the hydrogel showed a release rate in the range of 4%–8% of SIM after the first half an hour, increased to 51%–56% after 12 hours, and reached 68%–81% after 24 hours compared with the release of only 36% from the raw SIM Carbopol® gel (F20). The improvement in the dissolution rate of nanosuspension as compared to raw SIM Carbopol® gel reflects the advantages achieved by nanosizing. The solubility of SIM increased after formulation as nanosized particles resulted in a higher dissolution rate.
F20 showed the highest dissolution rate in the aqueous media, as 8% of the active ingredient was released after the first half an hour, 56% was released after 12 hours, and 81% was released after 24 hours; according to these data and the previously mentioned data (drug content, size, and zeta potential), F20 was selected for ex vivo permeation study. Figure 5 graphically illustrates the in vitro release data.
  | Figure 5 (A) Release rate of SIM from hydrogels in phosphate buffer pH 7.4, (B) Release rate of SIM from hydrogels in aqueous media at pH 5.5. |
Although the SIM solubility is pH-dependent and the highest solubility of SIM is achieved at pH =7,10 it was clear that the release was much better in the aqueous media, as the aqueous media contained methanol, which can dissolve SIM easily,48 and Tween-80, which can improve the solubility of the poorly soluble drugs.49
Ex vivo permeation study
The assessment of percutaneous permeation of the drug is very important in the evaluation of dermal delivery systems. Ex vivo animal skin models can be used rather than human skin for ethical, safety, and economic reasons. This study provides the essential data about the ability of the prepared formulae to permeate the skin layers.49
After the first half an hour, 2.07% of SIM passed through the skin, 44.12% permeated through the skin after 12 hours, and after 24 hours 69.19% of SIM permeated through the skin; Figure 6 illustrates the results. The ex vivo skin permeation results were much lower than those obtained from in vitro dissolution (8% released after the first half an hour and 81% released after 24 hours); the difference was statistically significant at P<0.05. This may be due to the complex structure of the skin that widely differs from cellophane membrane.50
  | Figure 6 Percentage of SIM permeated through the rat skin from the prepared nanosized hydrogel. |
It is important to mention that the release data for F20 were fitted to the first-order release kinetics model as it showed the best linearity based on the regression coefficients (R2=0.9695).
Study on the wound healing activity of SIM hydrogel and LEV hydrogel in rats
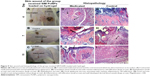
The area of wounds that received medicated gel and those that received plain gel were measured on days, 0, 4, 7, and 11 after wound creation as shown in Figure 7. The aim of this study was to get accurate data on the wound healing activity of the prepared SIM PoNPs; therefore, in addition to SIM PoNPs alone, LEV was added in another formulation to SIM PoNPs to exclude the retardation of wound closure caused by microbial contamination. LEV itself was evaluated for any wound healing properties.
Figure 7 showed that the wounds that received medicated gel in the three different groups showed a reduction in the wound area compared with the area of wounds received plain gel on the same day. The group received SIM-LEV hydrogel showed complete healing on day 11. However, the wound that received a plain gel in the same group showed also a remarkable improvement as SIM could permeate the skin, providing a systemic wound healing activity.51
According to Figure 8, the medicated wounds in the three groups showed a time-dependent improvement in the percent of wound contractions. Its contraction percent was also higher than that produced by the plain gel.
On day 4, group A showed a wound contraction percent of 33.98±1.3 (which was statistically significant as compared to the control group) as shown in Figure 9A, while the values were 29.86%±1.1% and 36.01%±0.8% in LEV group and SIM-LEV group, respectively, with the latter producing an insignificant percent contraction when compared to SIM group as shown in Figures 10A and 11A. Neither redness nor edema was detected around the wound area in the three groups.
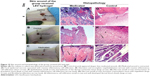
On day 7, the wound contraction percent values in the three groups were 76.24%±1.5%, 59.37%±0.9%, and 82.98%±1.4% for SIM group, LEV group, and SIM-LEV group, respectively. Wounds in the SIM group that received the medicated gel appeared contracted without hard or abnormal tissue on its surface, while control wounds showed a hard, abnormal tissue on the wound surface as shown in Figure 9B. In the LEV group, both wounds that received medicated gel and those that received plain gel showed a decrease in wound diameter without having hard tissue; however, the control wounds appeared deeper than medicated wounds as shown in Figure 10B. In the SIM-LEV group, both wounds that received the medicated gel and those that received plain gel showed decreases in the diameter; however, wounds that received the medicated gel started to epithelize as shown in Figure 11B.
