Back to Journals » International Journal of Nanomedicine » Volume 15
Formulation and Characterization of Sertaconazole Nitrate Mucoadhesive Liposomes for Vaginal Candidiasis
Authors Abdellatif MM , Khalil IA , Elakkad YE, Eliwa HA, Samir TM, Al-Mokaddem AK
Received 23 February 2020
Accepted for publication 11 May 2020
Published 11 June 2020 Volume 2020:15 Pages 4079—4090
DOI https://doi.org/10.2147/IJN.S250960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Menna M Abdellatif,1 Islam A Khalil,2 Yara E Elakkad,2 Hesham A Eliwa,3 Tamer M Samir,4 Asmaa K Al-Mokaddem5
1Department of Industrial Pharmacy, College of Pharmaceutical Sciences and Drug Manufacturing, Misr University for Science and Technology, Giza, Egypt; 2Department of Pharmaceutics, College of Pharmaceutical Sciences and Drug Manufacturing, Misr University for Science and Technology, Giza, Egypt; 3Department of Pharmacology and Toxicology, College of Pharmaceutical Sciences and Drug Manufacturing, Misr University for Science and Technology, Giza, Egypt; 4Department of Microbiology and Immunology, College of Pharmaceutical Sciences and Drug Manufacturing, Misr University for Science and Technology, Giza, Egypt; 5Department of Pathology, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
Correspondence: Menna M Abdellatif Tel +201005647945
Email [email protected]
Purpose: The aim of this study is to develop efficient localized therapy of sertaconazole nitrate for the treatment of vaginal candidiasis.
Methods: Sertaconazole nitrate-loaded cationic liposomes were prepared by thin-film hydration method and coated with different concentrations of pectin (0.05%, 0.1% and 0.2%) to develop mucoadhesive liposomes. The formulated mucoadhesive vesicles were characterized in terms of morphology, entrapment efficiency, particle size, zeta value, mucoadhesive properties and drug release. The selected formula was incorporated into a gel base and further characterized by an ex vivo permeation study in comparison with conventional sertaconazole gel. Also, the in vivo study was performed to assess the efficacy of sertaconazole mucoadhesive liposomal gel in treating rats with vaginal candidiasis.
Results: The mucoadhesive liposomes were spherical. Coating liposomes with pectin results in increased entrapment efficiency and particle size compared with uncoated vesicles. On the contrary, zeta values were reduced upon coating liposomes with pectin indicating efficient coating of liposomes with pectin. Mucoadhesive liposomes showed a more prolonged and sustained drug release compared with uncoated liposomes. Ex vivo study results showed that mucoadhesive liposomal gel increased sertaconazole tissue retention and reduced drug tissue penetration. In the invivo study, the mucoadhesive liposomal gel showed a significant reduction in the microbial count with a subsequent reduction in inflammatory responses with the lowest histopathological change compared with conventional gel.
Conclusion: The study confirmed the potentiality of employing mucoadhesive liposomes as a successful carrier for the vaginal delivery of antifungal drugs.
Keywords: sertaconazole nitrate, vaginal candidiasis, mucoadhesive liposomes
Introduction
Vaginal candidiasis is one of the most common reasons for women seeking healthcare and it has been estimated that about 75% of women will have an episode of candida vaginitis at least once during their lifetime, 40 to 50% of them will suffer from a recurrence and 5 to 8% will develop chronic vaginal candidiasis.1 For the treatment of such infection, local administration of antifungal drugs is desirable to avoid the several side effects caused by the systemic administration of antifungal drugs.2,3 However, conventional vaginal dosage forms as creams, tablets, pessaries, and solutions have several drawbacks as leakage, messiness and short residence time, this could be overcome by mucoadhesive formulations that increase drug retention time and enhance the absorption of poorly absorbable drugs through vaginal mucosa.4
Mucoadhesive nanoparticles where mucoadhesive polymers are used to enhance the mucoadhesive properties of nanocarriers have been extensively investigated for prolonging residence time of drugs at the site of application/absorption.5
Liposomes represent an attractive and physiologically acceptable drug delivery nanosystem, which was used in the topical delivery of drugs by all mucosal routes of administration.6 Liposomes can be coated by electrostatic deposition of an oppositely charged polymer.7 By coating, the liposomes with a mucoadhesive polymer such as chitosan, pectin and eudragit, not only the mucoadhesive properties and consequently the residence time on the mucosal surface could be increased but also the stability of the liposomes could be optimized.8 Pectin is a promising polymer in drug delivery due to the lack of toxicity and the low production cost. Pectin is an anionic polysaccharide, contains a certain amount of negatively charged carbonyl ends therefore used for coating positively charged liposomes.
