Back to Journals » International Journal of Nanomedicine » Volume 14
Formulation and evaluation of multilamellar vesicles ropivacaine in pain management
Authors Kao HW, Lin YY, Gwathney WJ , Hong K
Received 16 May 2019
Accepted for publication 15 August 2019
Published 27 September 2019 Volume 2019:14 Pages 7891—7901
DOI https://doi.org/10.2147/IJN.S215952
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Hao-Wen Kao,1 Yi-Yu Lin,1 Walter J Gwathney,1 Keelung Hong1,2
1TLC Biopharmaceuticals, Inc., South San Francisco, CA 94080, USA; 2Taiwan Liposome Company, Ltd., Taipei 11503, Taiwan
Correspondence: Yi-Yu Lin
TLC Biopharmaceuticals, Inc., South San Francisco, CA 94080, USA
Tel +1 650 872 8816
Fax +1 650 872 8817
Email [email protected]
Purpose: The improvement of postoperative pain control plays an important role in recovery outcomes and patient satisfaction. Multilamellar vesicles ropivacaine, MVR, is being developed to sustain the release of ropivacaine in situ while maintaining the local concentration of ropivacaine within the therapeutic window.
Methods: These studies summarized the processes of MVR formulation development and the evaluation of its releasing profile in vitro and the pharmacokinetics and anesthetic effect in vivo.
Results: The MVR demonstrates a sustained-release profile in an in vitro serum environment model after 24 hrs of incubation which translates in the in vivo rat pharmacokinetic profile of ropivacaine as a prolonged half-life that is 10-fold longer in duration than plain ropivacaine solution. The anesthetic effect of single-dose MVR is apparent by providing a prolonged analgesia effect compared to plain ropivacaine solution in an in vivo guinea pig pin-prick wheal model after a single intracutaneous injection. From a safety evaluation, MVR is well tolerated after a subcutaneously injection at a dose level of 20 mg/kg in rats, with no observable changes in clinical observation, body weight, organ weight, hematology and serum chemistry analysis.
Conclusion: These results suggest that single administration of MVR is a promising candidate in postoperative pain management.
Keywords: multilamellar vesicles, postoperative pain management, ropivacaine, sustained release
Introduction
Over 80% of patients experience significant postoperative pain particularly in the first few days following surgery.1 Effective pain management after surgery not only plays a critical factor in improving the patient satisfaction but also in reducing surgical complications. To improve patient recovery, various recommended strategies in postoperative pain management are employed in the clinical setting including oral administration of non-opioids and/or opioids, wound infiltration of local anesthetics, neuraxial or regional anesthesia, patient-controlled analgesia and multimodal treatments.2–4
Ropivacaine, an amino-amide long-acting local anesthesia drug, is widely used in pain management by reversibly inhibiting the voltage-gated sodium channels in nerve fibers. In 1996, ropivacaine, a pure S(‒)isomer compound, is introduced as a long-acting local anesthetic and in 2000, is approved by the United States Food and Drug Administration under the trade name Naropin® (Ropivacaine HCl Injection). Although local anesthesia drugs are useful for providing the analgesic effect in the clinic, they have relatively short half-lives and the potential acute toxicity on both the central nervous system and cardiovascular system limits the dosage in postoperative pain management. Therefore, a strategy of continuous infusion and continuous wound instillation of low-concentration ropivacaine is reported to mitigate postoperative pain while restricting the systemic exposure.5–7
Phospholipids are the major components of the cell membrane and have been widely used as a biodegradable, non-toxic excipient to formulate a lipid-based drug carrier in pharmaceutical drug products. In aqueous solutions, phospholipids spontaneously self-assemble into multilamellar vesicles, which have been developed to increase the payload of drug and improve the pharmacokinetic and safety profile of a pharmaceutically active ingredient. The multilamellar vesicles ropivacaine (MVR) formulation and characteristics of that formulation reported here demonstrate that this MVR serves as a sustained-release depot which may treat postoperative acute pain with single injection based on the anesthesia efficacy of ropivacaine.
Materials and methods
Materials
1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) was purchased from NOF (Tokyo, Japan). Cholesterol, solvents and other chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA). Ropivacaine base was purchased from Dishman (Veenendaal, Netherlands). Sprague Dawley rats (with and without jugular vein cannulated) and guinea pigs were obtained from Charles River Laboratories (Wilmington, MA, USA).
