Back to Journals » Drug Design, Development and Therapy » Volume 14
Focus on Notoginsenoside R1 in Metabolism and Prevention Against Human Diseases
Authors Liu H , Yang J , Yang W , Hu S , Wu Y , Zhao B , Hu H , Du S
Received 29 November 2019
Accepted for publication 28 January 2020
Published 7 February 2020 Volume 2020:14 Pages 551—565
DOI https://doi.org/10.2147/DDDT.S240511
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Hai Liu,1,2 Jianqiong Yang,3 Wanqing Yang,1 Shaonan Hu,1 Yali Wu,1 Bo Zhao,1 Haiyan Hu,1 Shouying Du1
1School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2College of Pharmacy, Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China; 3Department of Clinical Research Center, The First Affiliated Hospital of Gannan Medical University, Ganzhou, Jiangxi, People’s Republic of China
Correspondence: Shouying Du
School of Chinese Materia Medica, Beijing University of Chinese Medicine, 11 North 3rd Ring East Road, Chaoyang District, Beijing, People’s Republic of China
Tel +86 17812165051
Fax +86 10 64286231
Email [email protected]
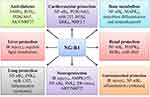
Abstract: Notoginsenoside (NG)-R1 is one of the main bioactive compounds from Panax notoginseng (PN) root, which is well known in the prescription for mediating the micro-circulatory hemostasis in human. In this article, we mainly discuss NG-R1 in metabolism and the biological activities, including cardiovascular protection, neuro-protection, anti-diabetes, liver protection, gastrointestinal protection, lung protection, bone metabolism regulation, renal protection, and anti-cancer. The metabolites produced by deglycosylation of NG-R1 exhibit higher permeability and bioavailability. It has been extensively verified that NG-R1 may ameliorate ischemia-reperfusion (IR)-induced injury in cardiovascular and neuronal systems mainly by upregulating the activity of estrogen receptor α-dependent phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and nuclear factor erythroid-2-related factor 2 (NRF2) pathways and downregulating nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways. However, no specific targets for NG-R1 have been identified. Expectedly, NG-R1 has been used as a main bioactive compound in many Traditional Chinese Medicines clinically, such as Xuesaitong, Naodesheng, XueShuanTong, ShenMai, and QSYQ. These suggest that NG-R1 exhibits a significant potency in drug development.
Keywords: notoginsenoside (NG)-R1, Panax notoginseng, micro-circulatory hemostasis, permeability, bioavailability
Introduction
Panax notoginseng (PN), a member of the family Araliaceae, has been widely used as a Traditional Chinese Medicine (TCM) for thousands of years. Particularly, its root is often clinically prescribed to maintain the micro-circulatory homeostasis in the human body and manage various diseases, including cardiovascular,1 neuronal,2 and diabetic dysfunctions.3 Xu et al (2018) reviewed the progress of PN in protection against inflammation-related chronic diseases.4 Xie et al (2018) discussed the mechanisms of PN in anti-depressant or anxiolytic effects.2 The bioactive compounds are the main factors responsible for the benefiting effects of TCM. More than 20 notoginsenosides (NGs), mainly belong to dammarane-type triterpenoidal saponins, have been identified and act as the main bioactive compounds responsible for the pharmacological effects of PN. These NGs include NG-R1, -R2, -R3, -R4, -R6, -Fa, -Fc, and -Fe, and ginsenoside-Rg1, -Rg2, -Rb1, -Rb2, -Rb3, -Rc, -Rd, -Re, -Rh, and -F2. Among them, NG-R1 (Figure 1), ginsenoside-Rg1, -Rd, and -Rb1 have been demonstrated to be the most effective.5 Increasing evidence shows that NG-R1 exhibits a variety of biological activities, including cardiovascular protection,6,7 neuroprotection,8,9 anti-diabetes,10,11 liver protection,12 gastrointestinal protection,13 lung protection,14 bone metabolism regulation,15 renal protection,16 and anti-cancer.17 Very recently, the effects of NG-R1 on organs ischemia/reperfusion injury have been discussed by meta-analysis, and NG-R1 has been indicated to be a novel drug candidate for ischemic diseases.18 The versatile properties of NG-R1 have been discussed.19 In this article, we will mainly discuss the metabolism and biological activities of NG-R1.
 |
Figure 1 The chemical structure of NG-R1, Rg1, F1, and PPT. |
Metabolism of NG-R1
Generally, compounds with high concentration are responsible for the pharmacological activity of the herbs. However, the most abundant compounds in herbs are not necessary to produce the highest concentrations after administration. This might be associated with the different pharmacokinetic and distribution characteristics of each constituent in vivo. Expectedly, the concentrations of many ingredients in blood plasma are closely related to their pharmacological activity.20–22 Pharmacokinetic research plays a crucial role in the development of drugs. The half-life of triterpenoid saponins is influenced by the number of sugar. Specifically, more sugar moieties indicate lower bioavailability and large polarity.23 Deglycosylation of NGs has been observed as the major metabolic pathway in rats.21 The absolute bioavailability of NG-R1, Rg1, and Rb1 in rats is 9.29%, 6.06%, and 1.18%, respectively5 (Table 1). During the metabolism of NG-R1 (Figure 1), the metabolites ginsenosides Rg1, F1, and 20(S)-protopanaxatriol (PPT) are produced by intestinal bacteria on a Caco-2 monolayer model, and the stepwise hydrolysis by gut bacteria via NG-R1→Rg1→F1→PPT→dehydrogenated PPT reveals an ascending permeability.24,25
 |
Table 1 The Biological Value of NG-R1 in Protection Against Diseases |
It has been demonstrated that NG-R1 exhibits poor membrane permeability and relatively low bioavailability and its transport primarily occurred by passive diffusion through the cell monolayer. The apparent permeability coefficient (Paff) of NG-R1 was about 1×10−7 cm/s, and the Cmax of NG-R1 is significantly increased after oral administration combined with aspirin.26 Borneol may act as a paracellular permeability enhancer to promote absorption. It has been demonstrated that the values of Tmax and Cmax of NG-R1 after oral administration to rats are 0.5 h and 6.45 μg/L, respectively. In borneol-included Fufang Danshen adhesive pellets (FDP) group, the Tmax and Cmax of NG-R1 are increased to 2.19 h and 10.13μg/L, respectively.27 The tissue distribution study by measurement of PN saponin concentrations indicates that the overall trend of PN saponin concentration is as follows: liver > kidney > lung > spleen > heart.28