Formulation of cationic liposomes involved the incorporation of quaternary ammonium compounds such as cetyltrimethylammonium bromide (CTAB), dimethyldidodecylammonium bromide (DDAB) and Dioctadecyldimethylammonium bromide (DODAB). These compounds are not only antibacterial but also possess antifungal, antiviral and anti-matrix metalloproteinase capabilities.9
Liposomes coated with pectin showed higher mucin adsorption and prolonged residence time at the site of application than uncoated liposomes.10,11
Sertaconazole nitrate is an imidazole derivative and which is effectively used for the treatment of the skin and mucosa mycoses, such as cutaneous, genital and oral candidiasis and tinea pedis. Sertaconazole is available under different formulations (tablets, pessaries, 2% solution and 2% cream) that have been adapted to the main clinical manifestations of superficial candidiasis and other mycoses.12
Therefore, the main goal of this research was the formulation of mucoadhesive liposomes where DDAB containing cationic liposomes loaded with the antifungal drug (sertaconazole) were coated with different concentrations of pectin and characterized for morphology, entrapment efficiency, particle size, zeta-potential, and drug release profile. Furthermore, an ex vivo permeation study was conducted to evaluate drug permeation and accumulation into the vaginal tissues. An in vivo study was conducted where antimicrobial efficacy of prepared formula was evaluated and confirmed with histopathological study and by measuring serum inflammatory biomarkers, immunoglobulin (IgM and IgG) and beta-D-Glucan (BDG).
Materials
Sertaconazole nitrate was kindly gifted by October Pharma (Cairo, Egypt), Soy phosphatidylcholine, Dimethyldidodecylammonium bromide (DDAB), Mucin from porcine stomach type II, Cellulose membrane (Molecular weight cut off: 12,000) and Cholesterol were purchased from Sigma-Aldrich (St. Loius, MO, U.S.A.). Pectin (Mwt: 30,000–100,000) was purchased from Techno Pharmchem (Haryana, India). Potassium dihydrogen phosphate, Disodium hydrogen phosphate, and Sodium chloride were purchased from El- Nasr Pharmaceutical Co. (Cairo, Egypt). Assay kit for tumor necrosis factor-alpha Cat. No: CSB- E1252r was purchased from Cusabio Biotech (Wuhan, China), Interleukin-23 Cat. No: 20,058 was purchased from RayBio (GA, USA), Immunoglobulin M Cat. No: E-EL-R0547, Immunoglobulin G (Cat. No: E-ELR0534) were purchased from Elabscience (Atlanta, USA) and 1,3-beta-D-glucan (BDG) Cat. No: MBS750634 was purchased from MyBioSource (San Diego, CA, USA). All other reagents and solvents were of analytical grade.
Methods
Formulation of Cationic Liposomes
Cationic liposomes were prepared by a thin-film hydration method where the mixture of soy phosphatidylcholine, cholesterol and the cationic surfactant (DDAB) in a molar ratio of 8:2:1 was dissolved methanol/chloroform mixture (2:1, v/v).13 Sertaconazole was added to the organic phase then the organic solvent system was slowly removed under reduced pressure, using a rotary evaporator, at (40◦ C) such that a very thin film of dry lipid was formed on the inner surface of the flask. The dry lipid film was slowly hydrated with 10 mL phosphate buffer saline (PBS) at pH 7.4.
Formulation of Mucoadhesive Liposomes
To prepare mucoadhesive liposomes, an equal volume of the liposomal suspension and pectin dispersion (at the concentration of 0.1, 0.2 and 0.4 % w/v in phosphate buffer saline, pH 7.4) was mixed where the liposomes dispersion was added to the pectin solution in a dropwise manner afterward the mixed suspension was stirred for an additional five minutes by vortexing to obtain 0.05, 0.1, and 0.2% w/v of pectin as a final concentration as shown in Table 1. The formulated formulae were stored in the refrigerator overnight then the excess pectin was removed by centrifugation at 20,000 rpm for 15 min at 4°C using a cooling centrifuge (Sigma 3K 30, Germany). The pellets were then resuspended in PBS, pH 7.4 and stored in the refrigerator for further characterization.
 |
Table 1 Composition and Characteristics of Different Formulations |
Characterization of Mucoadhesive Liposomes
Morphology
The morphology of different formulae (uncoated and coated) was analyzed using a transmission electron microscope (TEM) (JEM-1230, Joel, Japan). Samples were placed on a carbon-coated grid surface and negatively stained with a 1% aqueous solution of phosphotungstic acid and dried at room temperature before visualization.14
Determination of Entrapment Efficiency (EE)
One milliliter of each formula was centrifuged at 20,000 rpm for 1 hour at 4°C using a cooling centrifuge (Sigma 3K 30, Germany). Following centrifugation, the sediment was lysed using methanol and analyzed at 261nm using a UV-Vis spectrophotometer (Shimadzu UV 1650 Spectrophotometer, Japan). Entrapment was determined using the following equation:
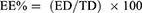
Where EE% is the percent encapsulation efficiency, ED is the concentration of entrapped drug and TD is the total drug concentration.
Particle Size, Distribution, and Zeta Potential
Particle size, polydispersity index, and zeta potential for formulae were measured by the dynamic light scattering (DLS) technique at 25°C using zetasizer (Malvern Instruments Ltd., UK). The dispersion was properly diluted with purified water in a ratio (1:10). Means and standard deviations were calculated for triplicates measurements.