On day 11, in the SIM group, medicated wounds appeared very narrow in diameter with normal epithelial tissue appearance, and the wound contraction percent reached 96.49±0.7 as shown in Figure 9C. This result emphasizes the SIM wound healing activity. In particular, it has been formulated as a hydrogel of SIM nanosized particles, which have a good ability to penetrate the skin.52 In addition, the presence of glycerol in the SIM PoNPs (F20) may play a role in the healing activity and may improve the healing and accelerate the healing rate. The glycerol can also relieve the inflammation and reduce the wound contamination.28 While the control wounds had formed a hard tissue covering the wound completely, in the LEV group, the wounds that received the medicated gel showed a very narrowed diameter compared with the control wounds, without hard or abnormal tissue formation in both medicated and control wounds. The wound contraction was 91±1.1% as shown in Figure 10C. This result corresponded to the reported LEV efficacy in the treatment of human soft tissue infection, and its ability to penetrate into tissues was not affected by local inflammation.53
In the SIM-LEV group, the medicated wounds had been completely cured and the wound contraction percent reached 99.9±0.2, while control wounds showed a small sign on the wound site as shown in Figure 11C. This elevated percentage of wound contraction may be revealed in the presence of SIM as PoNPs with its wound healing activity besides its antimicrobial activity against Gram-positive bacteria, in addition to the presence of LEV, which is a broad-spectrum antimicrobial and effective in soft skin and tissue infection.
Visual and histological examination of different skin samples
The histopathological examination of the medicated wound sample taken on day 4 (Figure 9D) showed formation of granulation tissue (small arrow) with massive inflammatory cell infiltration (large arrow). The control sample taken on the same day (Figure 9G) showed focal epidermal necrosis associated with massive inflammatory cell infiltration. The medicated wound sample taken on day 7 (Figure 9E) showed epithelization (small arrow) and scab formation (large arrow), while the control wound sample taken on the same day (Figure 9H) showed focal necrosis (small arrow) and formation of granulation tissue (large arrow).
On the eleventh day, the medicated wound sample (Figure 9F) showed epithelization (arrow), while the control wound sample taken on the same day (Figure 9I) showed formation of granulation tissue (small arrow) with massive inflammatory cell infiltration (large arrow).
The histopathological examination of the medicated wound sample taken on day 4 (Figure 10D) showed focal epidermal necrosis associated with massive inflammatory cell infiltration. The control sample taken on the same day (Figure 10G) showed necrosis (small arrow) and formation of granulation tissue (large arrow) with massive inflammatory cell infiltration (arrow head).
The medicated wound sample taken on day 7 (Figure 10E) showed ulceration and incomplete reepithelialization (arrow) with massive inflammatory cell infiltration (short arrow), while the control wound sample taken on the same day (Figure 10H) showed necrosis (small arrow), formation of granulation tissue with angioblasts (large arrow), and fibroblast proliferation (arrow head).
On the eleventh day, the medicated wound sample (Figure 10F) showed epithelization (small arrow) and hyalinosis of the dermal connective tissue (large arrow), while the control wound sample taken on the same day (Figure 10I) showed inflammatory cell infiltration (small arrow) and well-developed dermal blood vessels (large arrow).
The histopathological examination of the medicated wound sample taken on day 4 (Figure 11D) showed focal necrosis (small arrow) and inflammatory cell infiltration (large arrow), while the control sample taken on the same day (Figure 11G) showed necrosis (small arrow).
The medicated wound sample taken on day 7 (Figure 11E) showed well-vascularized (small arrow) formation of granulation tissue (large arrow), while the control wound sample taken on day 7 (Figure 11H) showed well-vascularized (small arrow) granulation tissue formation (large arrow).
On the eleventh day, the medicated wound sample (Figure 11F) showed normal hair follicle formation, while the control wound sample taken on the same day (Figure 11I) showed incomplete epithelization (arrow) and severe inflammatory cell infiltration in the dermal layer and focal areas of hemorrhage in the subepithelial region (small arrow).
