Back to Journals » Cancer Management and Research » Volume 14
Follicular Lymphoma in China: Systematic Evaluation of Follicular Lymphoma Prognostic Models
Authors Li Y, Zhang Y , Wang W, Wei C , Zhao D, Zhang W
Received 21 December 2021
Accepted for publication 31 March 2022
Published 8 April 2022 Volume 2022:14 Pages 1385—1393
DOI https://doi.org/10.2147/CMAR.S349193
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Harikrishna Nakshatri
Yanan Li, Yan Zhang, Wei Wang, Chong Wei, Danqing Zhao, Wei Zhang
Department of Hematology, Peking Union Medical College Hospital, Beijing, People’s Republic of China
Correspondence: Wei Zhang, Email [email protected]
Objects: To systematically evaluate the baseline characteristics, tumour microenvironment indicators such as the lymphocyte/monocyte ratio (LMR) and treatment response (POD24) as prognostic predictors of follicular lymphoma (FL) among Chinese patients.
Methods: We retrospectively analysed 112 FL patients from 2000 to 2017, whose pathology grading included 1– 3a. Absolute lymphocyte and monocyte counts were determined by cell blood counting. The Kaplan–Meier method was used to analyse the influence of prognostic predictors on progression-free survival (PFS) and overall survival (OS). Multivariate analysis was conducted using the Cox proportional risk model.
Results: The long-term survival of Chinese FL patients (median PFS, 74.8 months) was slightly better than that of patients in foreign countries. Among several prognostic models, the Follicular Lymphoma International Prognostic Index (FLIPI) score had a significant effect on both PFS and OS, while there were no independent prognostic predictors. Patients with LMR ≤ 3.6, ALC ≤ 0.6*109/L and AMC > 0.6*109/L had worse OS, among which LMR was an independent indicator of OS. The POD24 subgroup had a higher frequency of high-risk patients according to FLIPI and FLIPI2 scores (63.0% vs 35.1%, P = 0.013 and 18.5% vs 3.9%, P = 0.003) and had an markedly shorter OS (P < 0.0001).
Conclusion: This study is a systematic prognostic evaluation based on Chinese clinical data. We found a new factor, the LMR, that could independently predict prognosis compared with FLIPI or FLIPI2.
Keywords: follicular lymphoma, prognosis, clinical characteristics, lymphocyte/monocyte ratio, immunology
Introduction
Follicular lymphoma (FL) is a low-grade non-Hodgkin lymphoma (NHL) with follicular-like growth derived from B cells.1 The course of the disease is variable, usually starting from the upper mediastinum or abdominal lymph nodes and gradually involving extranodal tissues or organs such as bone marrow.2 Studies have found that many factors may affect the prognosis of the disease. The commonly accepted baseline prognostic models mainly include the Follicular Lymphoma International Prognostic Index (FLIPI) and Follicular Lymphoma International Prognostic Index 2 (FLIPI2) models proposed in the “post-rituximab era”3 and the extremely simplified PRIMA Prognostic Index (PRIMA-PI) score.4 The BioFLIPI score developed from the tumour immune microenvironment and biological characteristics are gradually being used for clinical evaluation.5 Recent studies on the tumour microenvironment of diffuse large B-cell lymphoma (DLBCL) have revealed the complex regulation of tumour growth and invasion, which involves lymphocytes and monocytes, and facilitate the identification of a more convenient biological prognostic marker.6,7 However, most of the abovementioned studies on various indicators are based on clinical data from Western countries, and a systematic and comprehensive evaluation of prognostic factors among the characteristics of Chinese patients has not been performed. In this study, we performed a single-centre retrospective analysis to systematically and comprehensively assess the baseline characteristics, immune microenvironment, and treatment response of Chinese FL patients to evaluate prognostic factors.
Materials and Methods
Patients
We retrospectively analysed data for a total of 112 FL patients in the Department of Haematology of Peking Union Medical College Hospital from January 2000 to August 2017. The inclusion criteria were as follows: clinical diagnosis of FL, hospitalization in our hospital, follicular grade 1 to grade 3A, and intact and traceable baseline data and imaging information. Data verification and regular follow-up visits were used to prevent missing data. All patients were followed up by telephone until August 12, 2020. The follow-up rate was 90.2%. Eleven patients were lost to follow-up. The median follow-up time was 64.5 months (3.2–146.3 months).