At least two phases of NG-R1 elimination by human intestinal bacteria in vitro are identified. NG-R1 is deglycosylated slowly at an average velocity of 0.18 μmol/h within the first 8 h (phase I). In Phase II, NG-R1 disappears 20 times faster than that in Phase I.24 The rate of elimination of NG-R1 is reduced by co-administration with aspirin.26 In addition, PPT formation rate is around 80% of NG-R1 elimination rate over the period of 8–48 h, and PPT is the most abundant metabolite after 12 h and reach > 70% of initial NG-R1 at 48 h.24 In rats, NG-R1 is eliminated by excreting into the bile in 2 h and can be detected within 12 h. This indicates that the time course of NG-R1 metabolism is long in rats.29 NG-R1 is quickly absorbed with the peak time (Tmax) of 0.2 h in rats.26 However, the pharmacokinetic parameters of NG-R1 in beagle dogs are showed that the value of T1/2 is 4.833±1.609 h, Tmax is 3.7±3.094 h, Cmax is 70.907±38.79 ng/mL, and AUC is 404.234±131.891 ng/Lh22 (Table 1). Another study in beagle dogs shows that the pharmacokinetic parameters of NG-R1 in the commercial capsules and the colon-specific osmotic pump capsules are different. In the commercial capsules, the value of T1/2 is 0.63 h, Tmax is 1.5 h, Cmax is 23.59 ng/mL, and AUC0-t is 75.29 ngh/mL. In contrast, in the colon-specific cosmotic pump capsules, they are 5.84 h, 11 h, 16.63 ng/mL, and 203.25 ngh/mL, respectively.30 Controversially, the pharmacokinetic parameters of NG-R1 in beagle dogs after a single oral dose of three-compound Danshen tablets are quite different. The value of T1/2 is 4.82 h, Tmax is 1.17 h, Cmax is 1.91 ng/mL, and AUC0-t is 6.84 ngh/mL.31 This difference might be explained by different species. However, more efforts are needed for better understanding the difference. NG-R1 has been demonstrated to show the inhibitory effects on cytochrome P450 1A2 (CYP1A2) but not on CYP2C11, CYP2D1, and CYP3A1/2, as indicated by increased caffeine Cmax (43.13%) and AUC0-∞ (40.57%) and decreased CL/F (62.16%).32
Due to the low bioavailability of NG-R1, its application in the clinic has been greatly affected. Many strategies have been investigated for improving drug bioavailability, such as extending the time of pharmaceutical preparations’s effects on target sites, increase of the contact with the absorption membrane, change of membrane fluidity, and increase of drug penetration to the epithelial cells. Recently, it has been reported that core-shell hybrid liposomal vesicles (HLV) nanocarriers for NG-R1 dramatically influence its release profiles, inhibiting brain edema and reducing the infarct volume, accompanied by downregulation of lactate dehydrogenase (LDH), malondialdehyde (MDA), and H2O2 and upregulation of superoxide dismutase (SOD).33 Consistently, poly (D,L-lactide-co-glycolide acid) (PLGA) nanoparticles (NPs) carrying NG-R1 have been showed to greatly improve pharmacokinetic activity and enhance bioavailability, as showed that the value of AUC0-t is 4 folds greater and Cmax is 14.4 folds higher.34 Another study reports that the bio-adhesive tablet prepared by chitosan as the main excipient significantly improves the bioavailability of PN saponins in beagle dogs than the normal tablet. Compared with that of the normal tablets, the relative bioavailability of bio-adhesive tablets after oral administration is 204.05% for NG-R1. Similar improvements for Tmax, Cmax, and AUC are observed.35
Drug–drug interaction may occur during the process of absorption. Borneol may enhance the bioavailability of NG-R1 by attenuating the intercellular tight junctions, as showed by increase of intestinal absorption and distribution and decrease of NG-R1 metabolism in Caco-2 cells.36 Similarly, aspirin and salicylic acid may negatively affect the integrity and configuration of tight junction proteins and positively increase the permeability, and PN NGs increase the stability of gastrointestinal tract, absorption, and clinical efficacy in MDCK-MDR1 monolayer cells.26 In Caco-2 cell monolayer, the AUC of NG-R1 may also be increased 2.24 folds by sodium N-[8-(2-hydrobenzoyl)amino]caprylate (SNAC), which facilitates passive transport of polar charged drugs, indicating improvement of absorption.37 Sodium glycocholate (SGC)-mediated liposomes (@SGC-Liposomes) have been designed as a nanovesicle, which may improve the intestinal permeability and absorption of NG-R1 with a 2.68-fold higher AUC0-t after oral administration in rats.38 Interestingly, NG-R1 may increase the intestinal absorption and subsequent oral bioavailability of geniposide by inhibiting P-glycoprotein (P-gp) activity in a way similar to that of a P-gp inhibitor.39
Roles of NG-R1 in Cardiovascular Diseases
A comprehensive overview of the protective effects of PN saponins on cardiovascular diseases has been discussed.6 NG-R1 has been demonstrated to improve the cardiac functions, as showed by attenuation of NF-κB signaling and subsequent reduction of inflammation and apoptosis responses (Figure 2). The possible mechanisms might be associated with the stimulatory activity of NG-R1 on estrogen receptor α and PI3K/AKT signaling pathways7 (Figure 3). Consistently, NG-R1 protects against endotoxin-induced toxicity by upregulating the expression of estrogen receptor α, but not estrogen receptor β, leading to blockage of inflammation and apoptosis in rat H9c2 cardiomyocytes40 (Table 1). The anti-inflammatory effects of NG-R1 contribute to the improvement of isoproterenol (ISO)-induced hypertrophied hearts of atherosclerosis-prone mice. In ApoE−/- mice, NG-R1 suppresses ISO-induced cardiac dysfunction, atherosclerotic lesions, and accumulation of inflammatory cytokines. These can be partially attenuated by the induction of CC chemokine receptor 2 (CCR2), which plays a critical role in recruiting pro-inflammatory monocytes to the inflamed hypertrophic heart tissues.41
In an ischemia-reperfusion (IR)-induced myocardial injury rabbit model, NG-R1 exhibits protective effects against IR injury by deactivating TGFβ1/TGFβ1 activated kinase 1 (TAK1) signaling pathway, as indicated by reduced expression of caspase-3, −8, and −9.42 Consistently, NG-R1 has showed cardioprotective effects against IR injury by amelioration of oxidative stress and endoplasmic reticulum (ER) stress in H9c2 cardiomyocytes and Langendorf-perfused rat hearts, as demonstrated by decreased expression of GRP78, PERK, ATF6, IRE1, CHOP, JNK, and caspase-1243 (Figure 2). Oxygen-glucose deprivation (OGD) produces negative effects on cardiomyocytes. In OGD-induced H9c2 cells, NG-R1 at the dose of 20 μM promotes cell viability and reduces the expression of Bax, cleaved caspase-3, and caspase-9. The possible underlying mechanism might be associated with the effects of NG-R1 on upregulation of miR-21 expression and downregulation of PTEN (a target of miR-21) expression and PI3K/AKT signaling pathway44 (Table 1). Consistently, NG-R1 decreases myocardial infarction and injury and cardiac dysfunction induced by OGD in H9c2 cells, as showed by the improvement of cell viability, maintenance of actin skeleton and mitochondrial morphology, and attenuation of apoptosis. These might be related to the effects of NG-R1 on inhibiting the ROCK signaling pathway and increasing ATP synthase δ-subunits activity.45
To investigate the effects of NG-R1 on the hemostatic system, the fibrinolytic parameters of cultured HUVECs have been studied. The antigen production of tissue-type plasminogen activator (TPA) and the complex formation of TPA and plasminogen activator inhibitor-1 (PAI-1) have been upregulated by NG-R1, while no effects on the expression of urokinase-type plasminogen activator and PAI-1 are found46 (Table 1). In human aortic smooth muscle cells (HASMCs), NG-R1 dose-dependently decreases TNFα-induced PAI-1 expression by suppressing the activity of extracellular regulated protein kinases (ERK) and protein kinase B (PKB) signaling pathways.47 Consistently, NG-R1 attenuates TNFα-induced ROS generation and ERK activation, leading to suppression of fibronectin expression and HASMC migration.48 The protective effects of NG-R1 on advanced glycation end products (AGEs)-treated H9c2 cardiomyocytes and diabetic db/db mice have been investigated. Expectedly, NG-R1 significantly ameliorates AGEs-induced mitochondrial damage, decreases reactive oxygen species (ROS) production, and attenuates apoptosis by activating estrogen receptor α-dependent AKT and nuclear factor-erythroid 2-related factor 2 (NRF2) signaling pathways, leading to suppression of cardiac fibrosis and hypertrophy49 (Figure 3).