From the previously mentioned histopathological investigation, it was evident that SIM PoNPs hydrogel showed an acceptable wound healing effect with the formation of the normal epithelial layer on day 11 after wound creation. The addition of LEV to the SIM hydrogel produced higher healing results with the presence of a normal hair follicle and the restoration of all normal skin layers, which is a positive sign of complete healing, besides the antibacterial properties of both SIM and LEV.
Conclusion
The solubility of SIM has been improved up to 23.4-fold after formulation as nanoparticles using the nanoprecipitation technique. The nanosuspension showed a good solubility due to the effect of glycerol (equivalent to 6.11 M) as a cosolvent and acetone as an organic solvent. The formulated SIM gel showed highly promising results in healing of the rat wound in 11 days with complete epithelialization, minimal inflammatory cell infiltration, mature collagen fiber formation, and more activated hair follicle growth.
Acknowledgments
The authors would like to thank Dr Kawkab Mostafa, Ms Asmaa Tarek, and Dr Mohamed Gamal for their generous contribution in the animal wound experiment and histopathological study.
Disclosure
The authors report no conflicts of interest in this work.
References
Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care. 2015;4(9):560–582. doi:10.1089/wound.2015.0635 | ||
Wang CC, Yang PW, Yang SF, Hsieh KP, Tseng SP, Lin YC. Topical simvastatin promotes healing of Staphylococcus aureus-contaminated cutaneous wounds. Int Wound J. 2015:1–8. doi:10.1111/iwj.12431 | ||
Firoz S, Sirisha MN, Rajalakshmi R. Hydrogels in topical drug delivery – a review. Innov Drug Discov. 2016;6(2):87–93. | ||
Boekhoven J, Brizard AM, Stuart MCA, et al. Bio-inspired supramolecular materials by orthogonal self-assembly of hydrogelators and phospholipids. Chem Sci. 2016;7(9):6021–6031. doi:10.1039/c6sc01021k | ||
Majumder P, Baxa U, Walsh STR, Schneider JP. Design of a multicompartment hydrogel that facilitates time-resolved delivery of combination therapy and synergized killing of glioblastoma. Angew Chemie. 2018;130(46):15040–15044. doi:10.1002/anie.201806483 | ||
Rezvanian M, Mohd Amin MCI, Ng SF. Development and physicochemical characterization of alginate composite film loaded with simvastatin as a potential wound dressing. Carbohydr Polym. 2016;137:295–304. doi:10.1016/j.carbpol.2015.10.091 | ||
Soni A, Dandagi P, Gadad A, Mastiholimath V. Simvastatin-loaded PLGA nanoparticles for improved oral bioavailability and sustained release: effect of formulation variables. Asian J Pharm. 2011;5(2):57. doi:10.4103/0973-8398.84545 | ||
Sameh N, Aly UF, Abou-Taleb HA, Abdellatif AAH. Prospective role of simvastatin on wound healing: review of the literature. J Bioequiv Bioavailab. 2018;10(2):36–42. doi:10.4172/0975-0851.1000375 | ||
Thangamani S, Mohammad H, Abushahba MF, et al. Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci Rep. 2015;5:16407. doi:10.1038/srep16407 | ||
Murtaza G. Solubility enhancement of simvastatin: a review. Acta Pol Pharm. 2012;69(4):581–590. | ||
Pentek T, Newenhouse E, O’Brien B, Singh Chauhan A. Development of a topical resveratrol formulation for commercial applications using dendrimer nanotechnology. Molecules. 2017;22:1. doi:10.3390/molecules22010137 | ||
Pal SL, Jana U, Manna PK, Mohanta GP, Manavalan R. Nanoparticle: an overview of preparation and characterization. J Appl Pharm Sci. 2011;1(6):228–234. | ||
Salem HF, Kharshoum RM. Nanoprecipitation technique for preparation of sterically stabilized risperidone nanosuspension: in vitro and in vivo study. Int J Pharm Pharm Sci. 2016;8(5):136–142. | ||
