Back to Journals » International Journal of Nanomedicine » Volume 14
Formulation and characterization of chlorhexidine HCl nanoemulsion as a promising antibacterial root canal irrigant: in-vitro and ex-vivo studies
Authors Abdelmonem R , Younis MK , Hassan DH, El-Sayed Ahmed MAEG , Hassanien E , El-Batouty K, Elfaham A
Received 8 February 2019
Accepted for publication 4 May 2019
Published 1 July 2019 Volume 2019:14 Pages 4697—4708
DOI https://doi.org/10.2147/IJN.S204550
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Rehab Abdelmonem,1 Mona K Younis,1 Doaa H Hassan,1 Mohamed Abd El-Gawad El-Sayed Ahmed,2 Ehab Hassanein,3 Kariem El-Batouty,3 Alaa Elfaham3
1Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmacy, Misr University for Science and Technology, 6th of October City, Egypt; 2Department of Microbiology and Immunology, Faculty of Pharmacy, Misr University for Science and Technology, 6th of October City, Egypt; 3Department of Endodontics Faculty of Dentistry, Ain Shams University, Cairo, Egypt
Introduction and aim: Chlorhexidine Hydrochloride [Chx.HCl] has a broad-spectrum antibacterial effect, sustained action and low toxicity so it has been recommended as a potential root canal irrigant. The aim of this study was to improve the penetration ability, cleansing and antibacterial effect of Chx.HCl using a newly formulated Chx.HCl nanoemulsion and use it as root canal irrigant.
Methods: Chx.HCl nanoemulsions were prepared using two different oils; Oleic acid and Labrafil M1944CS, two surfactants; Tween 20 and Tween 80 and co-surfactant; Propylene Glycol. Pseudoternary phase diagrams were constructed to designate the optimum systems. The prepared nanoemulsion formulae were evaluated for their drug content, emulsification time, dispersibility, droplet size, in-vitro drug release, thermodynamic stability, In-vitro antibacterial activity and ex-vivo study for the selected formula. Comparisons were made of Chx.HCl nanoemulsion with two different concentrations 0.75% and 1.6% vs Chx.HCl normal particle size as root canal irrigant for their penetration ability, cleansing effect and antibacterial effect.
Results: The selected formula was F6 with composition of 2% Labrafil, 12% Tween 80 and 6% Propylene glycol. It has small particle size (12.18 nm), short emulsification time (1.67 seconds), and fast dissolution rate after 2 minutes. It was found to be a thermodynamically/physically stable system. The higher concentration of Chx.HClnanoemulsion1.6% shows the best penetration ability compared to Chx.HCl normal particle size due to the smaller particle size. Chx.HCl nanoemulsion 1.6% has the lowest mean value of the remaining debris surface area (2001.47 μm2,) when compared to normal particle size material (2609.56 μm2,).
Conclusion: Chx.HCl nanoemulsion preparation has better cleansing ability and antibacterial effect with high efficacy on Enterococcus faecalis, where high reduction rate or complete eradication of bacterial cells has been achieved.
Keywords: chlorhexidine hydrochloride, nanoemulsion, root canal irrigant, penetration ability, cleansing effect, antibacterial irrigant
Introduction
Nanoemulsions are a class of emulsions with a droplet size in the range of 50–500 nm and have attracted a great deal of attention in recent years because of their unique characteristics.1 The growing use of non-ionic surfactants in nanoemulsions is related to their good detergent performance, which is not affected by water hardness, and, in most cases, to their low toxicity and they do not give rise to electrostatic interactions.2 Nanotechnology with the ultra-small particle sizes, large surface area/mass ratio and unique physicochemical properties compared with their bulk counterparts hold new prospects for the treatment and prevention of dental infections.3 Chlorhexidine hydrochloride (Chx.HCl) is sparingly soluble in water very slightly soluble in alcohol, it is gradually colored by light.4,5 Chx.HCl has a broad-spectrum antibacterial effect, sustained action and low toxicity. Because of these properties, it has also been recommended as a potential root canal irrigant. The major advantages of Chx.HCl are its lower cytotoxicity and lack of foul smell and bad taste.6–9 Several types of laser have been used to improve root canal disinfection. Bactericidal action of the laser depends on the wavelength and energy, and thermal effects that produce alteration of the cell wall of the bacteria, leading to changes in osmotic gradients up to cell death. The interaction between laser and the irrigant in the root canal outlines a new area of interest in the field of endodontic disinfection.10 Ultrasonic energy produces high frequencies but low amplitudes. The files are designed to oscillate at ultrasonic frequencies of 25–30 kHz, which are beyond the limit of human auditory perception (>20 kHz). They operate in a transverse vibration, setting up a characteristic pattern of nodes and antinodes along their length. The term passive ultrasonic irrigation (PUI) is an irrigation scenario where there is no instrumentation or contact of the canal walls with an endodontic file or instrument. During PUI, the energy is transmitted from an oscillating file to the irrigant in the root canal by means of ultrasonic waves. The latter induces acoustic streaming and cavitation of the irrigant.11 Based upon the above evidence, it was thought that evaluation by using nanotechnology to improve the penetration ability and cleansing effect of Chx.HCl is of value.