Data Collection
FL diagnosis is based on clinical manifestations; imaging examinations such as lymph node colour Doppler ultrasound, CT and PET-CT; and histopathology including immunohistochemistry. Antibodies included CD20, CD5, CD21, CD23, BCL2, BCL6, CD10, cyclin D1, MUM-1, and the Ki67 index (Ki67%) was assessed as a cell proliferation marker.
The absolute lymphocyte count (ALC) and absolute monocyte count (AMC) were determined through whole blood cell analysis, and the lymphocyte/monocyte ratio (LMR) was calculated at the same time. Other possible prognostic predictors included baseline/mid/end PET-CT performance, lactate dehydrogenase (LDH), β2 microglobulin (β2-MG) and other indicators. The samples were classified according to the WHO lymphoma classification and diagnostic criteria and staged according to the Ann Arbor staging scheme. The patients’ short-term efficacy evaluations included complete remission (CR), partial response (PR), stable disease (SD) and disease progression (PD). POD24 is defined as progression or relapse within 2 years from the start of chemotherapy. Survival outcomes including overall survival (OS), which is the time from the diagnosis of FL to death from any cause or the end of follow-up, and progression-free survival (PFS), which is the time from the beginning of FL treatment to the end of disease progression or follow-up, were evaluated.
Statistical Analysis
X-tile was used to select the discriminative cut-off values for the LMR, ALC and AMC, and IBM SPSS Statistics 20 was used to combine all quantitative data. For example, LMR, ALC, AMC, sex, age, clinical stage, FLIPI score, FLIPI2 score, β2-MG, LDH, complications, and extranodal involvement were all treated as binary/three-category variables, shown in the form of absolute values and percentages. The χ2 test was used to analyse the correlations between the LMR, ALC, AMC and other prognostic evaluation indicators and to evaluate the impact of the LMR, ALC, and AMC on short-term efficacy. The Spearman correlation analysis was used for the two-variable nonparametric tests, and the Pearson correlation test was used to assess continuous parameters. The Kaplan–Meier method was used to analyse the survival of patients over 10 years, and the Log rank test was used to calculate the effects of all prognostic predictors on PFS and OS. Multivariate analysis relied on the Cox proportional hazards model. P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical Statistics of All Patients
Of the 112 initially treated FL patients assessed in this study, 21.4% had an age of onset >60 years old; 54 patients were males, and 58 were females. 86.6% was diagnosed as Stage III-IV. The rest of the basic clinical information is shown in Table 1 and Supplemental Tables 1-2.
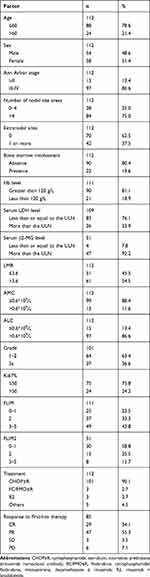 |
Table 1 Basic Clinical Characteristics of the 112 FL Patients |
Extranodal Involvement and Complications
Of the 112 patients, 29.5% (33/112) exhibited extranodal involvement based on imaging findings, pathology, and bone marrow examination. The bone marrow was the most common site for extranodal involvement, accounting for 19.6% (22/112) of cases. Other involved organs and common complications probably affecting the prognosis are listed in Supplemental Tables 1-2.
Prognostic Factor Analysis
Statistics of Patients’ Clinical Baseline Characteristics
The most significant cut-off value of the LMR was determined to be 3.6, and the critical cut-off value of the ALC and AMC was determined to be 0.6*109/L. Supplemental Table 3 shows the correlations between the LMR, ALC, AMC and other baseline clinical characteristics and prognostic factors. There was no significant difference in the clinical baseline data of patients with different LMR levels, suggesting that LMR acted as the immune status of patients could predict independent of other clinical factors.
The Impact of Baseline Characteristics on Survival
Kaplan–Meier survival analysis was performed for indicators that might influence survival (Table 2). The FLIPI score had a significant effect on both PFS and OS (P<0.05), the FLIPI2 score only had a significant effect on PFS (P<0.05), and the PRIMA-PI did not significantly affect survival. In addition, pathological grade 3a and Ki67%>50 could also indicate poor prognosis.
 |
Table 2 General Survival Analysis of Risk Factors for Prognostic Assessment |
Multivariate analysis of the above survival factors using the Cox proportional hazards model showed that none of the three indicators could be used as an independent prognostic factor.