Atherosclerosis, the leading cause of stroke and cardiovascular disease, is characterized by the accumulation of lipids and fibrous lesions. It has been reported that NG-R1 attenuates lipid deposition, oxidative stress, inflammation, and fibrosis and increases the levels of glutathione (GSH) and SOD in ApoE−/- mice. Additionally, NG-R1 pretreatment may induce downregulated expression of miR-21, miR-26a, and miR-126 and upregulated expression of miR-20a50 (Table 1). In human endothelial EA.hy926 cells, NG-R1 exhibits protective effects against oxidized low-density lipoprotein (oxLDL)-induced inflammatory damage, as showed by suppression of NF-κB and MAPK signaling pathways and enhancement of PPARγ expression51 (Figure 2). Neointimal hyperplasia-induced restenosis produces decreased long-term efficacy for dealing with coronary and peripheral arterial diseases. Abnormal proliferation and migration of vascular smooth muscle cells (VSMCs) is one of the main factors for the formation of neointima. It has been demonstrated that NG-R1 inhibits the proliferation and migration of VSMCs by mediating the activity of actin cytoskeleton dynamics and inhibiting the activation of the PI3K/AKT signaling pathway52 (Figure 3).
Hypertension may contribute to the development of cardiovascular accidents. NG-R1 has been investigated for its role in vasodilation and found that it dose-dependently induces vessel relaxation by regulating NO pathway, but not cyclooxygenase (COX) pathway, in isolated aortic rings with intact endothelium.53 Another report shows that NG-R1 may induce the expression of inducible nitric oxide synthase (iNOS)/NO signaling by downregulating the expression of LncRNA AK094457 in vascular endothelial cells from spontaneously hypertensive rats.54 Furthermore, NG-R1 may dramatically attenuate lipopolysaccharide (LPS)-induced red blood cell velocity, leukocyte adhesion, mast cell degranulation, cytokine production, and neutrophil CD11b/CD18 expression, blocking LPS-induced micro-circulatory disturbance.55
Roles of NG-R1 in Neuronal Diseases
PN is commonly used for treating micro-circulatory diseases in China. Recently, Nasal administration of PN saponin has been developed, and this indicates the benefiting roles of PN saponin in the brain.9 PN exhibits protective effects against spinal cord IR-induced injury by downregulating inflammatory cytokine expression and decreasing edema and cell apoptosis56 (Figure 2). Focal cerebral IR surgery may change intestinal flora and decrease the population of Bifidobacterium longum, which may induce the downregulated expression of GABA receptor in rats. These effects can be compromised by treatment with PN extracts.57 NG-R1 may dramatically reduce the volume of cerebral infarction and hippocampal neuron apoptosis and protect against cerebral IR-induced injury by activating the expression of brain-derived neurotrophic factor (BDNF) and Bcl-2 and inhibiting the expression of Bax8 (Table 1). It has been demonstrated NG-R1 prevents from IR-induced injury by inhibiting inflammation and ER stress-induced apoptosis, as showed by downregulating the expression of TNFα, intercellular cell adhesion molecule-1 (ICAM-1), p-JAK, caspase-12, p-STAT1, and nuclear translocation of NF-κB and upregulating the expression of glucose-regulated protein 78 (GRP78). In addition, NG-R1 also increases the contents of ATP, ADP, AMP, the total adenine nucleotides (TAN), the phosphorylation of AMPK1/2, and the expression of glucose transporter 3 (GLUT3) (Figure 2). Combination of Astragaloside IV, Rg1, Rb1, and NG-R1 may further strengthen these benefiting effects.58,59 LPS may be neurotoxic, decrease cell viability, induce apoptosis, and increase the expression of IL-6, −8, and TNFα in PC-12 cells. NG-R1 may compromise the effects of LPS by upregulating the expression of miR-132 and suppressing the activity of the JNK signaling pathway in PC-12 cells60 (Figure 3).
ER stress, known to be mediated by estrogen, is critical for the development of cell apoptosis during ischemia. The neuroprotective activity of NG-R1 by amelioration of ER stress has been demonstrated in neonatal rat hypoxia-ischemic encephalopathy, as showed by decreased expression of protein kinase R-like ER kinase (PERK)/C/EBP homologous protein (CHOP), inositol-requiring enzyme 1α (IRE1α), endoplasmic reticulum oxidoreductin 1-α (ERO1α), and caspase-12 and increased expression of Bcl-2. However, the inhibitor of estrogen receptor ICI-182780 may attenuate the protective effects of NG-R161 (Figure 3). ER maintains the homeostasis of calcium, and imbalance of calcium may induce ER stress. Further study shows that NG-R1 may positively improve the binding of GRP78 to PERK and IRE1α, suppress PLC/IP3R signaling pathway, and decrease cell apoptosis in oxygen-glucose deprivation/reoxygenation (OGD/R) rat model.62 Consistently, NG-R1 protects against brain damage in OGD/R rat model, upregulates the expression of PI3K/AKT/mTOR signaling pathway, and downregulates JNK signaling pathway by activating estrogen receptors63 (Figure 2). NG-R1 has been demonstrated to prevent OGD/R-induced oxidative stress by inhibiting the generation of NADPH oxidase- and mitochondria-based superoxide, malondialdehyde, protein carbonyl, and 8-hydroxydeoxyguanosine in vivo and in vitro. These changes are associated with activation of estrogen receptor-dependent AKT/NRF2 signaling pathways by NG-R1, which significantly improves neurologic outcomes and decreases cerebral infarct volume.64
Oxidative stress contributes to the development of neurodegenerative diseases. NG-R1 exhibits neuroprotective effects in PC12 cells by inhibiting ROS generation, lipid peroxidation, protein oxidation, DNA fragmentation, and mitochondrial membrane depolarization induced by H2O2. The underlying mechanism might be associated with crosstalk between AKT and ERK1/2 signaling pathways in an estrogen receptor-dependent manner, and subsequently with upregulation of NRF2/ARE signaling and Phase II antioxidant enzyme expression65 (Figure 2). The neurotoxicity of glutamate (Glu) has been associated with various intracellular signals in neurons. NG-R1 has been reported to significantly attenuate Glu-induced increase of intracellular Ca2+ concentration and ROS production and depolarization of mitochondrial membrane potential by inhibiting the activity of N-methyl-D-aspartate (NMDA) receptor, leading to inhibition of cell apoptosis in HEK293 cells66 (Table 1).