Preparation of multilamellar vesicles ropivacaine
A mixture of DMPC, cholesterol and ropivacaine base (DMPC/cholesterol/ropivacaine 1/0.5/1.5 molar ratio) was dissolved in tert-butanol at a concentration of 50 mM DMPC. The samples were frozen in −80°C freezer for 30 mins and then were lyophilized overnight. During lyophilization, the samples were placed in an ice bath for the first 4 hrs and then allowed to reach ambient temperature overnight on the laboratory bench. The lyophilized lipid cake was reconstituted with the reconstitution solution at an equal volume of tert-butanol at ambient temperature to obtain the MVR. The reconstitution solutions used in this study included 50 mM histidine solution at pH 5.5, pH 6.0, pH 6.5 and pH 7.0, to prepare the MVR 1, MVR 2, MVR 3 and MVR 4, respectively.
To determine the non-vesicle-associated ropivacaine in the MVR, 200 µL of MVR was centrifuged at 3000 g for 5 mins to separate the MVR from the aqueous suspension medium. After decanting the supernatant, MVR pellet was resuspended with reconstitution solution to a final solution volume of 200 µL. The ropivacaine concentrations of MVR and resuspended MVR were measured with a DU 530 UV/Vis spectrophotometer (Beckman, Brea, CA, USA) at 262 nm. The average particle size was determined using an LA-950V2 laser scattering particle size distribution analyzer (Horiba, Kyoto,
Japan). The surface morphology was measured by an FEI Nova 200 (Hillsboro, OR, USA) Dual Beam Focused Ion Beam scanning electron microscopy (SEM). The percentage of non-vesicle-associated ropivacaine was calculated according to the following formula:

In vitro release study
Drug release from MVR 2 was studied using a dialysis method. Briefly, a dialysis bag (MWCO 25 kDa; Spectrum Laboratories, Rancho Dominguez, CA, USA) containing 500 µL of MVR 2 or plain ropivacaine solution (control group) plus 500 µL of human-recovered plasma (Valley Biomedical, Winchester, VA, USA) was placed inside a 50 mL centrifuge tube containing 25 mL of phosphate-buffered saline (PBS), pH 7.4 (t=0). The samples were incubated at 37±1°C with gentle agitation and 1 mL aliquot outside of dialysis bag were collected at 2, 4, 8 and 24 hrs post-incubation to measure the released ropivacaine concentration using a high-performance liquid chromatography system (Waters, Milford, MA, USA) consisting of an Alliance 2695 Separations Module and 2996 Photodiode Array Detector. Chromatographic separation was performed at ambient temperature on a Zorbax 300SB-C18 analytical column (4.6×250 mm, 5 μm; Agilent, Santa Clara, CA, USA) with a Gemini C18 guard column (4.0×3.0 mm; Phenomenex, Torrance, CA, USA) using acetonitrile/20 mM phosphate buffer, pH 6.9=60/40 (v/v) as a mobile phase at a flow rate of 1 mL/min. The wavelength for UV detection was set at 262 nm and the injection volume was 50 µL.
A one-way ANOVA was performed for inter-group comparison; when the result of ANOVA suggested a significant inter-group difference, the treatment groups differences were further compared using Dunnett’s test.
Single-dose pharmacokinetic studies
The study protocol and all the procedures were approved by the Animal Ethics and Legal Committee of Bayside Biosciences, Inc. (CA, USA) and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Bayside Biosciences, Inc. (Approval No. 2016-002). Sixteen male Sprague Dawley rats (jugular vein cannulated, 290±40 g) were housed in group cages with 4 animals per cage, with standard rat food and water ad libitum in order to ensure proper nurturing and enrichment. The housing condition was controlled at 65–75°F (~18–23°C) with a 12 hrs light/12 hrs dark circadian cycle. The rats were randomly divided into four groups (n=4 per group): plain ropivacaine solution, MVR 1, MVR 2 and MVR 3. Each formulation was injected subcutaneously at a dose of 20 mg/kg into the dorsal flank and about 200 µL of blood was collected in K2-EDTA-containing tubes at 0.25, 0.5, 1, 2, 4, 6, 8 and 24 hrs post-injection for plain ropivacaine solution and 0.25, 1, 2, 4, 8, 24, 48 and 72 hrs post-injection for MVR 1, MVR 2 and MVR 3. The blood samples were then centrifuged at 6000 g for 10 mins at 4°C to obtain the plasma samples, which were then stored in −80°C freezer and shipped on dry ice before analysis.