Prognostic Evaluation of the LMR, ALC, and AMC
The Kaplan–Meier method was used to analyse the survival of all patients over a 10-year period. Table 2 and Figures 1–3 (detailed in Supplemental Table 4) show that patients with LMR≤3.6, ALC≤0.6*109/L and AMC>0.6*109/L had significantly worse OS than other patients and that the PFS of patients with a high AMC (AMC>0.6*109/L) was also significantly shortened. A subsequent multivariate analysis with the Cox risk model (Table 2) showed that LMR could be used as an independent prognostic predictor for OS.
Impact of POD24 on Survival
In terms of POD24 evaluation, 26% (27/104) of patients exhibited POD24 after treatment, and there was a higher frequency of patients with high risk according to the FLIPI and FLIPI2 scores in the POD24 subgroup than in the non-POD24 subgroup (63.0% vs 35.1%, P value=0.013 and 18.5% vs 3.9%, P value=0.003) (Supplemental Table 5). The median survival of the POD24 group was 34.5 months, which was significantly different from that of the non-POD24 group (P<0.0001) (Figure 4), suggesting that POD24 has good predictive value for the long-term survival of patients.
Discussion
Analysis of the clinical data of this study showed that the median age of onset for FL in China was 52 years old, which was lower than that in foreign countries. Stage III–IV was the most common stage, accounting for 86.8% of cases in China. The incidence of stage III–IV disease was much lower compared with cohort study in Sweden (60%). The immunity levels before treatment were weaker in China than in Western countries (median AMC of 0.35*109/L vs median ALC of 1.24*109/L).8,9 It has been suggested that the diagnosis of FL in China is relatively late, which may be associated with poor public physical examination awareness. However, with the application of immune-targeted therapeutics such as rituximab, prognosis has improved significantly. During our research, more than 80% of the patients received treatment regimens including rituximab, and their long-term survival was excellent; the median PFS was 74.8 months and the 10-year PFS rate was 43.1%, and these values were slightly better than those in foreign countries in the same period.10 The 5-year OS rate was 88.1%, and the 10-year OS rate was 75%, and these values were similar to those of studies abroad with the same treatment conditions.9
Regarding the prognostic impact of baseline characteristics, due to the long time span of more than 10 years, β2-MG was not commonly used, and the clinical value of FLIPI2 may have been undervalued;11 however, the predictive value of FLIPI2 in the post-rituximab era should be brought to the attention of clinicians. Therefore, we suggest following a standardized diagnosis process by obtaining the baseline data, such as β2-MG, bone marrow examination and even gene rearrangement, before initial assessment to provide the conditions for individual prognosis evaluation. In terms of tumour microenvironment indicators, peripheral blood LMR evaluation is a simple process. Previous studies have found that in patients with DLBCL and FL, OS after chemotherapy was significantly shortened in those with a low LMR (LMR≤2.6).12–14 Our study showed that the LMR is the only independent predictor of the long-term OS of FL patients in the Chinese population. ALC or AMC may also have some implications for OS. These results suggest that the immune microenvironment, especially cellular immunity, plays an important role in the progression of FL. Especially in recent years, drugs targeting the tumour microenvironment, such as lenalidomide and chidamide, have emerged. The LMR may serve as an important reference for clinical treatment decision making and may become a more popular indicator. In terms of the mechanism, previous literature has reported that peripheral blood lymphocytes can activate a variety of antigen-presenting cells, thus facilitating the recognition, uptake, presentation and immune system activation of tumour surface antigens. However, M2-type macrophages (tumour-associated macrophages [TAMs]) in peripheral blood are enriched around tumours under the guidance of regulatory T cells (Tregs), which directly promote tumour progression by endowing tumour tissues with functional polarity.15,16 In terms of treatment response prediction, POD24 status demonstrated strong predictive power for long-term survival and strong associations with baseline risk stratification status. Overall, considering the accuracy of prognosis prediction with the M7-FLIPI and health economics concerns, POD24 may be an important inclusion criterion for further gene expression profiling of patients.
This study is a systematic and comprehensive prognostic evaluation based on clinical data of Chinese patients. Prognostic predictors cover three categories: clinical baseline characteristics, tumour microenvironment characteristics and treatment response. All patients were followed up for more than 3 years, and the longest follow-up time was 146.3 months. We found a new factor, the LMR, that could predict prognosis independent of FLIPI or FLIPI2 status. The first drawback of this study is that the PRIMA-PI is affected by β2-MG and the small number of cases with bone marrow involvement, so the stratification results are not accurate. Second, due to the lack of gene expression profiling data for all of our patients, the prognostic assessment of tumour biological characteristics was incomplete. This study can be supplemented and corrected through prospective experiments in future clinical practice. Finally, because the sample size was insufficient, it was difficult to establish a prognostic evaluation model. Perhaps in the future, a more optimized FL prognostic model can be established with a larger cohort of patients from multiple centres with available data on the LMR and other independent prognostic predictors; this optimized model could be a simple, economical, and easy-to-apply evaluation system for guiding individualized treatment.