Amyloid β (Aβ) accumulation promotes the development of Alzheimer’s disease (AD), which is indicated by neurodegeneration and synaptic dysfunction. Aβ may induce oxidative stress and cell death in neuronal cells. It has been showed that NG-R1 can compromise the effects of Aβ, as indicated by increased viability, reduced oxidative damage, restored mitochondrial membrane potential, and attenuated MAPK signaling pathway67 (Table 1). The effects of NG-R1 on the neuronal excitability and synaptic and memory dysfunction under the condition of Aβ accumulation are investigated. NG-R1 may significantly enhance the membrane excitability of CA1 pyramidal neurons by inhibiting voltage-gated K+ currents and lowering the spike threshold. Furthermore, NGR-R1 protects against impairment in long-term potentiation induced by Aβ1-42 and improves learning performance in the APP/PSI mice models.68
Roles of NG-R1 in Diabetes
Recently, it has been reported that NG-R1 produces protective effects against diabetes and its clinical complications. NG-R1 may prevent against high glucose-stimulated damage in RSC96 cells, as showed by downregulation of caspase-3 and miR-503, decrease of PARP cleavage and ROS generation, and activation of PI3K/AKT and Wnt/β-catenin signaling pathways10 (Figure 3). The pro-inflammatory factor TNFα may produce damage in Min6 and rat primary islet β cells. NG-R1 has been demonstrated to ameliorate the effects of TNFα by positively regulating the expression of miR-29a, which is also mediated by Wnt/β-catenin and PI3K/AKT/GSK-3β signaling pathways, alleviating cell dysfunctions69 (Table 1). Diabetic encephalopathy is aggravated by oxidative stress and inflammation. NG-R1 has been showed to activate AKT/NRF2 signaling pathway and inhibit the activation of NLRP3 inflammasome in db/db mice and high-glucose triggered HT22 hippocampal neurons.11
In AGEs-treated db/db mice, NG-R1 greatly increases the levels of serum lipid, β2-microglobulin, creatinine, and blood urea nitrogen and reduces glomerular volume and fibrosis. In vitro, NG-R1 protects HK-2 cells against AGEs-induced mitochondrial damage, ROS generation, and cell apoptosis by upregulating the NRF2-HO-1 signaling pathway and downregulating the expression of TGFβ and collagen70 (Table 1). In high glucose-treated rat retinal capillary endothelial cells (RCECs), NG-R1 may monitor the cellular redox status and protect RCECs against damage, as showed by reduced production of ROS and nitrotyrosine, decreased activity of NADPH oxidase and PARP, and increased CAT activity.71 Diabetic retinopathy may be managed by the involvement of NG-R1, which exhibits inhibitory effects on VEGF expression, oxidative stress, inflammation, and apoptosis in rat retinal Muller cells induced by high glucose and in the retinas of db/db mice. In addition, NG-R1 may also increase the expression of PTEN induced putative kinase 1 (PINK1) and Parkin and the ratio of LC3-II/LC3-I and decrease the expression of p62/SQSTM1.72
Deficiency of α3β1 integrin induces reduced adhesion to the glomerular basement membrane, resulting in the development of diabetic kidney disease. NG-R1 has been showed to improve the expression of α3β1 integrin and increase podocyte adherence impaired by high glucose.73 Consistently, NG-R1 ameliorates podocyte injury by activation of PI3K/AKT signaling pathway and inhibition of inflammation and apoptosis, as indicated by increased phosphorylation of PI3K and AKT and decreased phosphorylation of p65 in streptozotocin-induced rat diabetic nephropathy.74 Furthermore, NG-R1 promotes the activity of the PI3K/AKT/mTOR signaling pathway and protects podocytes from high glucose-induced apoptosis75 (Figure 3).
Roles of NG-R1 in Liver Diseases
Gut IR-induced injury in the liver may initiate the rate-limiting step of recruiting leukocytes to vascular endothelium, disturbing hepatic microcirculation. NG-R1 has been showed to ameliorate the effects of IR-induced injury (Figure 3), as indicated that NG-R1 attenuated the expression of E-selectin, CD18, LDH, ALT, and AST and the activity of leukocyte rolling and adhesion12 (Table 1). Furthermore, PN and NG-R1 may regulate lipid metabolism by increasing the content of CYP7α and decreasing the levels of HMG-CoAR and SREBP-2 in steatotic L02 hepatocytes76 (Table 1).
Roles of NG-R1 in Bone Metabolism Regulation
Recently, it has been demonstrated that PN saponins promote osteogenesis, including proliferation, differentiation, and mineralization activity in osteoblasts.77 Accordingly, NG-R1 significantly induces bone development by inhibition of RNAKL-mediated MAPK and NF-κB signaling pathways and suppression of osteoclastogenesis and bone resorption.15 In addition, NG-R1 may significantly induce the expression of Runx-2 and Osx as well as the activity of ALP in MC3T3-E1 cells dose-dependently.78,79 The possible mechanism of NG-R1 in promotion on MC3T3-E1 differentiation is related to increased phosphorylation of MAPK, JAK1, and STAT3 and expression of miR-23a78 (Table 1). Furthermore, the promoting effects of NG-R1 on osteoblast differentiation and mineralization have been demonstrated to be associated with activation of estrogen receptor signaling pathway, as showed that the transcriptional activity of phosphorylated estrogen response element-luciferase is activated and knockdown by the antagonist ICI-182780 may abolish the effects of NG-R1 on human BMSCs80 (Figure 3).
Roles of NG-R1 in Gastrointestinal Diseases
IR-induced injury may occur in various conditions, including mesenteric artery embolism, abdominal aortic aneurysm surgery, and hemorrhagic shock. Intestinal IR-induced injury often causes high mortality clinically. NG-R1 significantly ameliorates IR-induced microvascular hyperpermeability, inflammatory cytokine generation, gap junction protein loss, and NF-κB activation13 (Figure 3). The effects of NG-R1 on chronic atrophic gastritis in rats have been investigated, and it demonstrates that NG-R1 may increase the levels of somatostatin, gastrin, motilin, secretory IgA, GSH, and Bcl-2 and decrease IL-1β, IL-6, PGE2, NOS, endothelin, and Bax.81 Inflammatory bowel disease (IBD) occurs in the gastrointestinal tract. It has been demonstrated that NG-R1 may significantly ameliorate the expression of inflammatory cytokines and the activity of myeloperoxidase and the NF-κB signaling pathway by acting a ligand to activate pregnane X receptor (PXR) and upregulating the expression of xenobiotic-metabolizing enzymes82 (Table 1).