Evaluation of Mucoadhesion Properties of Mucoadhesive Liposomes Using Mucin Test
The mucoadhesion properties of different formulae were evaluated where 1% w/v of porcine mucin solution was mixed with an equivalent amount of each formula dispersion in a dropwise manner under stirring. The stirring was conducted for an additional five minutes and the mixture was allowed to equilibrate overnight at room temperature. The surface charge of mucin particles and mucoadhesive liposomes in the presence of mucin were detected by zeta potential with a zetasizer.15
In vitro Release Study
Drug release was studied using locally fabricated Franz’s diffusion cell with an effective permeation area and receptor cell volume of 3.14cm2 and 12mL, respectively. The temperature of the receiver vehicle (vaginal simulated fluid containing 25% methanol to ensure sink condition, pH 4.5) was maintained at 37±1ºC. The cellulose membrane was mounted on a receptor compartment and mucoadhesive liposomal dispersions (equivalent to 10 mg sertaconazole) were added in the donor chamber and the system was properly sealed. The samples (500 µL) were collected every hour for 8 hours. The samples were replaced by an equal volume of fresh media upon removal of the sample from the receiver chamber. The sertaconazole content was determined by the HPLC method, where the mobile phase was composed of acetonitrile: disodium phosphate buffer (pH 4) (40:60, v/v). The drug was separated on a Luna C18 column using a flow rate of 1.8 mL/min. The retention time of the drug was around 21 minutes. The experiments were performed in triplicates.16
Formulation of Mucoadhesive Liposomal Gel
The selected mucoadhesive liposomes formula was incorporated into 1% HPMC gel where the weighted amount of HPMC was dissolved gradually in buffer solution (pH = 5.5) by the aid of magnetic stirrer at nearly 80 ○C then left to cool to room temperature then mixed with mucoadhesive liposomes dispersions. A control conventional gel was preformed where the accurately weighed amounts of sertaconazole were added to 1% HPMC gel to have a final sertaconazole concentration of 2% w/w in both gels.17
Ex vivo Permeation Study
Ex vivo permeation studies were carried out using locally fabricated Franz’s diffusion cell with a diffusion area of 0.785 cm2. Fresh sheep vaginal tissue was fixed between the donor and receptor compartments. Accurately measured 0.5 gm of sertaconazole mucoadhesive liposomal gel, equivalent to 10 mg of sertaconazole, was placed in the donor cell. The receptor compartment filled with 12 mL of phosphate buffer saline solution (pH 7.4) containing 25% methanol to ensure sink condition and maintained at 37 ± 1 °C. The system was properly sealed with parafilm. At an appropriate time, 0.5 mL of permeation media was withdrawn, and an equal volume of fresh media was added into the receiver cell. The samples were filtered through a 0.45 µm membrane and analyzed by using a validated HPLC method. Sertaconazole conventional gel was used as a control.
In vivo Evaluation of Mucoadhesive Liposomal Gel
Induction of Vaginal Candidiasis and Microbiological Assessment of Mucoadhesive Liposomal Gel Efficacy
Twenty-four ovariectomized female Sprague-Dawley rats (200 gm) were divided into four groups, each of six; the first group GP (I) was set as negative control while the other groups were subjected to vaginal candidiasis using Candida Albicans isolate. The isolate was taken as a part of routine laboratory procedure from a patient with acute vaginitis, therefore there was no written informed consent from the patient. The procedures were approved by the Ethical Committee of Misr University for science and technology (reference number: PT05) and are in accordance with the guidelines for the care and use of laboratory animals published by US NIH publication No. 85–23. In brief, all animals were subjected to estrus state with which was induced by subcutaneous administration of ß -estradiol- 17-valerate (Sigma Chemical Co., St. Louis, MO, USA) dissolved in 0.1 mL sesame oil and given in a dose of 0.5 mg/Kg for six days before infection and subsequently maintained by subcutaneous estradiol at a dose of 4 mg/kg weekly throughout the experiment.18 Furthermore, rats were administrated with dexamethasone 1 mg/L (Merck, Darmstadt, Germany) as immunosuppressant and amoxicillin (250 mg/L) in drinking water and continued during the experiment.19 On day 7 all the rats in groups were anesthetized through an I.M. injection of ketamine (30mg/kg) and rats in groups (II, III and IV) inoculated intravaginally with 107 yeast cells per 0.1 mL of sterile saline for the establishment of vaginal C. Albicans infection. By using a micropipette with disposable tips, inoculation was performed. The control group GP (I) rats were inoculated intravaginally with saline. The vaginal C. Albicans burden was evaluated at day 3 post-infection, 24 h before the start of treatment the basal level of infection was obtained by calculating the log10 CFU and standard deviation per vaginal sample to ensure that all animals involved in the study were homogeneously infected. Group II was set as the positive group receiving no treatment while group III receive sertaconazole control gel and group IV receive sertaconazole mucoadhesive liposomal gel. Antifungal therapy was started 4 days post-infection and was administered every 8 hours for 5 consecutive days. After completion of the treatment interval, vaginal swabs using sterile cotton were taken from each rat then suspended in 1 mL of sterile phosphate saline and cultured in duplicate on bengal rose chloramphenicol agar (Merck, Darmstadt, Germany) plate. Plates were incubated at 37°C for 48 hours.