Materials and methods
Materials
Chlorhexidine hydrochloride Chx.HCl was kindly provided by the Arab Drug Company for Pharmaceuticals (Cairo, Egypt). Labrafil M 1944 CS (oleoyl polyoxyl-6 glycerides), was generously supplied by Gattefosse (Saint-Priest, France). Tween 20 (polyoxyethylene (20) sorbitanmonolaurate), Tween 80 (polyoxyethylene (80) sorbitanmonooleate), Oleic acid, Propylene glycol were supplied by Gomhorya Company (Cairo, Egypt). Noncarious single rooted human teeth were extracted due to periodontal diseases or for orthodontic treatment in the oral and maxillofacial department in the Faculty of Dentistry, Ain Shams University, Cairo, Egypt. Pure culture of E. faecalis (strain ATCC 29212) grown in brain heart infusion (BHI) broth (RC CLEANER, IIchung Dental Ltd., Seoul, Korea).
Methods
Solubility studies
Solubility of Chx.HCl in different media (Oleic acid, Labrafil M 1944CS, Tween 20, Tween 80, Propylene glycol and water) were studied. Large excess of Chx.HCl (50 mg) was taken in centrifuge tubes and 5.0 g media phase was added. The mixture was shaken in a vortex mixer for 15 minutes and then stored at room temperature. After 24 hours, in order to obtain clear supernatant, the undissolved drug precipitates in the tubes were centrifuged for 5 minutes at 3000 rpm. Adequate sample solution was collected and diluted with n-butanol. Whatman 102 filter paper was used to filter the diluted sample which was further suitably diluted with n-butanol to determine the concentration of drug in saturated solution. The samples were analyzed with UV spectrophotometer at 260 nm against n-butanol as blank.12,13
Construction of a pseudoternary phase diagram
A pseudoternary phase diagram was constructed to determine the exact proportion of each component needed in the formulation to obtain the best parameters for the ideal nanoemulsion.14 Formulations were prepared using oil, namely Oleic acid and Labrafil M1944CS, surfactants, namely Tween 20 and Tween 80 and co-surfactant, namely Propylene glycol. Firstly, a mixture of surfactant alone (with no co-surfactant) and oil was prepared in different volume ratios (from 1:9 to 9:1). When aqueous titration (dropwise addition of water) was performed on the mixture, the observation of clear to turbid mixture, which was the end point, was closely monitored. These end points were then marked on a pseudoternary phase diagram. The whole procedure was repeated for a mixture of surfactant and co-surfactant (Smix) which were prepared in ratios 2:1 and 3:1 and combined with the selected oil15,16 Figure 1.
 |
Figure 1 Pseudoternary phase diagrams. |
Preparation of Chx.HCL nanoemulsion
Nanoemulsion systems containing Chx.HCl were prepared using Labrafil M 1944 CS as oil phase and surfactants Tween 80 or 20 as well as, Propylene glycol as cosurfactant, and, lastly, water, Table 1. The drug was dissolved in the Labrafil M 1944 CS, the water combined with surfactant and a cosurfactant is then added at a slow rate with gradual stirring. The amount of surfactant and cosurfactant to be added and the percent of oil phase that can be incorporated were determined with the help of pseudo-ternary phase diagram. Ultrasonicator (Ultrasonic LC 60 H, Elma, Germany) was used to achieve the desired size range for dispersed globules. It was then allowed to equilibrate.17
 |
Table 1 Compositions of Chx.HCl nanoemulsion formula |
Characterization of Chx.HCL nanoemulsion
Dispersibility tests
Dispersibility tests were done using a dissolution apparatus (Dr. Schleuniger Pharmaton, type Diss 6000, Thun, Switzerland) where 1 mL of each formula was added to 500 mL of water at 37±0.5ºC. A standard stainless-steel dissolution paddle rotating at 50 rpm provided gentle agitation. The type of emulsion formed was visually inspected and categorized as either clear, translucent with bluish tone, milky or turbid emulsion. The formulae that have clear appearance were selected for further studies.18,19
Determination of drug content
Chx.HCl from an optimized nanoemulsion formula based on the pseudoternary phase diagram results was extracted in n-butanol using the sonication technique. The extract was analyzed for Chx.HCl content spectrophotometrically at a wavelength of 260 nm after suitable dilution.20
Test for self-emulsification time of Chx.HCL nanoemulsion
To test the self-emulsification time, 1 mL volume of each formula was introduced into a beaker containing 250 mL of distilled water, maintained at 37±1°C under continuous stirring at 50 rpm. The self-emulsification time was taken as the time for a pre-concentrate to form a homogenous mixture upon dilution.21
Droplet size analysis
For the droplet size analysis 50 mg of the optimized formula were diluted with water to 1000 mL in a flask and gently mixed by hand. The droplet size distribution was determined by Malvern Zetasizer 2000 (Malvern Instruments Ltd., Malvern, UK) with the conditions of backscatter detection at 173º; temperature of 25ºC; refractive index of 1.330 were used.22
In-vitro release study
The in-vitro dissolution studies were carried out using USP apparatus type II (paddle) (Dr. Schleuniger Pharmaton, type Diss 6000) at 50 rpm. The dissolution medium used was distilled water (500 mL) maintained at 37±0.5ºC, and 5 mL of prepared formula was instilled to the dissolution medium. Then 5 mL of dissolution media were withdrawn at different time intervals and the content of drug released was determined spectrophotometrically at 254 nm. The experiments were conducted in triplicate.23
Kinetic study of the release data of ChxHCL nanoemulsions
The kinetic parameters of the in-vitro release of Chx.HCl from its prepared nanoemulsions were then measured. Zero, first and second order kinetics and the Higuchi-diffusion model were tried to choose the most suitable kinetic order for Chx.HCl release.