Roles of NG-R1 in Lung Diseases
Another report shows that NG-R1 exhibits protective effects against neonatal pneumonia. In LPS-treated WI-38 cells, NG-R1 decreases the expression of IL-1β, TNFα, and IL-6, decreases cell viability, and promotes apoptosis. These might be related to enhanced expression of miR-181a (targeting TLR4) and decreased activity of NF-κB and TAK1/JNK signaling pathways14 (Figure 3). In MRC-5 cells, NG-R1 protects against LPS-induced cell proliferation inhibition, apoptosis, and increased production of inflammatory cytokines by increasing the expression of miR-132 and inactivating NF-κB and JNK signaling pathways83 (Table 1).
Roles of NG-R1 in Renal Diseases
IR-induced injury, a major cause of renal failure, is associated with activation of inflammatory responses and apoptosis. NG-R1 has been reported to attenuate IR-induced renal dysfunction by suppressing inflammatory responses and inhibiting apoptosis in rats, as showed by downregulated the expression of p38 MAPK and NF-κB signaling pathway and upregulated the expression of Bcl-216 (Figure 3). In human renal proximal tubular epithelial cell lines HK-2 and RPTECs, NG-R1 protects against LPS-induced viability reduction, ROS generation, inflammatory stress, and apoptosis by enhancing the expression of miR-26a and inactivating NF-κB signaling pathway84 (Table 1).
Roles of NG-R1 in Cancer
PN saponins can maintain immune homeostasis and inhibit the proliferative activity of cancer cells. The anti-cancer potentials of PN saponins have been reviewed by Wang et al (2016).85 PN saponins have been reported to reduce the survival of Lewis lung carcinoma (LLC) cells and decreasetumor growth. The bioinformatics analysis of gene expression profiles indicates that PN saponins may decrease the expression of genes responsible for tumorigenesis and progression, including Hgf, Met, Notch3, Scd1, Epas1, Col1a1, Raf1, Braf1, and CDK6, and factors responsible for tumor suppression, including p27 and PTEN.86 The fermentation medium containing 10 g of raw PN powder and 90 mL of water has been demonstrated to counteract the proliferation against hepatoma Hep3B cells. However, the production of NG-R1 has been found to be decreased during fermentation, and ginsenosides Rh1 and Rg3 increase.87 Hepatocellular carcinoma (HCC) is one of the most common malignancies with high morbidity and mortality. NG-R1 may inhibit cell viability, increase LDH release, suppress invasion, and enhance caspase-3/-7 activity in HCC cells by inactivation of PI3K/AKT and downregulation of miR-21.88 Consistently, assays of notoginseng extracts and saponins in regulating SW480 cell apoptosis show that not NG-R1 but ginsenosides Rb1, Rb3, and Rg1 are the main bioactive compounds responsible for the suppression of human colorectal cancer cells.89
The regulatory activity of NG-R1 on the migration and invasion of cancer cells has been investigated. It indicates that NG-R1 may significantly reduce the expression of metalloproteinase (MMP)-9, E-selectin, ICAM-1 in HCT-116 cells and increase the capacity of the trans-epithelial electrical resistance in EA.hy926 endothelial cell monolayer17 (Table 1). Angiogenesis is a morphogenetic process of neovessel growth, which is tightly controlled by angiogenic factors and inhibitors. The pro-angiogenic action of NG-R1 has been involved in the proliferation of HUVECs, as indicated by the increased number of cross-membrane, enhanced tube formation by activation of VEGF/KDR signaling and upregulating the activity of PI3K/AKT signaling. In addition, NG-R1 triggers pro-angiogenic activity in chemically induced blood vessel loss model in zebrafish.90
Other Biological Activities of NG-R1
Autologous fat tissue transplantation has been popularly used in surgery. However, the accompanied responses of inflammation may negatively aggravate graft volume maintenance and survival rate. NG-R1 has been demonstrated to improve the quality of autologous fat graft and survival rate by increasing the expression of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), angiogenin (ANG), and hepatocyte growth factor (HGF)91 (Table 1).
Due to side effects and antibody responses, many adjuvants are limited for their application in vaccines. NG-R1 has been found to exhibit significant adjuvant effects on immune responses and specific antibody against ovalbumin in mice, accompanied by a slight hemolytic activity. Structural activity relationship study shows that the type of sugar at the terminal of the C-6 chain of PPT may regulate the hemolytic activity, adjuvant potentials, and immune responses.92
Burn injury shows dramatically negative effects on patients by triggering inflammation at least. NG-R1 has been demonstrated to counteract inflammatory injury in LPS-treated human keratinocyte HaCaT cells, as indicated by downregulation of MyD88 expression, inactivation of p38 MAPK and NF-κB signaling pathways, and suppression of cell apoptosis93 (Table 1).
Clinical Perspectives
Returning back to nature becomes a trend throughout the world, and it dramatically poses great enthusiasm in the exploration of traditional medicines for the management of complex diseases. However, traditional medicines generally contain complex mixtures of different chemical constituents, and this might raise a serious issue with quality uncertainty. Nowadays, characterization and quantification of the substances in traditional medicines are not of difficulty. Individual chemical markers may ensure the quality control of phytotherapeutic agents. NG-R1 has been identified as one of the bioactive chemical markers of Xuesaitong Injection (XST),94 which is made of the saponins from P. notoginseng and has been extensively employed in clinic for managing cardiovascular and cerebrovascular diseases in China.95 Naodesheng (NDS) is the TCM prescription widely used for clinic management of cerebral infarction in China. NG-R1 is one of the bioactive compounds in NDS, which has been demonstrated to improve neurobehavioral activity, decrease the cerebral infarct area, and attenuate pathological features in middle cerebral artery occlusion (MCAO) rat models. In addition, NDS also exhibits significant antioxidative activity, as showed by a decrease of LDH and MDA production, increase of SOD generation in plasma, and enhancement of brain levels of leucine, isoleucine, choline, and myo-inositol.96 NG-R1 is also the main bioactive and circulating compound of XueShuanTong, which has been predicted to show high potentials for drug interactions mediated by organic anion-transporting polypeptide (OATP)1B.97
Conventional pharmacotherapy has been implicated in the treatment of complex diseases, and the use of herbal medicinal products or botanical dietary supplements is prevalent in China. NG-R1 is one of the main bioactive compounds from a traditional Chinese medicine ShenMai Injection (SMI). In the excretion study, high concentration of prototype of NG-R1 has been observed in the rat kidney, and NG-R1 has been showed to be exclusively detected in rat urine, but not in feces.98 QiShenYiQi (QSYQ) pill, a Chinese medicine, contains NG-R1 as the bioactive ingredient and contributes to anti-hypertrophic effects in the management of cardiac hypertrophy. It has been verified that each ingredient such as NG-R1 exhibits similar effects as QSYQ but to a lesser extent in rats. The possible mechanism might be associated with the enhancement of energy metabolism and amelioration of oxidative stress.99 Further study shows that QSYQ may decrease IR-induced infarct size, ameliorate myocardial fibrosis, and inhibit monocyte infiltration and macrophage polarization towards M2 by downregulating the expression of TGFβ and TGFβRII in rats.100 The clinically therapeutic effects of NG-R1 have been observed, the chemical basis for understanding the underlying mechanisms responsible for NG-R1-drug interactions is still limited.