Assessment of Serum Inflammatory Biomarkers, Immunoglobulin (IgM and IgG) and BDG
At the end of the experiment, rats were anesthetized, and blood samples were collected into tubes by cardiac puncture for evaluation of rat tumor necrosis factor-alpha, BDG, immunoglobulin (IgM and IgG) and interleukin-23 using enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions. The assay procedure was followed as mentioned in the manuals and the absorbance of the yellow color was measured at 450 nm. The sample concentration was determined from the corresponding standard curve.
Histopathological Study
After euthanasia, vaginal samples were dissected out and kept in 10% neutral buffered formalin. After fixation, samples were routinely processed by the passage in grades of alcohol, xylene and finally embedded in paraffin. Five microns sections were cut and stained with hematoxylin and eosin.20 Olympus BX43 light microscope connected to Olympus DP 27 digital camera was used to examine the sections and capture images.
Statistical Analysis of Data
To investigate the significant difference between the results of studied formulae, the one-way analysis of variance (ANOVA) test was used. The level of significance was set at 0.05, and (*p<0.05) was considered to be statistically significant.
Results and Discussion
Characterization of Mucoadhesive Liposomes
Morphology
The liposomes (uncoated and coated) had a spherical shape as shown in Figure 1. The size of the mucoadhesive liposomes formula was larger than the uncoated liposomes indicating efficient coating of liposomes with pectin. Pectin coating was shown in the image as an external black layer on the surface of the liposomes.
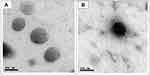 |
Figure 1 TEM images of the sertaconazole-loaded vesicles displaying (A) liposomes, (B) mucoadhesive liposomes. |
Entrapment Efficiency
The increased entrapment efficiency of the mucoadhesive liposomes compared to noncoated liposomes (F1) as shown in Table 1 and Figure 2 suggested that the pectin coating around the liposomes may have hindered the diffusion of the drug into the surrounding aqueous phase or that some of the drugs were physically trapped between the pectin layer and liposome surface. These results are in agreement with Li et al21 who found that the entrapment of bufalin was a little higher after coating liposomes with pectin. Similar results were also obtained by Haghighi et al22 who found that the entrapment efficiency of phloridzin increased after liposomes coating with pectin.
 |
Figure 2 Entrapment efficiency of different formulae (n=3). ****P < 0.0001. |
Particle Size, Distribution, and Zeta Potential
The coating of liposomes with pectin increased particle size as shown in Table 1 and Figure 3. This is in agreement with the results reported by Thirawong et al23 who found that the size of cationic liposomes was significantly increased after coating with pectin and owed this to the interaction between cationic liposomes and pectin. The mean particle size of F3 and F4 was of great importance as values were 193.4±10 and 229.8±11, respectively, where Jøraholmen et al24 stated that vesicles with a mean particle size around 200 nm assure both a depot effect at the vaginal site as well as being stable and avoiding potential precipitation during the cell studies. The polydispersity index (PI) increased with the increase of pectin concentration used for coating liposomes, indicating an elevated polydispersity of mucoadhesive liposomes.
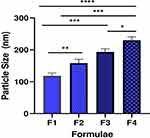 |
Figure 3 Particle size diameter of different formulae (n=3). *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. |
The zeta potential value of uncoated liposomes (F1) was 49.7 mv ± 0.58 which decreased to 19.7 mv ± 0.19 upon coating liposomes with 0.05% pectin (F2), on increasing pectin concentration to 0.1% (F3) value further decreased to 9.56 mv ± 0.34, on increasing pectin concentration to 0.2% (F4) value became to 10.4 mv ±0.71. The differences in zeta potential on the liposomal surface between non-coated and pectin-coated liposomes confirmed that liposomes were indeed coated with pectin. The lower zeta-potential values obtained by mucoadhesive liposomes as these vesicles are prepared using an electrostatic deposition method, which involves the deposition of anionic polysaccharides of pectin onto cationic liposomes through electrostatic attraction. If the liposomes were only stabilized by electrostatic repulsion, then the magnitude of the zeta-potential would be too low to ensure good stability. However, if they are coated by a layer of pectin molecules, it may be possible that these systems also remain stable for a specific period due to steric hindrance. These results agree with Feng et al25 who studied the stability of low methoxy pectin coated liposomes and found that the steric hindrance potentially exerted by surface-adsorbed pectin enhances the stability of the liposomes.
There was no significant difference between the zeta potential value of (F3) and (F4) this indicates that when the concentration of pectin exceeded 0.1% the zeta potential of liposomes did not change, which was probably due to that the liposomal surface became saturated with pectin molecules. Nguyen et al26 prepared pectin–liposome nanocomplexes exhibited that when pectin concentration exceeded 0.3 % there was no change in zeta value of pectin coated liposomes.