Pandya V, Patel J, Patel D. Formulation, Optimization and characterization of Simvastatin Nanosuspension prepared by nanoprecipitation technique. Der Pharm Lett. 2011;3(2):129–140. Available from: http://scholarsresearchlibrary.com/DPL-vol3-iss2/DPL-2011-3-2-129-140.pdf. Accessed April 12, 2019. | ||
Ghaderi S, Ghanbarzadeh S, Hamishehkar H. Evaluation of different methods for preparing nanoparticle containing gammaoryzanol for potential use in food fortification. Pharm Sci. 2015;20:130–134. | ||
Eckmann CDM. Treatment of complicated skin and soft-tissue infections caused by resistant bacteria: value of linezolid, tigecycline, daptomycin and vancomycin. Eur J Med Res. 2013;15(12):2345–2352. doi:10.1186/2047-783x-15-12-554 | ||
Adlin Jino Nesalin J, Gowthamarajan K, Somashekhara CN. Formulation and evaluation of nanoparticles containing flutamide. Int J ChemTech Res. 2009;1(4):1331–1334. | ||
Tandale P, Joshi D, Gaud RS. Formulation and evaluation of extended release solid dispersions conatining simvastatin. Asian J Biomed Pharm Sci. 2011;1(4):13–19. | ||
Narkhede K. Devlopement and evaluation of simvastatin nanoparticles using nanosuspension technique. J Pharm Sci Biosci Res. 2015;5:529–534. | ||
Kalimuthu S, Yadav A. Formulation and evaluation of Carvedilol loaded Eudragit e 100 nanoparticles. Int J PharmTech Res. 2009;1(2):179–183. | ||
Ubaid M, Ilyas S, Mir S, et al. Formulation and in vitro evaluation of carbopol 934-based modified clotrimazole gel for topical application. An Acad Bras Cienc. 2016;88(4):2303–2317. doi:10.1590/0001-3765201620160162 | ||
Bisht N, Goswami L, Kothiyal P. Preparation and evaluation of in-situ oral topical gel of levofloxacin by using combination of polymers. Indian J Drugs. 2014;2(4):142–151. | ||
Aly UF, Mansour HF. Novel pharmaceutical gels containing glyccerihizic acid ammonium salt for chronic wounds novel pharmaceutical gels containing glyccerihizic acid ammonium salt for chronic wounds. Br J Pharm Res. 2014;4(5):654–668. doi:10.9734/BJPR/2014/7591 | ||
Mandal S, Prabhushankar G, Thimmasetty M, Geetha M. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int J Pharm Investig. 2012;2(2):78. doi:10.4103/2230-973X.100042 | ||
Orgul D, Eroglu H, Hekimoglu S. Formulation and characterization of tissue scaffolds containing simvastatin loaded nanostructured lipid carriers for treatment of diabetic wounds. J Drug Deliv Sci Technol. 2017;41:280–292. doi:10.1016/j.jddst.2017.08.001 | ||
Shid RL, Dhole SN, Kulkarni N, Shid SL. Formulation and evaluation of nanosuspension formulation for drug delivery of simvastatin. Int J Pharm Sci Nanotech. 2014;7(4):2650–2665. | ||
Monica AS, Gautami J. Design and evaluation of topical hydrogel formulation of diclofenac sodium for improved therapy. Int J Pharm Sci Res. 2014;5(5):1973–1980. doi:10.13040/IJPSR.0975-8232.5(5).1973-80 | ||
Aly UF. Healing in diabetics. Int J Pharm Pharm Sci. 2012;4:76–77. | ||
National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington, DC. National Academies Press, 2012. | ||
Rego ACMD, Araújo Filho I, Damasceno BPGL, et al. Simvastatin improves the healing of infected skin wounds of rats. Acta Cir Bras. 2007;22(1):57–63. doi:10.1590/S0102-86502007000700012 | ||
Farsaei S, Khalili H, Farboud ES. Potential role of statins on wound healing: review of the literature. Int Wound J. 2012;9(3):238–247. doi:10.1111/j.1742-481X.2011.00888.x | ||
Sharma MC, Sharma S. Formulation and Spectroscopic Studies and Dissolution Behavior of Levofloxacin-β Cyclodextrins Inclusion Complex. Int J Pharm Tech Res. 2011;3(3):1883–1888. | ||
Vinay KB, Revanasiddappa HD, Xavier CM, Ramesh PJ, Raghu MS. A stability indicating UPLC method for the determination of tramadol hydrochloride: application to pharmaceutical analysis. Chromatogr Res Int. 2012;2012:1–9. doi:10.1155/2012/870951 | ||
Masilamani K, Ravichandiran V. Effect of formulation and procwss variables on drug content and Entrapment Efficiency of Aceclofenac Nanosuspension. Int Res J Pharm. 2012;3(3):315–318. | ||