Combinational, rather than single, chemotherapy strategy is often a more effective for the management of complex diseases. Botanical products, either as herbal medicines or as dietary supplements, can result in clinically important drug interactions. Increasing understanding of botanical products-drug interactions may ensure safe co-administration. Gap junctions (GJs), the specialized cell-cell junctions, have been linked to homeostasis maintenance, morphogenesis, cell differentiation, and growth control. Enhancement of GJs functions increases the cytotoxic activity of cisplatin, and both NG-R1 and Rg1 have been identified as the active compounds in PN for enhancing cisplatin-induced cytotoxic activity by inhibiting Cx32/Cx26 degradation and/or modulating translation in transfected HeLa cells. Unfortunately, Rb1 produces not any effects.101 A monoclonal antibody has been developed by immunization of NG-R1-bovine albumin conjugates with BALB/c male mice. Interestingly, this monoclonal antibody exclusively recognizes free NG-R1 during the indirect competitive ELISA, establishing a reliable system for various applications in pharmaceutical and food fields.102
Conclusion
Herbal medicines with multi-compounds have been considered to exhibit pharmaceutical effects on multi-targets in a co-operative manner. The integrated pharmaceutical activity produced by different compounds with the same mother nuclear structure may be more reasonable than that of adding up all compounds with different groups. In addition, the components of herb and their concentrations in the blood plasma are statistically associated with the pharmacological activity of the herb. Simultaneous, rather than solitary, determination of these compounds in the blood plasma may provide a holistic understanding of pharmacokinetic characteristics. Although the sugar moiety increases the polarity, it may decrease the bioavailability of NGs. However, the underlying mechanisms of the chemical and spatial structures of sugar moiety on regulating the biological activity of NGs are still unclear. NG-R1 may be degraded to be ginsenoside Rg1, F1, and PPT, which exhibit dramatic biological effects. Whether these metabolites are involved in the regulatory activity of NG-R1 in the management of human diseases is still unknown. Thus, the reported activity of NG-R1, particularly in vivo, might be derived from the parent compound or the metabolites, alone or combination. In addition, the specific targets of NG-R1 are not identified. The limitation of research on NG-R1 in this article is that all data are from Asia, and this might be explained by the situation that the herbs are mainly found and used in Asia. The human-benefiting functions of PN saponins and NG-R1 are versatile, and they should be known worldwide. More efforts are still needed.
Collectively, we update the biological activities of NG-R1 in cardiovascular protection, neuro-protection, anti-diabetes, liver protection, gastrointestinal protection, lung protection, bone metabolism regulation, renal protection, and anti-cancer.
Data Sharing Statement
The experimental data used to support the findings of this study are included within the article.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (81860261) and the Fundamental Research Funds for the Central Universities (100006122193).
Author Contributions
Shouying Du provided the idea of this paper and resolved the problems in research process. Hai Liu and Jianqiong Yang contributed equally to this manuscript for collecting materials and writing the paper, Wanqing Yang, Shaonan Hu, Yali Wu helped with literature screening and paper writing. Bo Zhao, Haiyan Hu organized the information and edited the article pictures. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflict of interests.
References
1. Zhang S, Chen C, Lu W, Wei L. Phytochemistry, pharmacology, and clinical use of panax notoginseng flowers buds. Phytother Res. 2018;32(11):2155–2163. doi:10.1002/ptr.v32.11
2. Xie W, Meng X, Zhai Y, et al. Panax notoginseng saponins: a review of its mechanisms of antidepressant or anxiolytic effects and network analysis on phytochemistry and pharmacology. Molecules. 2018;23(4):940. doi:10.3390/molecules23040940
3. Ju Z, Li J, Han H, Yang L, Wang Z. Analysis of bioactive components and multi-component pharmacokinetics of saponins from the leaves of panax notoginseng in rat plasma after oral administration by LC-MS/MS. J Sep Sci. 2018;41(7):1512–1523. doi:10.1002/jssc.v41.7
4. Xu Y, Tan HY, Li S, Wang N, Feng Y. Panax notoginseng for inflammation-related chronic diseases: a review on the modulations of multiple pathways. Am J Chin Med. 2018;46(5):971–996. doi:10.1142/S0192415X18500519
5. Li X, Wang G, Sun J, et al. Pharmacokinetic and absolute bioavailability study of total panax notoginsenoside, a typical multiple constituent traditional chinese medicine (TCM) in rats. Biol Pharm Bull. 2007;30(5):847–851. doi:10.1248/bpb.30.847
6. Yang X, Xiong X, Wang H, Wang J. Protective effects of panax notoginseng saponins on cardiovascular diseases: a comprehensive overview of experimental studies. Evid Based Complement Alternat Med. 2014;2014:204840. doi:10.1155/2014/204840
7. Sun B, Xiao J, Sun XB, Wu Y. Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic mice: an insight into oestrogen receptor activation and PI3K/Akt signalling. Br J Pharmacol. 2013;168(7):1758–1770. doi:10.1111/bph.12063
8. Zou S, Zhang M, Feng L, Zhou Y, Li L, Ban L. Protective effects of notoginsenoside R1 on cerebral ischemia-reperfusion injury in rats. Exp Ther Med. 2017;14(6):6012–6016. doi:10.3892/etm.2017.5268
9. Guo Q, Li P, Wang Z, et al. Brain distribution pharmacokinetics and integrated pharmacokinetics of panax notoginsenoside R1, ginsenosides Rg1, Rb1, Re and Rd in rats after intranasal administration of panax notoginseng saponins assessed by UPLC/MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;969:264–271. doi:10.1016/j.jchromb.2014.08.034