Abbreviations
LMR, lymphocyte/monocyte ratio; FL, follicular lymphoma; NHL, non-Hodgkin lymphoma; FLIPI, Follicular Lymphoma International Prognostic Index; FLIPI2, Follicular Lymphoma International Prognostic Index 2; PRIMA-PI, PRIMA Prognostic Index; DLBCL, diffuse large B-cell lymphoma; ALC, absolute lymphocyte count; AMC, absolute monocyte count; LDH, lactate dehydrogenase; β2-MG, β2 microglobulin; CR, complete remission; PR, partial response; SD, stable disease; PD, disease progression; OS, overall survival; PFS, progression-free survival; TAMs, tumour-associated macrophages.
Data Sharing Statement
No additional data available.
Ethics Approval and Informed Consent
Our study was approved by the PUMCH Institutional Review Board (protocal number: S-k1861). Because our study is a retrospective research, a waiver of patients consent was obtained from the PUMCH Institutional Review Board. We guaranteed the anonymity and privacy of patient information and the accessed data conformed to the data and privacy regulations of Declaration of Helsinki.
Funding
This study was supported by no agents.
Disclosure
The authors report no potential conflicts of interest in this work.
References
1. Chinese Society of Lymphoma, Chinese Anti-cancer Association; Chinese Society of Hematology, Chinese Medical Association. Chinese guidelines for diagnosis and treatment of follicular lymphoma. Zhonghua Xue Ye Xue Za Zhi. 2020;41(7):537–544.
2. Carbone A, Roulland S, Gloghini A, et al. Follicular lymphoma. Nat Rev Dis Primers. 2019;5(1):83.
3. Salles G, Barrett M, Foà R, et al. Rituximab in B-Cell Hematologic Malignancies: a Review of 20 Years of Clinical Experience. Adv Ther. 2017;34(10):2232–2273.
4. Bachy E, Maurer MJ, Habermann TM, et al. A simplified scoring system in de novo follicular lymphoma treated initially with immunochemotherapy. Blood. 2018;132(1):49–58.
5. Pastore A, Jurinovic V, Kridel R, et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 2015;16(9):1111–1122.
6. Leivonen SK, Pollari M, Brück O, et al. T-cell inflamed tumor microenvironment predicts favorable prognosis in primary testicular lymphoma. Haematologica. 2019;104(2):338–346.
7. Cioroianu AI, Stinga PI, Sticlaru L, et al. Tumor Microenvironment in Diffuse Large B-Cell Lymphoma: role and Prognosis. Anal Cell Pathol. 2019;2019:8586354.
8. Li X, Li G, Gao Z, Zhou X, Zhu Z, Chinese lymphoma pathological research cooperation group; Prognostic value of MYD88 L265P mutation in diffuse large B cell lymphoma via droplet digital PCR. Molecular medicine reports. Zhen Duan Xue Li Lun Yu Shi Jian. 2012;1(2):111–115.
9. Junlén HR, Peterson S, Kimby E, et al. Follicular lymphoma in Sweden: nationwide improved survival in the rituximab era, particularly in elderly women: a Swedish Lymphoma Registry study. Leukemia. 2015;29(3):668–676.
10. Dreyling M, Ghielmini M, Rule S, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(12):3109.
11. Federico M, Bellei M, Marcheselli L, et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol. 2009;27(27):4555–4562.
12. Armitage JO, Longo DL. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127(23):2804–2808.
13. Lee SF, Luque-Fernandez MA. Prognostic value of lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio in follicular lymphoma: a retrospective cohort study. BMJ Open. 2017;7(11):e017904.
14. Rambaldi A, Boschini C, Gritti G, et al. The lymphocyte to monocyte ratio improves the IPI-risk definition of diffuse large B-cell lymphoma when rituximab is added to chemotherapy. Am J Hematol. 2013;88(12):1062–1067.
15. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444.
16. Katoh D, Ochi Y, Yabushita T, et al. Peripheral Blood Lymphocyte-to-Monocyte Ratio at Relapse Predicts Outcome for Patients With Relapsed or Refractory Diffuse Large B-cell Lymphoma in the Rituximab Era. Clin Lymphoma Myeloma Leuk. 2017;17(12):e91–e97.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