The total plasma concentration of ropivacaine was analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS; Shimadzu, Tokyo, Japan). For each plasma sample, a 40 µL aliquot of plasma was mixed with 80 µL of ice-cold acetonitrile containing 50 ng/mL of dextromethorphan as an internal standard. After centrifugation at 6100 g for 30 mins, the extract was decanted from the plasma protein pellet and evaporated to dryness in a vacuum evaporation system at 25°C. The residue was reconstituted with an equal volume of 0.1% formic acid in water, and a 4 µL aliquot of the mixture was injected into LC-MS/MS system for analysis. The bioanalytical system employed a VP Series 10 high-performance liquid chromatography system (Shimadzu, Tokyo, Japan) and an API 4000 mass spectrometer (Sciex, Framingham, MA, USA) equipped with an electrospray ionization (ESI) interface. Chromatographic separation was performed at ambient temperature on a Proto™ 200 C18 analytical column (20×2 mm, 10 µm; Higgins, Mountain View, CA, USA) using 0.2% formic acid in water (mobile phase A)/0.2% formic acid in methanol (mobile phase B) as a gradient mobile phase at flow rate of 0.8 mL/min. The gradient conditions were 95% A and 5% B between 0 and 0.25 mins, returned to 5% A and 95% B between 0.25 and 1.50 mins, maintained at 5% A and 95% B between 1.50 and 1.70 mins, ramped to 95% A and 5% between 1.70 and 1.75 mins and maintained at 95% A and 5% between 1.75 and 2.00 mins. Data were acquired in the positive-ion mode with an ESI interface and multiple reaction monitoring (MRM) mode was used for quantitation. The MRM transition of ropivacaine was m/z 275.1→m/z 126.1, and the internal standard was m/z 272.2→m/z 215.2. The lower limit of quantification of the plasma sample was 2.0 ng/mL.
Pharmacokinetic analysis was performed using data from individual rats. A noncompartmental model was fitted to the plasma concentration versus time curve using WinNonlin software (Pharsight Corporation, Mountain View, CA, USA). The pharmacokinetic parameters, including maximal plasma concentration (Cmax), time to reach the Cmax (tmax), area under the plasma concentration–time curve from time zero to the last point (AUC0-t), area under the plasma concentration–time curve from time zero extrapolated to time infinity (AUC0-∞), half-life (t1/2), volume of distribution (Vd/F) and total body clearance (CL/F), were estimated.
Levene’s test of the homogeneity of variance was performed. If the variances were homogeneous, ANOVA was performed for inter-group comparison and when the result of ANOVA suggested a significant difference in an inter-group comparison, the treatment groups were compared to control group (plain ropivacaine solution groups), using Dunnett’s test. Alternatively, if the variances were heterogeneous, Kruskal–Wallis test was performed and when this test insinuated a significant difference, all groups were compared using Dunn’s test.
Evaluation of anesthetic effect in guinea pigs
The study protocol and all the procedures were approved by the Animal Ethics and Legal Committee of Bayside Biosciences, Inc. and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Bayside Biosciences, Inc (Approval No. 2016-006). For the in vivo efficacy study in guinea pig, eight male guinea pigs (8 weeks old, around 500 g) were housed in group cages with 2 animals per cage, with Guinea Pig food (Teklad Global guinea pig diet 2040; Envigo, Huntingdon, UK) and water ad libitum in order to ensure proper nurturing and enrichment. The housing condition was controlled at 65–75°F (~18–23°C) with a 12 hrs light/12 hrs dark circadian cycle. The local anesthetic effect was evaluated by the pin-prick wheal model in guinea pig which was modified according to the procedure described in the previous literature.8,9 The backs of guinea pigs were shaved 1 day before the experiment. On Day 1 of the experiment, four areas were drawn on the back of each guinea pig before administration of test articles and the sensibility of these areas were determined by a pin-prick to establish a subject-specific baseline. Each animal randomly received 4 designated regimens on the back via intracutaneous injection, which created 4 individually, distinct wheals; respectively. The injection volume of each drug formulation was controlled to introduce a similar amount, which was about 0.17 mL per regimen. Six different regimens were investigated in this study including (A) low dose MVR 2 (0.75 mg of 4-fold dilution of MVR 2 per wheal), (B) middle-low dose MVR 2 (1.5 mg of 2-fold dilution of MVR 2 per wheal), (C) middle-high dose MVR 2 (3.0 mg of MVR 2 per wheal), (D) high dose MVR 2 (6.0 mg of 2-fold concentrated MVR 2 per wheal), (E) low dose plain ropivacaine solution (1.5 mg per wheal), and (F) high dose plain ropivacaine solution (3.0 mg per wheal). Pin-pricks were standardized using a 5-mL water-filled syringe with 20G needle, which the total weight was consistently controlled to 9.2 g. The reaction to pin-pricks at the wheals was tested at 0.25, 1, 2, 4, 6, 12 and 23 hrs following the injections of test articles. The pin-pricks were applied first to a control area outside the wheal at each time point. After observing the animal’s normal reaction to the pin-prick outside the wheal, six pricks were applied on the wheal and the number of pricks which the guinea pig failed to react were recorded as non-responses. If an animal displayed a hundred percent response for all pin-pricks, that animal was no longer monitored at further time points. Fifty percent of maximum effect (EC50) was estimated using PKSolver add-in software (pharmacodynamic analysis using inhibitory effect sigmoid Emax model; Jiangsu, China) with data from individual wheal. Area under the non-responses time curve of each wheal was calculated using the linear trapezoidal rule served as an anesthetic score indicating the degree of local anesthesia.