Thermodynamic stability tests of prepared Chx.HCl nanoemulsions
Phase separation and drug precipitation
A quantity of 2 mL of each formula was stored for 48 hours at ambient temperature and observed thereafter for phase separation. Then 1 mL samples of each Chx.Hcl nanoemulsion formula was diluted to 10 mL and 100 mL with distilled water at 25°C stored for a period of 24 hours. and observed afterwards for phase separation.21
Refrigeration thaw cycle
Next, 2 mL samples of each formula were separately transferred to a transparent screw capped bottle and stored in a refrigerator at 2°C for 24 hours. After which they were removed and stored at 25°C and 40°C. A single refrigeration thaw cycle test was performed. The samples were then observed for phase separation and drug precipitation.21
Centrifugation
A 5 mL sample of each Chx.Hcl nanoemulsion formula formulation was transferred into a glass test tube and inserted into a laboratory centrifuge (Microcentrifuge Shanghai Surgical Instrument Factory, model 800, Shanghai, People's Republic of China) and centrifuged at 4,000 rpm for 5 min. Thereafter, the samples were observed for phase separation and drug precipitation.21
Irrigating solution examinations on extracted teeth
Penetration ability and cleansing effect
All experiments were approved by the Institutional Ethics Committee, Ain Shams University, Egypt. Fifty noncarious single rooted human teeth with mature apices were selected. The extracted teeth were used after taking signed written informed consent letters from patients. The teeth included maxillary and mandibular incisors and mandibular premolars. The external root surfaces were debrided using a curette and all teeth were placed in 0.5% NaOCl for 24 hours for surface disinfection and then stored in sterile saline until use. The crowns were removed using a safe sided diamond disc and the tooth length was standardized to 16 mm from the root apex to the coronal border.24,25 The teeth were divided into the following groups according to the solution of irrigation:
Group (A) (n=24) the samples were irrigated with Chx.HCl nanoemulsion. Subgroup (I) (n=12) the samples were irrigated with 5 mL Chx.HCl nanoemulsion 0.75% concentration. Subgroup (II) (n=12) the samples were irrigated with 5 mL Chx.HCl nanoemulsion 1.6% concentration. Group (B) (n=24) the samples will be irrigated with 5 ml 2% Chx.HCl normal particle size. Control groups: (n=2), irrigation with 5 mL saline No activation.
Each subgroup of A and B was divided according to the method of activation of the irrigant:Laser
- (n=4): Laser treatment was performed with a diode laser (Epic x diode laser 940, Biolase, Irvine, CA, USA) and the endodontic tip at a wavelength of 940 nm and output power of 1.5 w according to the instructions of the manufacturer. The laser irradiation was delivered for 30 seconds into the canal to the working length.
- Passive ultrasonic irrigation (PUI) (n=4): The ultrasonic tip was inserted in each canal full of irrigant for 30 seconds (Satelec P5, Acteon® Group, Merignac, France, Irrigation tip size 25).
- No activation (n=4): Mechanical preparation was done using standardized technique to a master apical file size 50. All the samples were grooved on the mesiodistal surfaces along their entire lengths and split longitudinally in two halves using a mallet and chisel. Then all samples were examined under scanning electron microscope (SEM).26–28
Antibacterial effect
Forty-four noncarious single-rooted human teeth with mature apices were selected. The teeth included maxillary and mandibular incisors and mandibular premolars. The external root surfaces were debrided using a curette and all teeth were placed in 0.5% NaOCl for 24 hours for surface disinfection and then stored in sterile saline until use. The crowns were removed using a safe sided diamond disc and the tooth length was standardized to 16 mm from the root apex to the coronal border.24,25,29
Mechanical preparation was done using standardized technique to a master apical file size 50. Sterile saline was used as irrigant during the procedure. The canals were finally flushed with 2 mL 17% EDTA for 1 min to remove the smear layer. The entire root surfaces, including the apical foramen of each sample were coated with two layers of nail polish (cyanoacrylate adhesive) to prevent leakage. The teeth were then mounted vertically in dental stone blocks to make handling and identification easier.29–33 The samples were then autoclaved at 121ºC and 15 lb pressure for 20 minutes. Subsequent to sterilization all samples were transported and manipulated under aseptic conditions using sterile instruments. Pure culture of E. faecalis (strain ATCC 29212) grown in brain heart infusion (BHI) broth for 24 hours at 37ºC was used to contaminate the root canals. An inoculum of pure suspension of E. faecalis was suspended into the prepared canals of all teeth by using sterile micropipette. The blocks were then placed inside sterile beakers and incubated at 37ºC for 24 hours.31,34,35
The teeth were divided into the following groups according to the solution of irrigation:
Group (A) (n=24) the samples were irrigated with Chx.HCl nanoemulsion. Subgroup (I) (n=12) the samples were irrigated with 5 mL Chx.HCl nanoemulsion 0.75% conc. Subgroup (II) (n=12) the samples were irrigated with 5 mL Chx.HCl nanoemulsion 1.6% conc.
Group (B) (n=12) the samples will be irrigated with 5 mL 2% Chx.HCl normal particle size.
Control Groups: Positive Control, (n=4) the contaminated root canals were irrigated with 5 mL normal saline and kept as positive control. Negative Control: (n=4) specimens did not receive suspension injections, ie, root canals were not contaminated with E. faecalis and were kept sterile to serve as negative controls to confirm sterilization and the reliability of the procedures. A volume of 5 mL of the test irrigant was used in each sample. Then final irrigation was done with 1 mL sterile normal saline for each sample.
