Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Folliculitis Decalvans and Lichen Planopilaris Phenotypic Spectrum: A Case Report
Authors Zhang X , Zhu M, Zhou J, Wu S, Liu J, Qin Q
Received 8 March 2022
Accepted for publication 26 May 2022
Published 31 May 2022 Volume 2022:15 Pages 993—996
DOI https://doi.org/10.2147/CCID.S365566
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Xi Zhang, Mingfang Zhu, Jia Zhou, Shuhui Wu, Juan Liu, Qiuyan Qin
Department of Dermatology, The Second Affiliated Hospital of Hunan University of Chinese Medicine, Changsha, People’s Republic of China
Correspondence: Mingfang Zhu, Department of Dermatology, The Second Affiliated Hospital of Hunan University of Chinese Medicine, 233 Cai E North Road, Kaifu District, Changsha, People’s Republic of China, Tel +86-13907317358, Fax +86-0731-84917727, Email [email protected]
Abstract: The folliculitis decalvans (FD) and lichen planopilaris (LPP) phenotypic spectrum combines biphasic features of FD and LPP. It is characterized by successive or concomitant occurrence of pustules, crusts, follicular tufts, perifollicular erythema, perifollicular scales, and cicatricial alopecia and includes mixed histologic features of both FD and LPP. Here, we report the case of a 33-year-old female patient with a 30-year history of FD-LPP phenotypic spectrum lesions.
Keywords: primary cicatricial alopecia, biphasic features, pustules, perifollicular erythema, follicular tufts
Introduction
Folliculitis decalvans (FD), classified as neutrophilic cicatricial alopecia, mainly presents with follicular pustules and follicular tufts. However lichen planopilaris (LPP) is classified as a lymphocytic cicatricial alopecia, which mainly presents with perifollicular erythema and perifollicular scales.1 In addition to some familiar common features (such as follicular tufts),2 these conditions may have compatible or biphasic clinical, histologic, and trichoscopic presentations during the active phase; this may make differential diagnosis difficult. Yip et al described this biphasic presentation as the FD-LPP phenotypic spectrum (FDLPPPS) and attributed the phenomenon to an abnormal hair follicle inflammatory response and collapse of the hair follicle immune privilege due to microbiome dysbiosis.3 Instead of administering separate treatments for FD and LPP, combined antibacterial and anti-inflammatory drugs (including corticosteroids and retinoids) should be prescribed as appropriate to obtain better therapeutic results. In the present report, we describe the case of a 33-year-old female patient with a 30-year history of FDLPPPS, that have been well controlled with oral antibiotics and corticosteroids during 6 months of follow-up.
Case Report
A 33-year-old female patient presented with intermittent pustules, follicular erythema, follicular scales, and alopecia on her scalp for 30 years. She had experienced pustules on the scalp for an unknown cause preceding extensive hair loss, 30 years ago. Following systemic antibiotic therapy, she made a gradual improvement with new hair grown. However, follicular erythema and scaling started to appear on her scalp, followed by intermittent pustules. The symptoms began to increase significantly 10 years previously; lesions of symmetrical cicatricial alopecia appeared on the scalp with follicular papules, pustules, hemorrhagic crusts, perifollicular erythema, and scale build-up. She was diagnosed with FD, discoid lupus erythematosus, psoriasis, and LPP at different stages and sequentially received topical/oral antibiotics, topical corticosteroids, and oral hydroxychloroquine, among other treatments. Nevertheless, her symptoms continued to progress slowly with increased follicular erythema, scaling, and recurrent pustules. The patient denied any history of chronic disease and sunburn, and had no relevant family medical history.
Dermatological examination showed two patches of symmetrical cicatricial alopecia on the scalp, measuring 7×10 cm and 8×10 cm, with perifollicular erythema, hyperkeratosis, and yellowish scales; the results of the hair pull test were negative. No lesions were found on the face, extremities, mouth, nails, or the remaining (Figure 1). Histopathological examination showed hyperkeratosis, mild epidermal hyperplasia, and epithelial basal layer destruction in the hair follicle. There was visible dense lymphocyte and histiocyte infiltration surrounding the hair follicle and significant plasma cell infiltration in some areas. On horizontal sections, partial destruction of hair follicles was seen with surrounding fibrosis; only the structure of the arrector pill muscle remained (Figure 2). Trichoscopy revealed loss of partial follicular opening, follicles surrounded by milky red areas, dilated blood vessels, pustules, and follicular tufts (Figure 3). These clinical, histopathological, and trichoscopic findings were consistent with the diagnosis of FDLPPPS. Following oral administration of prednisone tablets (20 mg/day, tapered over 2 months) and azithromycin (500 mg thrice weekly for 1 month), the patient ceased to experience pustules and had fewer erythematous lesions and scales with no progression of hair loss during 6 months of follow-up.