Levene’s test of the homogeneity of variance was performed. If the variances were homogeneous, ANOVA was performed for inter-group comparison; when the result of ANOVA showed a significant difference, the treatment groups were compared to control group (plain ropivacaine solution groups), using Dunnett’s test. Alternatively, if the variances were heterogeneous, Kruskal–Wallis test was performed; when this test shows a significant difference, all groups were compared using Dunn’s test.
Safety evaluation in rats
The study protocol and all the procedures were approved by the Animal Ethics and Legal Committee of Bayside Biosciences, Inc. and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Bayside Biosciences, Inc (Approval No. 2016-004). Twenty male Sprague Dawley rats (8 weeks old, 240±20 g) were housed in group cages with up to 3 animals per cage, with standard rat food and water ad libitum in order to ensure proper nurturing and enrichment. The housing condition was controlled at 65–75°F (~18–23°C) with a 12 hrs light/12 hrs dark circadian cycle.
The rats were randomly divided into four groups (n=5 per group) as shown in Table 1: (A) vehicle (containing all excipients except ropivacaine as a control group), (B) MVR 2 at doses of 10 mg/kg, (C) MVR 2 with additional 2-fold dilution at doses of 10 mg/kg, and (D) MVR 2 at doses of 20 mg/kg. All test articles were administrated to the rats subcutaneously in the back at Day 0. Observations were made twice daily at Day 0 and Day 1 followed by three times a week for mortality and changes in general appearance or behavior. All significant findings would be recorded, if any. Observations included, but not limited to, changes of the skin, fur and eye, somatomotor activity and behavior patterns. Individual body weight of each rat was recorded at the beginning of administration and monitored weekly.
 |
Table 1 The study groups of safety evaluation |
At the Day 14, blood sample was collected from each rat by cardiac puncture. Two milliliters of blood was transferred into a tube containing K2-EDTA and was used for hematology analysis. One milliliter of blood sample was centrifuged at 3000 rpm for 10 mins to obtain the plasma sample for clinical chemistry analysis. Hematological parameters containing white blood cell (WBC) count, red blood cell count, hemoglobin, hematocrits, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and absolute and percent differential WBC count (including neutrophil, lymphocyte, monocyte, eosinophil, and basophil) were determined using a Sysmex XT-2000iV hematology analyzer (Kungsbacka, Sweden). Serum chemistry parameters including alkaline phosphatase, aspartate aminotransferase, alanine aminotransferase, creatine kinase, total bilirubin, total protein, albumin, globulin, albumin/globulin ratio, blood urea nitrogen (BUN), creatinine, BUN/creatinine ratio, cholesterol, glucose, calcium, phosphorus, bicarbonate, chloride, potassium, sodium, ratio of sodium to potassium (Na/K ratio), bilirubin-conjugated and bilirubin-unconjugated were determined using a Beckman Coulter AU680 analyzer (Brea, CA, USA). A complete necropsy was conducted on all animals. The following tissues and organs were weighed and recorded: lungs, heart, liver, spleen, pancreas, kidneys, and testes.
Levene’s test of the homogeneity of variance was performed. If the variances were homogeneous, a one-way ANOVA was performed for inter-group comparison; when the result of ANOVA showed a significant difference, the treatment groups were compared to control group (vehicle group), using Dunnett’s test. Alternatively, if the variances were heterogeneous, Kruskal–Wallis test was performed; when this test shows a significant difference, all groups were compared using Dunn’s test.