Each subgroup of A and B was divided according to the method of activation of the irrigant:
- Laser (n=4), Laser treatment was performed with a diode laser (Epic x diode laser 940, Biolase) and the endodontic tip at a wavelength of 940 nm and output power of 1.5 w according to the instructions of the manufacturer. The laser irradiation was delivered for 30 seconds into the canal to the working length.
- Passive ultrasonic irrigation (PUI) (n=4), the ultrasonic tip was inserted in each canal full of irrigant for 30 seconds (Satelec P5, Irrigation tip size 25).
- No activation (n=4).
Evaluation of bacterial growth
Sterile paper point size 35 was used to collect samples from root canals. The paper point was inserted into the canals till the working length, left for 10 seconds and then transferred to agar plates to determine the number of colony-forming units (CFUs) per plate. The plates were incubated at 37ºC for 24 hours after which the plates were visually evaluated for occurrence of bacterial growth. Clear plates demonstrated complete sterilization. Blurred plates were considered showing positive growth. The mean number of CFUs in the areas of bacterial growth on each plate was determined and the number of CFUs was calculated. Survivors were measured primarily using viable counts on drop plates. Additionally, pour plates were used to count low colony-forming units and dilutions to 106 were used to count high CFU.36,37
Viable count method using the pour plate method38
A tube containing 15 mL of melted agar medium was prepared where the agar medium was pre-sterilized in an autoclave on the same day it was needed for the experiment. E. faecalis a facultative Gram positive anaerobic coccus has the capability of surviving at very high pH, acidity and high temperatures.39 The bacterial sample (E. faecalis ATCC 29212) was prepared by mixing cells from a colony into sterile saline solution. Then bacterial sample was diluted with saline to match McFarland 0.5 which is equivalent to 108 cfu/mL. The sample volume added was 10 µL.39 Preparation of Turbidity standard (McFarland 0.5)40 was achieved by pouring 0.6 mL of a 1% (10 g/L) solution of barium chloride dihydrate into a 100 mL graduated cylinder, and filling to 100 mL with 1% (10 mL/L) sulfuric acid. The turbidity standard was placed in a tube identical to the one used for the broth sample and stored in the dark at room temperature for 6 months and sealed well to prevent evaporation. The lid of the empty Petri dish was opened, and the sample was dispensed into the middle of the plate. If the agar had thoroughly solidified then the plate was inverted and incubated at 37°C for 24 hours.
Statistical methods
All data were collected, tabulated and subjected to statistical analysis. Statistical analysis was performed with IBM® SPSS® Statistical Version 17 for Windows (SPSS Inc., IBM Corporation, Armonk, NY, USA).
Results and discussion
Solubility studies
The solubility of Chx.HCl in various oily phases, surfactant solutions, cosurfactant solution, and water were studied. The highest solubility of Chx.Hcl was in Labrafil M and the least one was in Oleic acid. The higher solubility of the drug in the oil phase is important for the nanoemulsion as the ability of the nanoemulsion to maintain the drug in solubilized form means that higher solubility of the drug in oil will ensure a lesser amount of oil in the formulation and consequently lesser amounts of surfactants and co-surfactants are required for emulsification of drug loaded oil droplets.
Construction of a pseudoternary phase diagram
A pseudoternary phase diagram was constructed to identify the nanoemulsifying region and to optimize the concentration of the selected oil, surfactant, and co-surfactants (namely Labrafil M, Tween 80, Tween 20 and propylene glycol, respectively. Chx.Hcl showed very low solubility in oleic acid which resulted in a turbid appearance when titrating Oleic acid with water from the first drop. So oleic acid systems were excluded from this study. Other prepared formulae gave clear solutions with an infinite amount of water during titration when prepared with an oil and surfactant mixture in a ratio of 1:9. The surfactants used (Tween 20 and Tween 80) are non-ionic hydrophilic surfactants and were selected due to lower oral toxicity with better stability to emulsion over a wide range of pH and ionic strength
Characterization of Chx.HCL nanoemulsion
Dispersibility tests
All prepared formulae showed a clear appearance except system F2 which showed a turbid appearance, so it was excluded from further evaluation study.
Determination of drug content
The drug content of the prepared Chx.HCl nanoemulsion formulae ranged between 86.3 and 96.7%
Test for the self-emulsification time of Chx.HCL nanoemulsion
An ideal nanoemulsion formulation should have the ability to disperse completely and quickly when subjected to dilution under mild agitation. Chx.HCl nanoemulsion formulae showed short emulsification time ranged between 1.67 and 12.33 seconds. Tween 80 was found to have the least emulsification time. This could be explained by the higher solubilization capacity of Tween 80. Self emulsification time was increased by the increase in the concentrations of surfactant which may be due to the increase in the viscosity of the system by the surfactant.