Evaluation of Mucoadhesion Properties of Different Formulae Using Mucin Test
The zeta potential of the mucin suspension (−15.5 mV ± 0.22) changed upon mixing with mucoadhesive liposomes (F2-F4) as shown in Figure 4. A less negative value obtained from the complexes formed between mucin and pectin-coated liposomes where the negative charge of the mucin particles would be neutralized with the positive charge of the mucoadhesive liposomes adsorbed on their surface. Takeuchi et al27 stated that the surface property of the mucin particles might be changed by the adhesion of the polymer if the polymer has a mucoadhesive property. Mucoadhesive liposomal formula F3 (pectin concentration 0.1 %) showed the maximum shift (- 6.67 mV ± 0.10) of mucin zeta potential value.
 |
Figure 4 Zeta potential shift of mucin upon mixing with different mucoadhesive liposomes. |
Drug Release
Studying the sertaconazole in vitro release profile as shown in Figure 5 indicated that increasing pectin concentration for coating liposomes enhanced the sustained release of sertaconazole comparing to non-coating formula (F1). The drug release from coated and uncoated formulae was found to be best fitted by the Higuchi square root model which implies that the release of drugs as a square root of time-dependent process and diffusion controlled. The cumulative drug release percent after 8 hours from (F1) was about 89.07% ±1.91, Upon coating vesicles with 0.05% pectin (F2) the cumulative release of drug was decreased to 81.13% ± 1.93, increasing pectin concentration to 0.1% (F3) the value decreased to 68.15% ± 1.35, further increase of pectin concentration to 0.2% (F4) associated with a further decrease in cumulative percent release 58.24% ± 1.69. This is of great importance considering the vaginal administration, as a reduced frequency in need for repeated administration correlates with better patient compliance.28
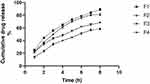 |
Figure 5 In vitro drug release profile. |
Ex vivo Permeation
The effectiveness of drug delivery at the mucosal site is dependent on a series of interconnected actions including distribution and retention of drug delivery system on the mucosal surface, its penetration into/through the mucus mesh, and release profile of the drug.29 Mucoadhesive liposomal formula (F3) was chosen to be incorporated into the gel base for further investigation as it showed maximum mucoadhesion property and satisfied entrapment efficiency, particle size, and in vitro drug release results. The ex vivo permeation experiments performed on the sheep vaginal tissue showed that coated liposomes assured increased sertaconazole tissue retention and reduced its penetration as compared to the control gel as shown in Figures 6 and 7. Where the permeability coefficient (Kp) of mucoadhesive liposomal gel and control gel was 0.00878 and 0.0190 cm/h, respectively as shown in Table 2. It is important to mention that this prolonged release may benefit the treatment of vaginal candidiasis due to the continuous drug release in the vaginal cavity. The drug retention in vaginal tissue was evaluated where the mucoadhesive liposomal gel retains the drug in vaginal tissues 1.75 times more than control gel while the amount of drug permeated was reduced by 2.47 times compared to control gel. For topical vaginal candidiasis treatment, it is desirable to limit the amount of systemic exposure of the user to the antifungal drug. Thus, when less drug passes through the vaginal mucosa, vaginal candidiasis treatment becomes more effective and less systemic effects are associated. The drug release from mucoadhesive gel and control gel were fitted in different mathematical models to understand the mechanisms of release. The regression coefficient values indicate that the permeation across vaginal tissue can best be fitted using the Higuchi release model.
 |
Table 2 Ex vivo Permeation Parameters of Mucoadhesive Liposomal Gel and Control Gel |
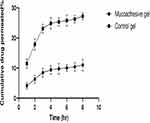 |
Figure 6 Ex vivo permeation drug profile. |
 |
Figure 7 Cumulative drug release and tissue retention of mucoadhesive liposomal gel and control gel. |
In vivo Evaluation
Assessment of Antimicrobial Efficacy of the Mucoadhesive Liposomal Gel
The study was performed, and no complications related to the induction of vaginal candidiasis or treatment techniques were observed. None of the animals in the uncontaminated group had signs of vaginal candidiasis. On the other hand, all the rats in the untreated contaminated group exhibited apparent infection evident by pus drainage at the infection site. The mean microbial count was compared for the four groups as shown in Figure 8. The uncontaminated group GP (I) showed no growth of candida, while the other three contaminated groups (II, III and IV) showed microbial growth of candida Albicans. The mean number of colonies forming unit (CFUs) before the intervention was 68 ± 1.73, 72 ± 1.3 and 70 ± 1.9 in three contaminated groups (II, III and IV), respectively. After 5 days, the number of CFUs significantly increased to 168.8 ± 5.8 in the positive control group (II), and significantly decreased to 42.4± 1.1 and 10 ± 1.9 in treated groups (III and IV), respectively. The superiority of mucoadhesive liposomal gel in terms of reduction magnitude of microbial infection in comparison to control gel might be due to firstly, the anticandidal effect of cationic surfactant DDAB. Lin et al30 studied the disinfectant effect of the mixture of glutaraldehyde and (DDAB) against C. Albicans and owed efficacy of DDAB as a quaternary ammonium compound which can actively adsorb and accumulate on cell surfaces and alter the charge characteristics and the charge density of the membrane. Also, can change the hydrophobicity of cell membranes leading to membrane decomposition. Secondly, mucoadhesive liposomes were able to assure the sustained release of sertaconazole and had prolonged the residence time in the vaginal cavity.