10. Wang W, Hao Y, Li F. Notoginsenoside R1 alleviates high glucose-evoked damage in RSC96 cells through down-regulation of miR-503. Artif Cells Nanomed Biotechnol. 2019;47(1):3947–3954. doi:10.1080/21691401.2019.1671434
11. Zhai Y, Meng X, Luo Y, et al. Notoginsenoside R1 ameliorates diabetic encephalopathy by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Oncotarget. 2018;9(10):9344–9363. doi:10.18632/oncotarget.v9i10
12. Chen WX, Wang F, Liu YY, et al. Effect of notoginsenoside R1 on hepatic microcirculation disturbance induced by gut ischemia and reperfusion. World J Gastroenterol. 2008;14(1):29–37. doi:10.3748/wjg.14.29
13. Li C, Li Q, Liu YY, et al. Protective effects of notoginsenoside R1 on intestinal ischemia-reperfusion injury in rats. Am J Physiol Gastrointest Liver Physiol. 2014;306(2):G111–G122. doi:10.1152/ajpgi.00123.2013
14. Qian D, Shao X, Li Y, Sun X. Notoginsenoside R1 protects WI-38 cells against lipopolysaccharide-triggered injury via adjusting the miR-181a/TLR4 axis. J Cell Biochem. 2019. doi:10.1002/jcb.29282
15. Zhao S, Yan L, Li X, Zhang Z, Sun Y, Wang J. Notoginsenoside R1 suppresses wear particle-induced osteolysis and RANKL mediated osteoclastogenesis in vivo and in vitro. Int Immunopharmacol. 2017;47:118–125. doi:10.1016/j.intimp.2017.03.018
16. Liu WJ, Tang HT, Jia YT, et al. Notoginsenoside R1 attenuates renal ischemia-reperfusion injury in rats. Shock. 2010;34(3):314–320. doi:10.1097/SHK.0b013e3181ceede4
17. Lee CY, Hsieh SL, Hsieh S, et al. Inhibition of human colorectal cancer metastasis by notoginsenoside R1, an important compound from panax notoginseng. Oncol Rep. 2017;37(1):399–407. doi:10.3892/or.2016.5222
18. Tong Q, Zhu PC, Zhuang Z, et al. Notoginsenoside R1 for organs ischemia/reperfusion injury: a preclinical systematic review. Front Pharmacol. 2019;10:1204. doi:10.3389/fphar.2019.01204
19. Guo S, Xi X, Li J. Notoginsenoside R1: a systematic review of its pharmacological properties. Pharmazie. 2019;74(11):641–647. doi:10.1691/ph.2019.9534
20. Zhou L, Xing R, Xie L, et al. Development and validation of an UFLC-MS/MS assay for the absolute quantitation of nine notoginsenosides in rat plasma: application to the pharmacokinetic study of panax notoginseng extract. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;995–996:46–53. doi:10.1016/j.jchromb.2015.05.022
21. He C, Li J, Xu N, et al. Pharmacokinetics, bioavailability, and metabolism of notoginsenoside Fc in rats by liquid chromatography/electrospray ionization tandem mass spectrometry. J Pharm Biomed Anal. 2015;109:150–157. doi:10.1016/j.jpba.2015.02.038
22. Wu H, Liu H, Bai J, Lu Y, Du S. Simultaneous determination of notoginsenoside R(1), ginsenoside Rg(1), ginsenoside Re and 20(S) protopanaxatriol in beagle dog plasma by ultra high performance liquid mass spectrometry after oral administration of a panax notoginseng saponin preparation. J Chromatogr B Analyt Technol Biomed Life Sci. 2015;974:42–47. doi:10.1016/j.jchromb.2014.10.025
23. Liu H, Yang J, Du F, et al. Absorption and disposition of ginsenosides after oral administration of panax notoginseng extract to rats. Drug Metab Dispos. 2009;37(12):2290–2298. doi:10.1124/dmd.109.029819
24. Ruan JQ, Leong WI, Yan R, Wang YT. Characterization of metabolism and in vitro permeability study of notoginsenoside R1 from radix notoginseng. J Agric Food Chem. 2010;58(9):5770–5776. doi:10.1021/jf1005885
25. Wei Y, Li P, Fan H, et al. Metabolism study of notoginsenoside R1, ginsenoside Rg1 and ginsenoside Rb1 of radix panax notoginseng in zebrafish. Molecules. 2011;16(8):6621–6633. doi:10.3390/molecules16086621
26. Tian Z, Pang H, Zhang Q, et al. Effect of aspirin on the pharmacokinetics and absorption of panax notoginseng saponins. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1074–1075:25–33. doi:10.1016/j.jchromb.2017.12.033
27. Chen XN, Li DQ, Zhao MD, et al. Pharmacokinetics of panax notoginseng saponins in adhesive and normal preparation of fufang danshen. Eur J Drug Metab Pharmacokinet. 2018;43(2):215–225. doi:10.1007/s13318-017-0433-y
28. Zhang B, Zhang Q, Liu M, et al. Increased involvement of panax notoginseng in the mechanism of decreased hepatotoxicity induced by tripterygium wilfordii in rats. J Ethnopharmacol. 2016;185:243–254. doi:10.1016/j.jep.2016.03.027
29. Wen XD, Yang J, Ma RH, et al. Analysis of panax notoginseng metabolites in rat bile by liquid chromatography-quadrupole time-of-flight mass spectrometry with microdialysis sampling. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;895–896:162–168. doi:10.1016/j.jchromb.2012.03.009
30. Jin D, Wang B, Hu R, et al. A novel colon-specific osmotic pump capsule of Panax notoginseng Saponins (PNS): formulation, optimization, and in vitro-in vivo evaluation. AAPS PharmSciTech. 2018;19(5):2322–2329. doi:10.1208/s12249-018-1068-2
31. Song M, Zhang S, Xu X, Hang T, Jia L. Simultaneous determination of three panax notoginseng saponins at sub-nanograms by LC-MS/MS in dog plasma for pharmacokinetics of compound danshen tablets. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878(32):3331–3337. doi:10.1016/j.jchromb.2010.10.007
32. Yin S, Cheng Y, Li T, Dong M, Zhao H, Liu G. Effects of notoginsenoside R1 on CYP1A2, CYP2C11, CYP2D1, and CYP3A1/2 activities in rats by cocktail probe drugs. Pharm Biol. 2016;54(2):231–236. doi:10.3109/13880209.2015.1029051
33. Zhang J, Han X, Li X, et al. Core-shell hybrid liposomal vesicles loaded with panax notoginsenoside: preparation, characterization and protective effects on global cerebral ischemia/reperfusion injury and acute myocardial ischemia in rats. Int J Nanomedicine. 2012;7:4299–4310. doi:10.2147/IJN.S32385
34. Cai H, Wen X, Wen L, et al. Enhanced local bioavailability of single or compound drugs delivery to the inner ear through application of PLGA nanoparticles via round window administration. Int J Nanomedicine. 2014;9:5591–5601. doi:10.2147/IJN