Droplet size analysis
The droplet size of the emulsion defines the rate and extent of drug release. Smaller emulsion droplet size results in lower emulsification time and a larger surface area provided for the drug absorption. The average droplet size of selected Chx.HCl nanoemulsion formulae were 711±0.44, 587±15.3, 10.97±0.11, 16.43±4.55 and 12.18±2.48 with PDI 0.76, 0.19, 0.61, 0.47, and 0.16 for F1, F2, F3, F4, F5, and F6 respectively. The formulation containing Tween 80 as surfactant showed smaller globule size. This might be attributed to its higher emulsifying ability. PDI values were low, representing that the system had a narrow size distribution. These formulae showed clear appearance as their droplet radii fell below the optical wavelength of visible light (390–750 nm) by which minimum light scattering took place.41
In-vitro release study
Figure 2 shows the percent of Chx.HCl release from the prepared formulae. The complete drug release for prepared Chx.HCl nanoemulsion formulae ranged between 2 and 7 mins. It was observed that the highest drug release rate was obtained in the case of Chx.HCl nanoemulsion formula F6 (2 minutes) and that may be attributed to the presence of Tween 80 which exhibits higher emulsifying, the resulting nano-level droplet size which provided a large surface area for the release of the drug, thus permitting a faster rate of drug release. In the same time the solubility property of propylene glycol allowed large quantities of the hydrophilic surfactant to dissolve in the oil.40
 |
Figure 2 In-vitro release of Chx.HCl from Chx.HCl powder and nanoemulsion formula. |
Kinetic study of the release data of Chx.HCL nanoemulsion
It was found that the in-vitro release of Chx.HCl followed different kinetic orders and no definite kinetic order could express the drug release from differently prepared nanoemulsion formulae. The kinetic release of drug from F4 was first order kinetics, which indicates that they release in a way that is proportional to the amount of drug remaining in its interior.42 The kinetic release of drugs from other formulae followed the Higuashi Diffusion model which indicates that the amount of drug release is proportional to the square root of the total amount of drug and the solubility of the drug in the nanoemulsion formula.42
Thermodynamic stability tests of prepared Chx.HCL nanoemulsion
The selected formulae were subjected to different thermodynamic stability by using heating cooling cycles, centrifugation and freeze-thaw cycle stress tests. It was observed that F3 and F4 formulae exhibited drug precipitation after a thaw cycle, and F1 exhibited thickening (gelation). F5 and F6 have passed the successive cycles of centrifugation, heating-cooling test and freezing-thawing test. Nanoemulsions are thermodynamically stable systems and are formed at a particular concentration of oil, surfactant, and water, with no phase separation, creaming or cracking. It is the thermo-stability which differentiates nanoemulsions from emulsions that have kinetic stability and will eventually phase separate.19 F3 exhibited larger particle size than other formulae (587 nm) which may be the cause of phase separation and drug precipitation in the thermodynamic stability test. F4 which includes Tween 80 with no cosurfactant exhibited drug precipitation which may indicate the need for using propylene glycol with Tween 80 for nanoemulsion formula stability. F1 which includes Tween 20 with no cosurfactant exhibited thickening (gelation) which is an increase in the viscosity or gel strength due to droplet aggregation.
The stability results confirm the importance of presence of the cosurfactant propylene glycol to increase dispersion of particles and prevent drug precipitation.43 F6 was the best formula as it has small particle size (12.18 nm), short emulsification time (1.67 seconds), and fast dissolution rate after 2 minutes. It was found to be a thermodynamically/physically stable system, so it was selected for further study.
Irrigating solution examinations on extracted teeth
Penetration ability in dentinal tubules
Failures after root canal treatment are increasingly frequent representing elevated risk of more complex infections in patients.44,45 The removal of the biofilm is mandatory in root canal disinfection and sealing.46,47 Due to the complex nature of the root canal system it becomes difficult for the complete debridement of canal containing bacteria with instrumentation and irrigation alone.48 Effectiveness of an endodontic irrigating solution is dependent on the penetration of irrigant in DT and duration of action against bacteria.49 Hence, newer modalities were tried and tested for thorough disinfection of root canals. Conventional irrigants cannot completely eliminate E. faecalis, because of lesser penetration depth into DT.50
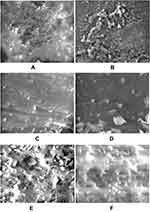
The mean of irrigant cleansing ability for nanoemulsion irrigants was 2001.47 µm2 while for normal particle size irrigants the mean was 2609.56 µm. The mean difference between nanoemulsion irrigants and normal particle size irrigants was 608.09 µm2. There was a statistically highly significant (P<0.001) difference between nanoemulsion irrigants and normal particle size irrigants with (P-value 0.00052). Nanoemulsion had a statistically highly significant difference showing a lower mean value of the remaining debris surface area when compared to normal particle size material, ie nanoemulsion materials had better cleansing ability as shown in Figure 3.
The mean surface area of remaining debris for Chx.HCl 1.6% was 2320.36 µm2 when compared to Chx.HCl 2% its mean was 2949.85 µm2. There was a statistically highly significant (P<0.001) difference between the higher concentration of nanoemulsion irrigants and the normal particle size irrigants (P-value 0.00000). Although the concentration of nanoemulsion irrigants was lower in comparison to the concentration of normal particle size irrigants, the effect of this low concentration on debris removal was significantly stronger and more effective in cleansing the canals.
PUI had a statistically highly significant difference (p<0.001) when compared to other activation methods. The mean value of remaining debris surface area was 1695.31 µm2 when activated by PUI. The mean difference between PUI and Laser was 987.89929 showing highly statistically significant (P<0.001) difference with (p-value 0.00000). The mean difference between PUI and no activation was 712.40643 showing a highly statistically significant (P<0.001) difference with a p-value of 0.00098).The use of either Laser activation or no activation was not significantly statistically (P>0.05) different with a P-value of 0.451211. The mean surface area of the remaining debris when Laser activation was used was 2683.21 µm2. The mean surface area of the remaining debris when no activation was used was 2407.72 µm2. PUI has a statistically lower mean value of debris surface area when compared to either laser activation or no activation, ie, better cleansing ability.