 |
Figure 8 Mean colony-forming unit (CFU) of each group before and after the treatment period (n=6). ***P < 0.001. |
Assessment of Serum Inflammatory Biomarkers, Immunoglobulin (IgM and IgG) and BDG
In this study, sertaconazole control gel GP (III) and sertaconazole mucoadhesive liposomal gel GP (IV) were assessed for their ability to improve the biochemical features of vaginal candidiasis in comparison with positive control GP (II) and negative control GP (I). Where the occurrence of vaginal candidiasis takes on rising tendency on account of the wide use of cytotoxic and immunosuppressive agents.31 Inflammatory cytokines are the main components between innate immunity and chronic infection, which produced by a fungal infection. It has been shown that IL-22, the key cytokine produced by the T helper 22 subset of lymphocytes (Th22), synergistically induces the production of hBD2, S100A7, and CXCL-10 together with TNF-alpha in keratinocytes. The IL-22 and TNF-α may provide a protective effect against C. Albicans infection.32 IL-23 had been found to selectively induce the proliferation of memory T cells. The ability of IL-23 to stimulate CD4 + T cells to produce IL-17 plays a dominant role in the development and persistence of autoimmunity. In general, IL-23 had been found to selectively induce the proliferation of memory T cells, by initiating and maintaining Th1 responses, which has long been thought to account for overreacting immune and autoimmune disorders.33
In our vaginal candidiasis model, local administration of sertaconazole mucoadhesive liposomal gel GP (IV) significantly decreased serum level of inflammatory cytokines (TNF-α and IL-23) as compared to sertaconazole control gel GP (III) and control positive GP (II), we investigated that rats with vaginal candidiasis showed a rapid elevation in the serum level of inflammatory cytokines (TNF-α and IL-23) as compared to control and treatment groups.
On the other hand, results of the present study showed that a significant increase in serum levels of immunoglobulin’s (IgM and IgG) after vaginal candidiasis as compared to control negative GP (I) and treatment groups, while local administration of sertaconazole mucoadhesive liposomal gel GP (IV) exhibited a significant decrease in serum (IgM and IgG) as compared to sertaconazole control gel GP (III) and control positive GP (II) as in Figure 9).
The Candida cell wall is composed of chitin, β -glucans, and mannoproteins. BDG is a polysaccharide composed of glucose monomers linked by beta 1–3 glucosidic bonds. BDG test is widely used in many studies for the detection of invasive fungal disease (IFDs),34 so that these tests performed in patients that are at high risk for the of IFDs.35 The results of BDG test may be used as a diagnostic marker for the presence of an IFDs.36 Finally, BDG serum level significantly elevated after induction of fungal infection by candida Albicans, while treatment with sertaconazole mucoadhesive liposomal gel GP (IV) showed a significant reduction in serum BDG as compared to sertaconazole control gel GP (III) and control positive GP (II).
Histopathological Study
Histological examination of vaginal samples from the negative control group revealed the normal histological structure of rat vagina as shown in (Figure 10A and B), in which the mucosa appeared intact and made up of stratified squamous epithelium rested on the dense sub-epithelial connective tissue that contained blood vessels and very few or no inflammatory cells. The positive group exhibited some histopathological alterations represented by heavy subepithelial inflammatory cells infiltrations with the damaged mucosal surface that appeared covered by necrotic tissue debris (Figure 10C and D). Moreover, the mucosa showed some areas of hyperplastic epithelium that appeared covered by a thickened keratin layer (Figure 10E). Heavy sub-epithelial infiltration with neutrophils and mononuclear cells was the frequently encountered feature of this group (Figure 10F). These findings were consistent with those of De Bernardis et al and Li et al.37,38 The presence of mononuclear inflammatory cells in the subepithelial tissue of Candida infected vagina could be attributed to activation of the subepithelial lymphocyte populations rather than being transmigrated under the effect of estrogen. Also, the observed epithelial damage due to Candida infection was explained as a consequence of the inflammatory process itself. Concerning the epithelial hyperplasia observed, it could be attributed to estradiol treatment as previously described by Carbonel et al.39 On the contrary, the group received mucoadhesive liposomal gel exhibited mild sub-epithelial inflammatory reaction (Figure 10G) with intact mucosa (Figure 10, h). The vagina of rats group receiving sertaconazole control gel (Figure 10I and J) showed intact mucosa with the presence of necrotic debris in the keratin layer in almost all the examined sections with mildly dispersed sub-epithelial connective tissue with dilated blood vessels due to edema.
Conclusions
Vaginal candidiasis is a common infection with a 40 to 50% recurrence rate in females. Local administration of antifungal is desirable to avoid side effects of systemic preparations, current conventional vaginal dosage forms have several drawbacks that affect the convenience of using them. In this study, we developed mucoadhesive liposomes capable of localizing antifungal drugs (sertaconazole) in the vaginal cavity. Cationic liposomes were rendered mucoadhesive by coating with different concentrations of pectin. Entrapment efficiency, particle size, zeta potential value, and mucoadhesion properties were dependent on pectin concentration used for coating liposomes. Mucoadhesive liposomes coated with pectin showed a prolonged and sustained drug release compared with uncoated liposomes. The ex vivo study showed increased drug tissue retention and reduced drug tissue permeability of mucoadhesive liposomal gel compared to control gel. Drug tissue retention and reduced systemic absorption serve developing local application form by minimizing drug systemic absorption. In vivo study results suggested that the cationic surfactant (DDAB) enhanced the anticandidal activity of mucoadhesive liposomal gel and led to its superiority in treating vaginal candidiasis in comparison with sertaconazole conventional gel. The current study points out that the mucoadhesive liposomes are a promising candidate for local vaginal drug delivery.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Albertini B, Passerini N, Di Sabatino M, Vitali B, Brigidi P, Rodriguez L. Polymer–lipid based mucoadhesive microspheres prepared by spray-congealing for the vaginal delivery of econazole nitrate. Eur J Pharm Sci. 2009;36(4–5):591–601. doi:10.1016/j.ejps.2008.12.009