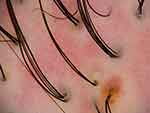 |
Figure 3 Trichoscopy showing follicular tufts, pustules and dilated blood vessels in an area of scarring alopecia. |
Discussion
According to the predominant inflammatory cell infiltrate on histopathology, FD and LPP are divided into two distinct types, namely, neutrophilic and lymphocytic primary cicatricial alopecia (PCA). However, the differential diagnosis of cases with mixed presentations of both remains a challenge for dermatologists. Based on sequential trichoscopic observations, Trüeb et al found a tendency for some cases of FD to gradually present as LPP.4 Early trichoscopic findings for FD include the presence of pustules, erythema, and follicular tufts; these are probably followed by lichen-like lesions of perifollicular erythema, scales, and fibrosis after repeated anti-microbial therapy. Morais et al reported on 13 cases of LPP with pustules, crusts, follicular tufts and histopathological findings of follicular fibrosis and lymphocyte and plasma cell infiltration.5 They suggested the difficulty in differentiating LPP and FD, which are often misdiagnosed, and the need for further study to elucidate whether LPP with pustules constitute a new subtype of LPP or even a different disease. Subsequently, Yip et al reported on 13 cases, where the lesions developed biphasic clinical and histologic features of FD and LPP over the period of change in clinical phenotype. FD presentations generally preceded those of LPP; They designated this biphasic presentation as FDLPPPS.3 Egger et al also suggested that clinical features of active disease and PCA with histopathological features of a multi compound follicular structure, follicular epithelial atrophy, and a prominent plasma cell component with no neutrophils, should be considered as part of FD-LPP, particularly in patients showing resistance to monotherapy with anti-inflammatory regimens, steroids, and/or other immunomodulatory agents.6 The reason for appearance of this spectrum is related to the respective pathogenesis. FD is a chronic inflammatory disease that is usually induced by colonization with bacteria such as Staphylococcus aureus (S. aureus). In contrast, LPP is an autoimmune disease mediated by destruction of immune privilege in the hair follicle epithelial stem cell niche and Th1-biased infiltration of cytotoxic lymphocytes.7 Matard et al found the associations between clinical improvement achieved in most FD cases with antibiotic treatment and the disappearance of S. aureus in all affected areas, an incomplete restoration of normal microbiota, as well as a significant increase in negative bacterial samples. Despite its possible effect on reducing the bacterial load below the threshold for triggering the innate immune system’s inflammatory response, antibiotic therapy fails to restore a normal composition due to persistent imbalances in the follicular microbiota, which may trigger inflammation and a different immune response.8 Moreno-Arrones et al found a statistically significant decline in levels of S. aureus in patients with FDLPPPS compared to those with typical FD.9 It can be speculated that persistent microbiota dysbiosis after anti-staphylococcal antibiotic treatment may lead to immune privilege collapse of hair follicles and Th1-mediated inflammation, leading to presentation with prominent features of FD or LPP depending on different inflammatory stages. Based on the summary of the opinions from the existing literature, FDLPPPS may manifest as another FD phenotype that finally presents with lichenoid features, or as overlapping FD and LPP, or bacterial infection subsequent to LPP. Further research to investigate whether FDLPPPS is another phenotype of FD that ultimately manifests with lichenoid features is meaningful. In fact, such difficult diagnoses caused by clinical phenotype change and biphasic presentation are not rare, but few have been reported and discussed in the literature. Reporting this phenotypic spectrum is important for dermatologists, especially for those who have not specialized in the management of scalp diseases; this will help them recognize this particular presentation and optimize treatment with combinations of antibiotics and anti-inflammatory therapies.