Cleansing ability
The Mean of irrigant cleansing ability for nanoemulsion irrigants was 2001.47 µm2 while for normal particle size irrigants the mean was 2609.56 µm. The mean difference between nanoemulsion irrigants and normal particle size irrigants was 608.09 µm2. There was statistically highly significant (P<0.001) difference between nanoemulsion irrigants and normal particle size irrigants with (P-value 0.00052). Nanoemulsion had statistically highly significant difference showing lower mean value of the remaining debris surface area when compared to normal particle size material, ie nanoemulsion materials had better cleansing ability as shown in Figure 3.
The mean surface area of remaining debris for Chx.HCl 1.6% was 2320.36 µm2 when compared to Chx.HCl 2% its mean was 2949.85 µm2. There was statistically highly significant (P<0.001) difference between the higher concentration of nanoemulsion irrigants and the normal particle size irrigants (P-value 0.00000). Although the concentration of nanoemulsion irrigants was lower in comparison to the concentration of normal particle size irrigants, the effect of this low concentration on debris removal was significantly stronger and more effective in cleansing the canals.
PUI had statistically high significant difference (p<0.001) when compared to other activation methods. The mean value of remaining debris surface area was 1695.31 µm2 when activated by PUI. The mean difference between PUI and Laser was 987.89929 showing highly statistically significant (P<0.001) difference with (p-value 0.00000).The mean difference between PUI and no activation was 712.40643 showing highly statistically significant (P<0.001) difference with (p-value 0.00098).The use of either Laser activation or no activation was none significantly statistical (P>0.05) different with (P-value 0.451211). The mean surface area of remaining debris when Laser activation was used was 2683.21 µm2.The mean surface area of remaining debris when no activation was used was 2407.72 µm2. PUI has statistically lower mean value of debris surface area when compared to either laser activation or no activation, ie better cleansing ability.
Comparison between irrigants cleansing ability
The mean effect of nanoemulsion irrigants on debris removal were statistically significantly higher than normal particle size irrigants. Chx.HCl 1.6% with PUI had 1938.77 µm2; while with the laser it was 2510.96 µm2. With no activation the mean was 2511.34 µm2. The worst results, largest amount of debris, was seen when Chx.HCl 2% was used and activated by Laser. The same results were also seen when Chx.HCl 0.75% had no activation. It is clear that the best results were achieved when the higher concentration of the irrigant in nanoemulsion dosage form was used. PUI has the most potent effect in activating the irrigants and flushing debris out as shown in Figure 3A–F)).
Antibacterial effect
As shown in Table 2 nanoemulsion Chx.HCl had a better result than normal particle size in terms of viable count with good correlation with the formulae penetration ability and cleansing effect according to the following parameters: Irrigant type either nanoemulsion or normal particle size, irrigant concentration, and methods of activation.
 |
Table 2 Results of viable count method using the pour plate method |
Complete bacterial elimination was achieved when the higher concentrations of irrigants were used. Chx.HCl 0.75% had the worst antibacterial effect even by activation with PUI. Laser activation had an adverse effect on the nanoemulsion irrigant. From all the previous results it was obvious that the use of laser has reduced the efficiency of Chx.HCl 0.75% nanoemulsion, where the CFU for nano Chx.HCl 0.75% was 195 which is very high count indicating that the reagents were not effective in this concentration with laser activation. The diode laser has photothermal effects so either the light or the heat may cause the nanoemulsion to lose its antibacterial effect. The results for the high concentrations were complete bacterial eradication. Nano Chx.HCl 1.6% showed negative bacterial growth with the presence of laser activation which means that laser did not affect the antibacterial ability of nano Chx.HCl 1.6%. It can be concluded that the higher concentration of nanoemulsion material has a better antibacterial effect.
Conclusion
In this work, Chx.HCl nanoemulsions were prepared using two different oils, two surfactants and co-surfactant; the optimum formula (F6) that displayed small particle size, short emulsification, and fast dissolution rate, was selected. Additionally, (F6) was subjected to thermodynamical/physical stability tests. In the Chx.HCl nanoemulsion in a concentration of 1.6% compared to traditional Chx.HCl as an irrigation solution, the Chx.HCl nanoemulsion shows the best penetrations in dentinal tubules, and cleansing ability with PUI as the activation methods. Moreover, the antibacterial study of Chx.HCl nanoemulsion shows complete bacterial elimination. The results confirmed that. Chx.HCl nanoemulsion could be considered as a promising irrigant solution.
Acknowledgments
We are so grateful to the staff members of the research laboratory, Misr University for Science & Technology, for their kind support.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Mandal A, Bera A. Surfactant stabilized nanoemulsion: characterization and application in enhanced oil recovery. World Acad Sci Eng Technol. 2012;67:21–26.
2. Patrycja S, Halina S. Water solubilization using nonionic surfactants from renewable sources in microemulsion systems. J Surfactants Deterg. 2012;15(4):485–494. doi:10.1007/s11743-011-1323-y
3. Mohan S, Gurtu A, Singhal A, Mehrotra A. Nanotechnology: its implications in conservative dentistry and endodontics. J Dent Sci Or Rehab. 2013;6:9–14.
4. Rowe RC, Sheskey PJ, Owen SC, editors. Handbook of Pharmaceutical Excipients. London: Pharmaceutical press; 2009:162–165.