2. Hainer BL, Gibson MV. Vaginitis: diagnosis and treatment. Am Fam Physician. 2011;83(7):807–815.
3. Vanić Ž, Hurler J, Ferderber K, Golja Gašparović P, Škalko-Basnet N, Filipović-Grčić J. Novel vaginal drug delivery system: deformable propylene glycol liposomes-in-hydrogel. J Liposome Res. 2014;24(1):27–36. doi:10.3109/08982104.2013.826242
4. Takeuchi H, Yamamoto H, Kawashima Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Adv Drug Deliv Rev. 2001;47(1):39–54. doi:10.1016/S0169-409X(00)00120-4
5. Netsomboon K, Bernkop-Schnürch A. Mucoadhesive vs. mucopenetrating particulate drug delivery. Eur J Pharm Biopharm. 2016;98:76–89. doi:10.1016/j.ejpb.2015.11.003
6. Erjavec V, Pavlica Z, Sentjurc M, Petelin M. In vivo study of liposomes as drug carriers to oral mucosa using EPR oximetry. Int J Pharm. 2006;307(1):1–8. doi:10.1016/j.ijpharm.2005.09.016
7. Laye C, McClements D, Weiss J. Formation of biopolymer-coated liposomes by electrostatic deposition of chitosan. J Food Sci. 2008;73(5):N7–N15. doi:10.1111/j.1750-3841.2008.00747.x
8. Smistad G, Bøyum S, Alund SJ, Samuelsen ABC, Hiorth M. The potential of pectin as a stabilizer for liposomal drug delivery systems. Carbohydr Polym. 2012;90(3):1337–1344. doi:10.1016/j.carbpol.2012.07.002
9. Jiao Y, Niu L-N, Ma S, Li J, Tay FR, Chen J-H. Quaternary ammonium-based biomedical materials: state-of-the-art, toxicological aspects and antimicrobial resistance. Prog Polym Sci. 2017;71:53–90. doi:10.1016/j.progpolymsci.2017.03.001
10. Sharma R, Ahuja M, Kaur H. Thiolated pectin nanoparticles: preparation, characterization and ex vivo corneal permeation study. Carbohydr Polym. 2012;87(2):1606–1610. doi:10.1016/j.carbpol.2011.09.065
11. Mahajan HS, Tatiya BV, Nerkar PP. Retracted: ondansetron loaded pectin based microspheres for nasal administration: in vitro and in vivo studies. Powder Technol. 2012;221:168–176. doi:10.1016/j.powtec.2011.12.063
12. Carrillo-Muñoz AJ, Tur-Tur C, Giusiano G, et al. Sertaconazole: an antifungal agent for the topical treatment of superficial candidiasis. Expert Rev Anti Infect Ther. 2013;11(4):347–358. doi:10.1586/eri.13.17
13. Makky A, Mohamed MI, Abdellatif MM. Liposomal gels as carriers for safer topical delivery of tazarotene. J Res Opin. 2016;3:3.
14. Hassan DH, Abdelmonem R, Abdellatif MM. Formulation and characterization of carvedilol leciplex for glaucoma treatment: in-vitro, ex-vivo and in-vivo study. Pharmaceutics. 2018;10(4):197. doi:10.3390/pharmaceutics10040197
15. Klemetsrud T, Jonassen H, Hiorth M, Kjøniksen A-L, Smistad G. Studies on pectin-coated liposomes and their interaction with mucin. Colloids Surf B Biointerfaces. 2013;103:158–165. doi:10.1016/j.colsurfb.2012.10.012
16. Muzaffar F, Sing UK. RP-HPLC and UV Spectrophotometric methods for estimation of sertaconazole nitrate in microemulsion. J Chem Pharm Res. 2016;8(7):740–745.
17. Abdellatif MM, Khalil IA, Khalil MAF. Sertaconazole nitrate loaded nanovesicular systems for targeting skin fungal infection: in-vitro, ex-vivo and in-vivo evaluation. Int J Pharm. 2017;527(1–2):1–11. doi:10.1016/j.ijpharm.2017.05.029
18. De Bernardis F, Boccanera M, Adriani D, Spreghini E, Santoni G, Cassone A. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect Immun. 1997;65(8):3399–3405. doi:10.1128/IAI.65.8.3399-3405.1997
19. Martinez A, Regadera J, Jimenez E, Santos I, Gargallo-Viola D. Antifungal efficacy of GM237354, a sordarin derivative, in experimental oral candidiasis in immunosuppressed rats. Antimicrob Agents Chemother. 2001;45(4):1008–1013. doi:10.1128/AAC.45.4.1008-1013.2001
20. Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. Elsevier health sciences; 2008.