Sreelola V, Sailaja AK, Pharmacy M. Preparation and characterisation of ibuprofen loaded polymeric nanoparticles by solvent evaporation technique. Int J Pharm Pharm Sci. 2014;6(8):416–421. | ||
Gupta MN, Batra R, Tyagi R, Sharma A. Polarity index: the guiding solvent parameter for enzyme stability in aqueous-organic cosolvent mixtures. Biotechnol Prog. 1997;13(3):284–288. doi:10.1021/bp9700263 | ||
Yoon JW, Kim JH. Establishment of a solvent map for formation of crystalline and amorphous paclitaxel by solvent evaporation process. Korean J Chem Eng. 2011;28(9):1918–1923. doi:10.1007/s11814-011-0060-2 | ||
Lipsky BA, Silverman MH, Joseph WS. A proposed new classification of skin and soft tissue infections modeled on the subset of diabetic foot infection. Infect Dis Am. 2016;4:1–8. doi:10.1093/ofid/ofw255 | ||
Kharia AA, Singhai AK, Verma R. Formulation and evaluation of polymeric nanoparticles of an antiviral drug for gastroretention. Int J Pharm Sci Nanotechnol. 2012;4(4):1557–1562. | ||
Eaton P, Quaresma P, Soares C, et al. A direct comparison of experimental methods to measure dimensions of synthetic nanoparticles. Ultramicroscopy. 2017;182:179–190. doi:10.1016/j.ultramic.2017.07.001 | ||
Uma Maheswari R, Mullaicharam A. Development and In-Vitro evaluation of nanosuspension formulation containing acyclovir for the treatment of ocular infections. Res J Pharm Biol Chem Sci. 2013;4(1):463–480. | ||
Kota S, Jahangir MA, Ahmed M, et al. Development and evaluation of ofloxacin topical gel containing wound healing modifiers from natural sources. Der Pharm Lett. 2015;7(10):226–233. | ||
Ono S, Imai R, Ida Y, Shibata D, Komiya T, Matsumura H. Increased wound pH as an indicator of local wound infection in second degree burns. Burns. 2015;41(4):820–824. doi:10.1016/j.burns.2014.10.023 | ||
Islam MT, Rodriguez-Hornedo N, Ciotti S, Ackermann C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm Res. 2004;21(7):1192–1199. | ||
Beaulieu A. Wound healing formulations containing human plasma fibronectin. US Patent 5,641,483, issued June 24, 1997. | ||
Garg A, Aggarwal D, Garg S, Singla AK. Spreading of semisolid formulations. Pharm Technol. 2002;26(9):84–105. doi:10.5138/ijdd.2010.0975.0215.02012 | ||
Amsa P, Tamizharasi S, Jagadeeswaran M, Sivakumar T. Preparation and solid state characterization of simvastatin nanosuspensions for enhanced solubility and dissolution. Int J Pharm Pharm Sci. 2014;6(1):2–6. | ||
Chavhan V, Ghante M. Stability indicating UV spectrophotometric method development and validation of simvastatin in bulk and tablet dosage form. J App Pharm. 2014;6(2):235–246. | ||
Seedher N, Agarwal P. Various solvent systems for solubility enhancement of enrofloxacin. Indian J Pharm Sci. 2009;71(1):82–87. doi:10.4103/0250-474X.51958 | ||
Chourasia MK, Kang L, Chan SY. Nanosized ethosomes bearing ketoprofen for improved transdermal delivery. Results Pharma Sci. 2011;1(1):60–67. doi:10.1016/j.rinphs.2011.10.002 | ||
Fitzmaurice GJ, McWilliams B, Nölke L, Redmond JM, McGuinness JG, O’Donnell ME. Do statins have a role in the promotion of postoperative wound healing in cardiac surgical patients? Ann Thorac Surg. 2014;98(2):756–764. doi:10.1016/j.athoracsur.2014.02.089 | ||
Yokota J, Kyotani S. Influence of nanoparticle size on the skin penetration, skin retention and anti-inflammatory activity of non-steroidal anti-inflammatory drugs. J Chinese Med Assoc. 2018;81(6):511–519. doi:10.1016/j.jcma.2018.01.008 | ||
Bellmann R, Kuchling G, Dehghanyar P, et al. Tissue pharmacokinetics of levofloxacin in human soft tissue infections. Br J Clin Pharmacol. 2004;57(5):563–568. doi:10.1111/j.1365-2125.2004.02059.x |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.