5. Senior N. Some observations on the formulation and properties of chlorhexidine. J Soc Cosmet Chem. 1973;24(4):259–278.
6. Venghat S, Hegde M, Shetty C. Irrigants used in endodontics. Int J Curr Microbiol App Sci. 2014;3(3):126–132.
7. Torabinejad M, Handysides R, Khademi A, Bakland LK. Clinical implications of the smear layer in endodontics: a review. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:658–666. doi:10.1067/moe.2002.128962
8. Murad CF, Sassone LM, Souza MC, et al. Antimicrobial activity of sodium hypochlorite, chlorhexidine and MTAD® against Enterococcus faecalis biofilm on human dentin matrix in vitro. RSBO Revista Sul-Brasileira de Odontologia. 2012;9(2):143–150.
9. Plotino G, Cortese T, Grande NM, et al. New technologies to improve root canal disinfection. Braz Dent J. 2016;27(1):3–8. doi:10.1590/0103-6440201600726
10. Gu LS, Kim JR, Ling J, et al. Review of contemporary irrigant agitation techniques and devices. J Endod. 2009;35(6):791–804. doi:10.1016/j.joen.2009.03.010
11. Roni MA, Jalil RU. Comparative study of ibuprofen solubility in synthetic and natural lipid vehicles. Dhaka Univ J Pharma Sci. 2011;10(1):65–66. doi:10.3329/dujps.v10i1.10018
12. Rushikesh J, Vipul M, Ravindra G, et al. Development and validation of UV-visible spectophotometic method for estimation of selected antiseptic drug in bulk and pharmaceutical dosage form. World J Pharm Pharm Sci. 2016;5(9):1197–1205.
13. Nornoo AO, Zheng H, Lopes LB, et al. Oral microemulsions of paclitaxel: in situ and pharmacokinetic studies. Eur J Pharm Biopharm. 2009;71(2):310–317. doi:10.1016/j.ejpb.2008.08.015
14. Patel J, Kevin G, Patel A, Raval M, Sheth N. Design and development of a self-nanoemulsifying drug delivery system for telmisartan for oral drug delivery. Int J Pharm Investig. 2011;1(2):112–118. doi:10.4103/2230-973X.82431
15. Drais HK, Hussein AA. Formulation and characterization of carvedilol nanoemulsion oral liquid dosage form. Int J Pharm Pharm Sci. 2015;7(12):209–216.
16. Thakur A, Walia MK, Kumar SL. Nanoemulsion in enhancement of bioavailability of poorly soluble drugs: a review. Pharmacophore. 2013;4(1):15–25.
17. Rajinikanth PS, Suyu Y, Garg S. Development and in-vitro characterization of self-nanoemulsifying drug delivery systems of valsartan. World Acad Sci Eng Technol. 2012;72:1418–1423.
18. Harika K, Debnath S, Babu MN. Formulation and evaluation of nanoemulsion of amphotericin B. IJNTPS. 2015;4(5):114–122.
19. Zhang P, Liu Y, Feng N, Xu J. Preparation and evaluation of self microemulsifying drug delivery system of oridonin. Int J Pharm. 2008;355(1–2):269–276. doi:10.1016/j.ijpharm.2007.12.026
20. Agubata CO, Nzekwe IT, Obitte NC, et al. Effect of oil, surfactant and co-surfactant concentrations on the phase behaviour, physicochemical properties and drug release from self-emulsifying drug delivery systems. J Drug Discov Develop and Deliv. 2014;1(1):1–7.
21. Patel HC, Parmar G, Seth AK, et al. Formulation and evaluation of O/W nanoemulsion of ketoconazole. Int J Pharma Sci. 2013;4(4):338–351.
22. Singh G, Pai RS. Optimized self-nanoemulsifying drug delivery system of atazanavir with enhanced oral bioavailability: in vitro/in vivo characterization. Expert Opin Drug Deliv. 2014;11(7):1023–1032. doi:10.1517/17425247.2014.913566
23. Xhevdet A, Stubljar D, Kriznar I, et al. The disinfecting efficacy of root canals with laser photodynamic therapy. J Lasers Med Sci. 2014;5(1):19.
24. Berber VB, Gomes BP, Sena NT, et al. Efficacy of various concentrations of NaOCl and instrumentation techniques in reducing Enterococcus faecalis within root canals and dentinal tubules. Int Endod J. 2006;39(1):10–17. doi:10.1111/j.1365-2591.2005.01038.x
25. Palazzi F, Blasi A, Mohammadi Z, et al. Penetration of sodium hypochlorite modified with surfactants into root canal dentin. Braz Dent J. 2016;27(2):208–216. doi:10.1590/0103-6440201600650
26. Polenik P, Netolicky J Deposition of fluorescent magnetic nanoparticles into dentinal tubules.
27. Bumb SS, Bhaskar DJ, Agali CR, et al. Assessment of photodynamic therapy (PDT) in disinfection of deeper dentinal tubules in a root canal system: an in vitro study. Jcdr. 2014;8(11):ZC67. doi:10.7860/JCDR/2014/6788.3956
28. Gomes BP, Ferraz CC, Me V, et al. In vitro antimicrobial activity of several concentrations of sodium hypochlorite and chlorhexidine gluconate in the elimination of Enterococcus faecalis. Int Endod J. 2001;34(6):424–428.