Results
Characterization of multilamellar vesicles ropivacaine
MVR was prepared using a lyophilization method and a range of 3–30% non-vesicle-associated ropivacaine of the initially added ropivacaine was obtained after reconstitution (Table 2). The concentration of ropivacaine in each MVR formulation was measured by a UV/Vis spectrophotometer in the range from 17.1 to 20.8 mg/mL. The average median particle sizes (D50) of MVR reconstituted with pH 6.0 solution (MVR 2) and pH 6.5 solution (MVR 3) were 9.7 and 11.1 µm, respectively. A particle size frequencies histogram and a typical SEM image of SEM were shown in Figure S1.
 |
Table 2 The characterizations of four MVR formulations after reconstituting the lyophilized lipid cake with reconstitution solutions at different pH values |
The in vitro release profiles of ropivacaine from MVR 2 in PBS are shown in Figure 1. Releasing rate of ropivacaine from the dialysis bag containing MVR was slower compared to that of plain ropivacaine solution. Greater than 90% of ropivacaine was released from the dialysis bags containing 5 mg/mL of plain ropivacaine solution within 2 hrs. In comparison, around 20% of ropivacaine was released from MVR within 2 hrs and gradually increased to around 45% over 24 hrs.
Single-dose pharmacokinetic studies
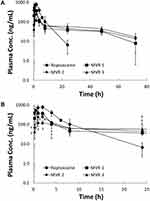
Ropivacaine concentration in plasma versus time profiles were obtained for all groups following a single subcutaneously injection, as depicted in Figure 2. Ropivacaine was rapidly absorbed after subcutaneous injection of plain ropivacaine solution with peak concentration reached in 2 hrs. Since there was about 30%, 20% and 10% non-vesicle-associated ropivacaine in MVR 1, MVR 2 and MVR 3, respectively; a rapid rise during the absorption phase was observed after subcutaneous injection of MVR and the peak concentration in plasma was consistent with the order of non-vesicle-associated ropivacaine percentage. After reaching the peak, plasma concentrations decreased in a mono-exponential manner in plain ropivacaine solution group and in a multi-exponential manner in MVR groups. At the same dosage of ropivacaine with 4 different formulations, ropivacaine in the plasma was detected (higher than the limit of quantification, 2.0 ng/mL) until 72 hrs in all MVR groups; however, ropivacaine was not detected in the plasma after 24 hrs in the plain ropivacaine solution group.
The pharmacokinetic parameters derived from the plasma concentration versus time curves in Figure 2 are summarized in Table 3. The pharmacokinetic data derived from one of the rats in MVR 1 group were excluded in this pharmacokinetic study due to the extremely long half-life (304 hrs). The Cmax of MVR 1, MVR 2 and MVR 3 were significantly lower compared to that of plain ropivacaine solution, which were only 55.5%, 35.5%, and 16.7% of the Cmax of plain ropivacaine solution. The Cmax levels of MVRs were in line with the percentage of non-vesicle-associated ropivacaine in the MVRs, which was caused by the alkalinity level of the reconstitution solution. The more alkaline the reconstitution solution employed, the lower the non-vesicle-associated ropivacaine was in the MVR. Based on the results of area under the curve (AUC0-t), 84.7–90.8% of the ropivacaine was released from MVR 72 hrs after injection of the MVR when comparing to the plain ropivacaine solution group, representing the exposures in the four groups were nearly the same. The half-life (t1/2) of all three MVR groups was significantly prolonged compared with that of plain ropivacaine solution group. Although no differences were observed in the clearance (CL/F) between the plain ropivacaine solution group and all MVR groups, the volume of distribution (Vd/F) in all MVR groups increased by 9.4- to 10.6-times compared to the plain ropivacaine solution group. Overall, these results indicate MVR form a depot that sustains the release of ropivacaine into circulation. To achieve a balance between the fast onset, which is attributed to non-vesicle-associated ropivacaine, and sustained anesthetic effect, the MVR 2, reconstituted with a pH 6.0 solution, was used for further animal efficacy studies and a safety study.