In this context, there are few reports on this spectrum of disease and childhood-onset PCA is rare. Ramos et al has previously reported on two cases of FDLPPPS diagnosed in dark-skinned children.10 The patient described in this report presented with the lesions at the age of 3 years and has had the condition for 30 years. To the best of our knowledge, this case has the youngest age of presentation and longest duration of all cases reported to date, and is therefore very rare. Despite multiple diagnoses and various prior treatments, her symptoms continued to progress. She is currently showing improvement while using systemic antibacterial drugs in combination with anti-inflammatory therapy. This is based on the fact that hair cannot regenerate in areas of cicatricial alopecia, The areas of hair loss do not shrink, but do not continue to expand either; control of the condition is therefore indicative of good results in control.11 It is noteworthy that corticosteroids have potent anti-inflammatory effects and induce a rapid response, which can be misleading. Recurrence remains possible after 6 months, requiring continuous follow-up to ensure provision of timely treatment.
Abbreviations
FD, folliculitis decalvans; LPP, lichen planopilaris; FDLPPPS, folliculitis decalvans and lichen planopilaris phenotypic spectrum; PCA, primary cicatricial alopecia.
Ethics Statement
The publications of images were included with the patient’s consent.
Consent Statement
Informed consent was provided by the patient for publication of the case. Institutional approval was not required to publish the case details.
Funding
This work was funded by the Hunan Province Leading Talents of Chinese Medicine Project (Xiang TCM [2022]4) and the Hunan Province High-Level Health Talents 225 Project (Xiangweihan [2019]196).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Olsen EA, Bergfeld WF, Cotsarelis G, et al. Summary of North American Hair Research Society (NAHRS)-sponsored Workshop on Cicatricial Alopecia, Duke University Medical Center, February 10 and 11, 2001. J Am Acad Dermatol. 2003;48(1):103–110. doi:10.1067/mjd.2003.68
2. Starace M, Vezzoni R, Alessandrini A, et al. Tufted hairs: a bouquet of flowers in different hair diseases. J Cosmet Dermatol. 2022. doi:10.1111/jocd.14712
3. Yip L, Barrett TH, Harries MJ. Folliculitis decalvans and lichen planopilaris phenotypic spectrum: a case series of biphasic clinical presentation and theories on pathogenesis. Clin Exp Dermatol. 2020;45(1):63–72. doi:10.1111/ced.13989
4. Trüeb RM, Rezende HD, Diaz M. Dynamic Trichoscopy. JAMA Dermatol. 2018;154(8):877–878. doi:10.1001/jamadermatol.2018.1175
5. Morais KL, Martins CF, Anzai A, Valente N, Romiti R. Lichen planopilaris with pustules: a diagnostic challenge. Skin Appendage Disord. 2018;4(2):61–66. doi:10.1159/000478268
6. Egger A, Stojadinovic O, Miteva M. Folliculitis decalvans and lichen planopilaris phenotypic Spectrum-A series of 7 new cases with focus on histopathology. Am J Dermatopathol. 2020;42(3):173–177. doi:10.1097/DAD.0000000000001595
7. Harries MJ, Meyer K, Chaudhry I, et al. Lichen planopilaris is characterized by immune privilege collapse of the hair follicle’s epithelial stem cell niche. J Pathol. 2013;231(2):236–247. doi:10.1002/path.4233
8. Matard B, Donay JL, Resche-Rigon M, et al. Folliculitis decalvans is characterized by a persistent, abnormal subepidermal microbiota. Exp Dermatol. 2020;29(3):295–298. doi:10.1111/exd.13916
9. Moreno-Arrones OM, Del Campo R, Saceda-Corralo D, et al. Folliculitis decalvans microbiologic signature is specific for disease clinical phenotype. J Am Acad Dermatol. 2021;85(5):1355–1357. doi:10.1016/j.jaad.2020.10.073
10. Ramos PM, Melo DF, Lemes LR, et al. Folliculitis decalvans and lichen planopilaris phenotypic spectrum: case report of two paediatric cases. J Eur Acad Dermatol Venereol. 2021;35(10). doi:10.1111/jdv.17379
11. Uchiyama M. Primary cicatricial alopecia: recent advances in evaluation and diagnosis based on trichoscopic and histopathological observation, including overlapping and specific features. J Dermatol. 2021;49:37–54. doi:10.1111/1346-8138.16252
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