21. Li Y, Zhao H, Duan L-R, et al. Preparation, characterization and evaluation of bufalin liposomes coated with citrus pectin. Colloids Surf a Physicochem Eng Asp. 2014;444:54–62. doi:10.1016/j.colsurfa.2013.12.006
22. Haghighi M, Yarmand MS, Emam-Djomeh Z, McClements DJ, Saboury AA, Rafiee-Tehrani M. Design and fabrication of pectin-coated nanoliposomal delivery systems for a bioactive polyphenolic: phloridzin. Int J Biol Macromol. 2018;112:626–637. doi:10.1016/j.ijbiomac.2018.01.108
23. Thirawong N, Thongborisute J, Takeuchi H, Sriamornsak P. Improved intestinal absorption of calcitonin by mucoadhesive delivery of novel pectin–liposome nanocomplexes. J Controlled Release. 2008;125(3):236–245. doi:10.1016/j.jconrel.2007.10.023
24. Jøraholmen MW, Basnet P, Tostrup MJ, Moueffaq S, Škalko-Basnet N. Localized therapy of vaginal infections and inflammation: liposomes-in-hydrogel delivery system for polyphenols. Pharmaceutics. 2019;11(2):53. doi:10.3390/pharmaceutics11020053
25. Feng S, Sun Y, Wang P, Sun P, Ritzoulis C, Shao P. Co-encapsulation of resveratrol and epigallocatechin gallate in low methoxyl pectin-coated liposomes with great stability in orange juice. Int J Eng Res Appl. 2019.
26. Nguyen S, Solheim L, Bye R, Rykke M, Hiorth M, Smistad G. The influence of liposomal formulation factors on the interactions between liposomes and hydroxyapatite. Colloids Surf B Biointerfaces. 2010;76(1):354–361. doi:10.1016/j.colsurfb.2009.11.020
27. Takeuchi H, Thongborisute J, Matsui Y, Sugihara H, Yamamoto H, Kawashima Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv Drug Deliv Rev. 2005;57(11):1583–1594. doi:10.1016/j.addr.2005.07.008
28. Jøraholmen MW, Škalko-Basnet N, Acharya G, Basnet P. Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur J Pharm Sci. 2015;79:112–121. doi:10.1016/j.ejps.2015.09.007
29. Vanić Ž, Škalko-Basnet N. Nanopharmaceuticals for improved topical vaginal therapy: can they deliver? Eur J Pharm Sci. 2013;50(1):29–41. doi:10.1016/j.ejps.2013.04.035
30. Lin W, Yuan D, Deng Z, Niu B, Chen Q. The cellular and molecular mechanism of glutaraldehyde-didecyldimethylammonium bromide as a disinfectant against Candida albicans. J Appl Microbiol. 2019;126(1):102–112.
31. Fidel PL, Luo W, Steele C, Chabain J, Baker M, Wormley F. Analysis of vaginal cell populations during experimental vaginal candidiasis. Infect Immun. 1999;67(6):3135–3140. doi:10.1128/IAI.67.6.3135-3140.1999
32. Eyerich S, Wagener J, Wenzel V, et al. IL-22 and TNF-α represent a key cytokine combination for epidermal integrity during infection with Candida albicans. Eur J Immunol. 2011;41(7):1894–1901. doi:10.1002/eji.201041197
33. Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202(1):96–105. doi:10.1111/j.0105-2896.2004.00214.x
34. Tian R, Fu M, Zhang Z, et al. In situ IgM production and clonal expansion of B-1 cells in peritoneal cavity promote elimination of C. albicans infection in IgH transgenic mice with VH derived from a natural antibody. PLoS One. 2013;8(4). doi:10.1371/annotation/bedab014-d393-4fad-aa07-0df1efb59038
35. Theel ES, Doernb CD. Point-counterpoint: β-d-glucan testing is important for diagnosis of invasive fungal infections. J Clin Microbiol. 2013;51(11):3478–3483. doi:10.1128/JCM.01737-13
36. Lu Y, Chen Y-Q, Guo Y-L, Qin S-M, Wu C, Wang K. Diagnosis of invasive fungal disease using serum (1→3)-β-D-glucan: a bivariate meta-analysis. Int Med. 2011;50(22):2783–2791. doi:10.2169/internalmedicine.50.6175
37. De Bernardis F, Santoni G, Boccanera M, et al. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect Immun. 2000;68(6):3297–3304. doi:10.1128/IAI.68.6.3297-3304.2000
38. Li T, Liu Z, Zhang X, Chen X, Wang S. Local probiotic lactobacillus crispatus and lactobacillus delbrueckii exhibit strong antifungal effects against vulvovaginal candidiasis in a rat model. Front Microbiol. 2019;10:1033. doi:10.3389/fmicb.2019.01033
39. Carbonel AF, Haidar MA, Simões RS, et al. Proliferation histomorphometric and immunohistochemical markers on the ovariectomized rat vagina after estrogen and/or isoflavones treatments. Microsc Microanal. 2010;16(42):1156–1157. doi:10.1017/S1431927610060253
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.