 |
Table 3 The pharmacokinetic parameters after subcutaneous injection of multilamellar vesicles ropivacaine and plain ropivacaine solution in rats |
Evaluation of anesthetic effect in guinea pigs
The local anesthetic effects of MVR 2 and plain ropivacaine solution were evaluated with different doses in guinea pigs as shown in Figure 3. The onset of all the dosage of MVR 2 was observed at the first time point, within 15 mins. The EC50 (Table 4), which was 4.3 hrs for Group A, 5.7 hrs for Group B, 9.2 hrs for Group C, and 13 hrs for Group D, respectively, was increased aligned with the dosage increase. The duration of time for MVR 2 to completely lose the anesthetic effect after intracutaneous injection, the number of non-responses went back to zero, was also longer when the dosage increased. These results indicated that the effective duration of anesthesia after injection of different doses of MVR 2 was dose-dependent. Compared to the plain ropivacaine solution groups, MVR 2 groups exhibited sustained anesthetic effect. When dosing the MVR 2 with half the amount of ropivacaine compared to the plain ropivacaine solution (Group A versus Group E), the results showed a statistically significant local anesthetic effect (P<0.05) for the MVR 2 group at the 4 hrs time point. At the same amount of ropivacaine per wheal (Group B versus Group E), MVR 2 also showed a significantly prolonged local anesthetic effect after 2 hrs post-injection. Intuitively, a significant prolonged local anesthetic effect was observed when dosing the MVR 2 with 2-times and 4-times amount of ropivacaine per wheal (Group C and Group D versus Group E). When comparing the anesthetic score within 23 hrs of MVR 2 groups to those of plain ropivacaine solution groups, the anesthetic score of Group C and Group D was significantly larger (P<0.05) than Group E and the anesthetic score of Group D was significantly larger than Group F as well.
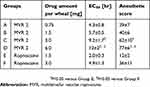 |
Table 4 Local anesthetic effect in guinea pigs with MVR 2 and plain ropivacaine solution for 23 hrs |
Safety evaluation in rats
No animal deaths or abnormal clinical signs were observed in all groups during the 14 days experimental period. Mean final body weights and body weight gains of rats receiving MVR 2 were similar to both control group, as shown in Figure 4. No treatment-related changes in the value of organ weights were noted following administration of MVR 2, as listed in Table 5.
 |
Figure 4 Body weight of rats at 0, 7 and 14 days post-administration in rats. Data represent mean value ± SD, n=5. |
 |
Table 5 Absolute organ weight (g) of rats at 14 days post-administration in rats |
The results of hematological analysis, as recorded in Table 6, suggest there was no significant difference in all groups and all these variations were marginal and within the normal laboratory ranges.10 The serum chemistry results, as shown in Table 7, found the phosphorus levels in MVR 2 treated groups were statistically, significantly lower than that in Group A (P<0.05), the differences were tiny and were not thought to be indicative of any toxic effect. In addition, these differences were not dose-related in their manner, and therefore, they were not considered to be test article-related.
 |
Table 6 Hematological values at 14 days post-administration in rats |
 |
Table 7 Serum biochemistry at 14 days post-administration in rats |
Discussion
Even though ropivacaine and bupivacaine are similar in chemical structure, the propyl group of ropivacaine causes it to be less lipophilic compared bupivacaine with its butyl group. This minor modification results in a slight decrease in the potency of ropivacaine compared to bupivacaine but it also increases ropivacaine’s margin of safety in clinical use. Ropivacaine showed from about 60% to almost the same analgesia potencies of bupivacaine via epidural administration.11 Even though this ratio will be slightly different depending on the injection routes, the doses where the central nervous system toxicity appeared after ropivacaine administration in different species are 140–220% of bupivacaine.12,13 It is for these safety and effectiveness reasons, ropivacaine has been selected as the candidate to be developed for the multilamellar vesicles, sustained-release formulation.
A MVR is prepared by lyophilization technology which can extend the shelf-life of product and can be reconstituted quickly prior to clinical use as demonstrated in the present studies. The MVR serve as a depot to carry considerable amount of ropivacaine and to control the release rate of the ropivacaine in order to maintain the local concentration of ropivacaine within its therapeutic window. This depot appears to stay in situ during the drug release period. Oussoren et al14 have reported that the particle size of the liposome is the most important factor influencing the lymphatic uptake and blood distribution after subcutaneous injection. At 52 hrs post-injection, more than 80% to almost 100% of the liposomes with a mean diameter of 400 nm and non-sized liposome (without any particle sizing process after liposome formed), respectively, will stay in the injection site. The D50 of our MVR was measured to be approximately 10 µm, and the D10 and D90 were about 6 and 20 µm, respectively, which the particles size distribution was larger than the reported size threshold. These results suggest that the multilamellar vesicles would remain in situ as a depot, and by extrapolation, the ropivacaine determined in circulation is not from the multilamellar vesicle-bound form.