29. Siqueira JF, Machado AG, Silveira RM, et al. Evaluation of the effectiveness of sodium hypochlorite used with three irrigation methods in the elimination of Enterococcus faecalis from the root canal, in vitro. Int Endod J. 1997;30(4):279–282.
30. Agrawal V, Rao MR, Dhingra K, Gopal VR, Mohapatra A, Mohapatra A. An in vitro comparison of antimicrobial effcacy of three root canal irrigants-BioPure MTAD, 2% chlorhexidine gluconate and 5.25% sodium hypochlorite as a final rinse against E. faecalis. J Contemp Dent Pract. 2013;14(5):842–847.
31. Saber SE, El-Hady SA. Development of an intracanal mature Enterococcus faecalis biofilm and its susceptibility to some antimicrobial intracanal medications; an in vitro study. Eur J Dent. 2012;6(1):43.
32. Mithra NH, Krishna RS, Shishir S, Veenna SA. Comparative evaluation of bactericidal effects on Enterococcus faecalis using diode laser irradiation, sodium hypochlorite and chlorhexidine gluconate irrigation”-an in vitro study. Oral Health Dent Manag. 2013;12(3):145–150.
33. Castelo-Baz P, Bahillo J, Seoane-Prado R, Gude F, DeMoor R. Combined sodium hypochlorite and 940 nm diode laser treatment against mature E. faecalis biofilms in-vitro. J Lasers Med Sci. 2012;3(3):116.
34. Bago I, Plečko V, GabrićPandurić D, et al. Antimicrobial efficacy of a high power diode laser, photo activated disinfection, conventional and sonic activated irrigation during root canal treatment. Int Endod J. 2013;46(4):339–347. doi:10.1111/j.1365-2591.2012.02120.x
35. Francescutti Murad C, Moura Sassone L, Souza MC, et al. Antimicrobial activity of sodium hypochlorite, chlorhexidine and MTAD against Enterococcus faecalis biofilm on human dentin matrix in vitro. RSBO Revista Sul-Brasileira de Odontologia. 2012;9(2):143–50.
36. Frough-Reyhani M, Ghasemi N, Soroush-Barhaghi M, et al. Antimicrobial efficacy of different concentration of sodium hypochlorite on the biofilm of Enterococcus faecalis at different stages of development. J Clin Exp Dent. 2016;8(5):e480.
37. Qazi JI, Asif HI, Shahid R. Economical method for estimation of bacterial viable count. Pakistan J Zool. 2008;40(4):289–294.
38. Moghadas L, Shahmoradi M, Narimani T. Antimicrobial activity of a new nanobased endodontic irrigation solution: in vitro study. Dent Hypotheses. 2012;3(4):142. doi:10.4103/2155-8213.106838
39. Benita S, editor. Microencapsulation: Methods and Industrial Applications. Boca Raton, CRC Press; 2005.
40. Mason TG, Wilking JN, Meleson K, et al. Nanoemulsions: formation, structure and physical properties. J Phys. 2006;18:R635–R666.
41. Costa P, Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13(2):123–133.
42. Sarpal K, Pawar YB, Bansal AK. Self-emulsifying drug delivery systems: a strategy to improve oral bioavailability. Crips. 2010;11(3):42–49.
43. Narayanan LL, Vaishnavi C. Endodontic microbiology. J Conserv Dent. 2010;13(4):233. doi:10.4103/0972-0707.73386
44. Zehnder M. Root canal irrigants. J Endod. 2006;32(5):389–398. doi:10.1016/j.joen.2005.09.014
45. Mohammadi Z, Soltani MK, Shalavi S. An update on the management of endodontic biofilms using root canal irrigants and medicaments. Iran Endod J. 2014;9(2):89.
46. Portenier I, Waltimo TM, Haapasalo M. Enterococcus faecalis– the root canal survivor and ‘star’in post treatment disease. Endod Topics. 2003;6(1):135–159. doi:10.1111/j.1601-1546.2003.00040.x
47. Marion J, FranzoniarruDa ME, de MoraiS CA. Chlorhexidine and its applications in Endodontics: a literature review. Dent Press Endod. 2013;3:36–54.
48. Kamath P, Kundabala M, Shenoy S, et al. An evaluation of horizontal depth of penetration of various irrigants into the dentinal tubules when used alone and in combination with diode laser: an in vitro study. J Interdisc Dent. 2014;4(3):130. doi:10.4103/2229-5194.147331
49. George S, Kishen A, Song P. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J Endod. 2005;31(12):867–872.
50. Menezes MM, Valera MC, Jorge AO, et al. In vitro evaluation of the effectiveness of irrigants and intracanal medicaments on microorganisms within root canals. Int Endod J. 2004;37(5):311–319. doi:10.1111/j.0143-2885.2004.00799
51. Ferraz CC, de Almeida Gomes BP, Zaia AA, et al. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J Endod. 2001;27(7):452–455.
52. Ahangari Z, Samiee M, Yolmeh MA, Eslami G. Antimicrobial activity of three root canal irrigants on enterococcus faecalis: an in vitro study. Iran Endod J. 2008;3(2):33.
53. Moritz A, Gutknecht N, Goharkhay K, Schoop U, Wernisch J, Sperr W. In vitro irradiation of infected root canals with a diode laser: results of microbiologic, infrared spectrometric, and stain penetration examinations. Quint Int. 1997;28(3):205–209.
54. Nusstein JM. Endodontic sonic and ultrasonic irrigant activation. Clin Dent Revi. 2018;2(1):22. doi:10.1007/s41894-018-0037-1
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.