To demonstrate the release rate of ropivacaine from the MVR, an in vitro release test using a dialysis method and an in vivo pharmacokinetic study in rats was performed. Both in vitro and in vivo results suggest that the MVR provided a sustained release profile for ropivacaine. The sustained-release hypothesis is particularly supported by the t1/2 of about 33 hrs obtained for the MVR groups versus a t1/2 of 3.07 hrs obtained for the plain ropivacaine solution group. According to literature results of clinical studies, the t1/2 of ropivacaine after intravenous injection of 14C-labeled ropivacaine in healthy volunteers is 2.0±0.3 hrs.15 Kopacz et al16 report that the elimination t1/2 of ropivacaine after intercostal nerve block is 2.3±0.8 hrs and the sensory blockade duration measured by pin-prick test are 6.0±2.5 hrs. Most surgical patients experience moderate to severe acute postsurgical pain during the first 2–4 days after surgery. Therefore, a pain management therapy that can successfully mitigate pain during this period will improve patient satisfaction and recovery. The MVR provides at least a 10-fold increase in t1/2, which implies that this sustained-release formulation has the potential to relieve acute pain for at least 60 hrs postoperatively.
The efficacy of MVR is demonstrated in the intracutaneous wheal model in a guinea pig, which was established in 1945 to test the potency of local anesthetics.9 The results of anesthetic scores increase with dosage from 0.75 mg to 6.0 mg of MVR 2 in a dose-dependent manner, as depicted in Figure 5, where the concentration of ropivacaine in MVR 2 ranged from 4.3 to 36 mg/mL. Likewise, the anesthetic scores increase from a dosage of 1.5 to 3.0 mg of unformulated ropivacaine with a slightly steeper slope. Though the high concentration of plain ropivacaine solution showed a higher anesthetic score than low dosage, the highest concentration of 1% Ropivacaine HCl Injection (8.3 mg/mL of ropivacaine base) available in clinic is almost equal to the concentration of low dosage in the present study. Some evidence suggests that the cell toxicity effect of local anesthetics may be determined in a concentration-dependent manner from in vitro testing with specific cell lines, such as Schwann cells,17 human melanoma cells18 or rabbit intervertebral disc cells.19 A comparative advantage of using a sustained-release formulation is it will decrease the local concentration of un-bound anesthetic by holding it in reserve in MVR to reduce the local toxicity.
The results of a toxicity study of single dose ropivacaine after subcutaneous administration was reported in the Pharmacology Review of Ropivacaine HCl Injection,20 and the maximum non-lethal dose (MNLD) was determined to be 62 mg/kg in Wistar male rats. For these studies, the dosage of ropivacaine in the high-dosage group of MVR 2 is 20 mg/kg, which is 3-fold lower than the reported MNLD. The recommended dosage of Ropivacaine HCl Injection for infiltration is in a range of 2 to 200 mg of ropivacaine HCl with an anesthesia duration of 2–6 hrs. The dosage of the high-dosage group of MVR 2 can be calculated to be about 200 mg human equivalent ropivacaine dose,21 which will be a feasible dosage in the clinic. However, the toxicity of higher dosage in rats or other species is further evaluated in other studies in supporting the application of clinical research.
Conclusion
Based on the findings in the in vitro release profiles, pharmacokinetic profiles and pharmacodynamic properties; MVR is able to provide a longer anesthetic effect than plain ropivacaine solution after a single administration. Furthermore, MVR at dose level of 20 mg/kg in rats did not show any dose-related toxicity. This sustained-release formulation has promising evidence that its inclusion in a regimen for postoperative pain management may offer relief for the critical 2–4 days period after surgery which may also reduce the use of opioids and opioid-induced adverse effects after surgical procedures.
Acknowledgments
Part of the results in this manuscript were presented at the 44th Annual Regional Anesthesiology & Acute Pain Medicine Meeting named “The Pharmacokinetic and Pharmacodynamic Evaluation of Multilamellar Vesicles Formulated Ropivacaine, TLC590, Administered by Local Injection in Rats” as a poster presentation with interim findings.
Disclosure
The authors were supported by TLC Biopharmaceuticals, Inc. (South San Francisco, USA) and Taiwan Liposome Company, Ltd. (Taipei, Taiwan). All authors are employees of TLC Biopharmaceuticals, Inc. The authors report no other conflicts of interest in this work.
References
1. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97(2):534–540. table of contents. doi:10.1213/01.ane.0000068822.10113.9e
2. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131–157. doi:10.1016/j.jpain.2015.12.008
3. Argoff CE. Recent management advances in acute postoperative pain. Pain Pract. 2014;14(5):477–487. doi:10.1111/papr.12108
4. Garimella V, Cellini C. Postoperative pain control. Clin Colon Rectal Surg. 2013;26(3):191–196. doi:10.1055/s-0033-1351138
5. Oxlund J, Clausen AH, Veno S, et al. A randomized trial of automated intermittent ropivacaine administration vs. continuous infusion in an interscalene catheter. Acta Anaesthesiol Scand. 2018;62(1):85–93. doi:10.1111/aas.13011
6. Ng FY, Ng JK, Chiu KY, Yan CH, Chan CW. Multimodal periarticular injection vs continuous femoral nerve block after total knee arthroplasty: a prospective, crossover, randomized clinical trial. J Arthroplasty. 2012;27(6):1234–1238. doi:10.1016/j.arth.2011.12.021
7. Brown SL, Morrison AE. Local anesthetic infusion pump systems adverse events reported to the Food and Drug Administration. Anesthesiology. 2004;100(5):1305–1307. doi:10.1097/00000542-200405000-00036
8. Yoshitomi T, Kohjitani A, Maeda S, Higuchi H, Shimada M, Miyawaki T. Dexmedetomidine enhances the local anesthetic action of lidocaine via an alpha-2A adrenoceptor. Anesth Analg. 2008;107(1):96–101. doi:10.1213/ane.0b013e318176be73
9. Bulbring E, Wajda I. Biological comparison of local anaesthetics. J Pharmacol Exp Ther. 1945;85:78–84.
10. Mary LA, Giknis CBC. Clinical Laboratory Parameters for Crl: CD(SD)rats. Charles River Laboratories; 2006.
11. Capogna G, Celleno D, Fusco P, Lyons G, Columb M. Relative potencies of bupivacaine and ropivacaine for analgesia in labour. Br J Anaesth. 1999;82(3):371–373. doi:10.1093/bja/82.3.371
12. Groban L. Central nervous system and cardiac effects from long-acting amide local anesthetic toxicity in the intact animal model. Reg Anesth Pain Med. 2003;28(1):3–11. doi:10.1053/rapm.2003.50014
13. Casati A, Putzu M. Bupivacaine, levobupivacaine and ropivacaine: are they clinically different? Best Pract Res Clin Anaesthesiol. 2005;19(2):247–268.
14. Oussoren C, Zuidema J, Crommelin DJ, Storm G. Lymphatic uptake and biodistribution of liposomes after subcutaneous injection. II. Influence of liposomal size, lipid compostion and lipid dose. Biochim Biophys Acta. 1997;1328(2):261–272. doi:10.1016/s0005-2736(97)00122-3
15. Halldin MM, Bredberg E, Angelin B, et al. Metabolism and excretion of ropivacaine in humans. Drug Metab Dispos. 1996;24(9):962–968.
16. Kopacz DJ, Emanuelsson BM, Thompson GE, Carpenter RL, Stephenson CA. Pharmacokinetics of ropivacaine and bupivacaine for bilateral intercostal blockade in healthy male volunteers. Anesthesiology. 1994;81(5):1139–1148. doi:10.1097/00000542-199411000-00007
17. Yang S, Abrahams MS, Hurn PD, Grafe MR, Kirsch JR. Local anesthetic Schwann cell toxicity is time and concentration dependent. Reg Anesth Pain Med. 2011;36(5):444–451. doi:10.1097/AAP.0b013e318228c835
18. Kang DK, Zhao LY, Wang HL. [Cytotoxic effects of local anesthesia through lidocaine/ropivacaine on human melanoma cell lines]. Rev Bras Anestesiol. 2016;66(6):594–602. doi:10.1016/j.bjan.2016.08.002
19. Cai XY, Xiong LM, Yang SH, et al. Comparison of toxicity effects of ropivacaine, bupivacaine, and lidocaine on rabbit intervertebral disc cells in vitro. Spine J. 2014;14(3):483–490. doi:10.1016/j.spinee.2013.06.041
20. Fresenius Kabi, LLC, USA. NAROPIN (Ropivacaine hydrochloride); 1996. Available from: http://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020533.
21. Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7(2):27–31. doi:10.4103/0976-0105.177703
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.