35. Feng H, Chen W, Zhu C. Pharmacokinetics study of bio-adhesive tablet of panax notoginseng saponins. Int Arch Med. 2011;4(1):18. doi:10.1186/1755-7682-4-18
36. Wang S, Zang W, Zhao X, et al. Effects of borneol on pharmacokinetics and tissue distribution of notoginsenoside R1 and ginsenosides Rg1 and Re in Panax notoginseng in rabbits. J Anal Methods Chem. 2013;2013:706723. doi:10.1155/2013/706723
37. Li Y, Yang D, Zhu C. Impact of sodium N-[8-(2-Hydroxybenzoyl)amino]-caprylate on intestinal permeability for notoginsenoside R1 and salvianolic acids in Caco-2 cells transport and rat pharmacokinetics. Molecules. 2018;23:11. doi:10.3390/molecules23112990
38. Fan Q, Zhang Y, Hou X, et al. Improved oral bioavailability of notoginsenoside R1 with sodium glycocholate-mediated liposomes: preparation by supercritical fluid technology and evaluation in vitro and in vivo. Int J Pharm. 2018;552(1–2):360–370. doi:10.1016/j.ijpharm.2018.10.005
39. Chula S, Hang L, Yinying B, Jianning S, Shi R. The effects of notoginsenoside R(1) on the intestinal absorption of geniposide by the everted rat gut sac model. J Ethnopharmacol. 2012;142(1):136–143. doi:10.1016/j.jep.2012.04.027
40. Zhong L, Zhou XL, Liu YS, et al. Estrogen receptor alpha mediates the effects of notoginsenoside R1 on endotoxin-induced inflammatory and apoptotic responses in H9c2 cardiomyocytes. Mol Med Rep. 2015;12(1):119–126. doi:10.3892/mmr.2015.3394
41. Xiao J, Zhu T, Yin YZ, Sun B. Notoginsenoside R1, a unique constituent of panax notoginseng, blinds proinflammatory monocytes to protect against cardiac hypertrophy in ApoE(-/-) mice. Eur J Pharmacol. 2018;833:441–450. doi:10.1016/j.ejphar.2018.07.004
42. Ge ZR, Xu MC, Huang YU, Zhang CJ, Lin JE, Ruan CW. Cardioprotective effect of notoginsenoside R1 in a rabbit lung remote ischemic postconditioning model via activation of the TGF-beta1/TAK1 signaling pathway. Exp Ther Med. 2016;11(6):2341–2348. doi:10.3892/etm.2016.3222
43. Yu Y, Sun G, Luo Y, et al. Cardioprotective effects of notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress- related signaling pathways. Sci Rep. 2016;6:21730. doi:10.1038/srep21730
44. Liu Z, Wang H, Hou G, Cao H, Zhao Y, Yang B. Notoginsenoside R1 protects oxygen and glucose deprivation-induced injury by upregulation of miR-21 in cardiomyocytes. J Cell Biochem. 2019;120(6):9181–9192. doi:10.1002/jcb.v120.6
45. He K, Yan L, Pan CS, et al. ROCK-dependent ATP5D modulation contributes to the protection of notoginsenoside NR1 against ischemia-reperfusion-induced myocardial injury. Am J Physiol Heart Circ Physiol. 2014;307(12):H1764–H1776. doi:10.1152/ajpheart.00259.2014
46. Zhang W, Wojta J, Binder BR. Effect of notoginsenoside R1 on the synthesis of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in cultured human umbilical vein endothelial cells. Arterioscler Thromb. 1994;14(7):1040–1046. doi:10.1161/01.ATV.14.7.1040
47. Zhang HS, Wang SQ. Notoginsenoside R1 from panax notoginseng inhibits TNF-alpha-induced PAI-1 production in human aortic smooth muscle cells. Vascul Pharmacol. 2006;44(4):224–230. doi:10.1016/j.vph.2005.12.002
48. Zhang HS, Wang SQ. Notoginsenoside R1 inhibits TNF-alpha-induced fibronectin production in smooth muscle cells via the ROS/ERK pathway. Free Radic Biol Med. 2006;40(9):1664–1674. doi:10.1016/j.freeradbiomed.2006.01.003
49. Zhang B, Zhang J, Zhang C, et al. Notoginsenoside R1 protects against diabetic cardiomyopathy through activating estrogen receptor alpha and its downstream signaling. Front Pharmacol. 2018;9:1227. doi:10.3389/fphar.2018.01227
50. Jia C, Xiong M, Wang P, et al. Notoginsenoside R1 attenuates atherosclerotic lesions in ApoE deficient mouse model. PLoS One. 2014;9(6):e99849. doi:10.1371/journal.pone.0099849
51. Su P, Du S, Li H, Li Z, Xin W, Zhang W. Notoginsenoside R1 inhibits oxidized low-density lipoprotein induced inflammatory cytokines production in human endothelial EA.hy926 cells. Eur J Pharmacol. 2016;770:9–15. doi:10.1016/j.ejphar.2015.11.040
52. Fang H, Yang S, Luo Y, et al. Notoginsenoside R1 inhibits vascular smooth muscle cell proliferation, migration and neointimal hyperplasia through PI3K/Akt signaling. Sci Rep. 2018;8(1):7595. doi:10.1038/s41598-018-25874-y
53. Wang Y, Ren Y, Xing L, et al. Endothelium-dependent vasodilation effects of panax notoginseng and its main components are mediated by nitric oxide and cyclooxygenase pathways. Exp Ther Med. 2016;12(6):3998–4006. doi:10.3892/etm.2016.3890
54. Yang Y, Xi P, Xie Y, Zhao C, Xu J, Jiang J. Notoginsenoside R1 reduces blood pressure in spontaneously hypertensive rats through a long non-coding RNA AK094457. Int J Clin Exp Pathol. 2015;8(3):2700–2709.
55. Sun K, Wang CS, Guo J, et al. Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci. 2007;81(6):509–518. doi:10.1016/j.lfs.2007.06.008
56. Ning N, Dang X, Bai C, Zhang C, Wang K. Panax notoginsenoside produces neuroprotective effects in rat model of acute spinal cord ischemia-reperfusion injury. J Ethnopharmacol. 2012;139(2):504–512. doi:10.1016/j.jep.2011.11.040
57. Li H, Xiao J, Li X, et al. Low cerebral exposure cannot hinder the neuroprotective effects of panax notoginsenosides. Drug Metab Dispos. 2018;46(1):53–65. doi:10.1124/dmd.117.078436
58. Huang XP, Ding H, Lu JD, Tang YH, Deng BX, Deng CQ. Effects of the combination of the main active components of astragalus and panax notoginseng on inflammation and apoptosis of nerve cell after cerebral ischemia-reperfusion. Am J Chin Med. 2015;43(7):1419–1438. doi:10.1142/S0192415X15500809
59. Huang XP, Ding H, Wang B, et al. Effects of the main active components combinations of astragalus and panax notoginseng on energy metabolism in brain tissues after cerebral ischemia-reperfusion in mice. Pharmacogn Mag. 2015;11(44):732–739. doi:10.4103/0973-1296.165572
60. Sun Y, Liu B, Zheng X, Wang D. Notoginsenoside R1 alleviates lipopolysaccharide-triggered PC-12 inflammatory damage via elevating microRNA-132. Artif Cells Nanomed Biotechnol. 2019;47(1):1808–1814. doi:10.1080/21691401.2019.1610414
61. Wang Y, Tu L, Li Y, Chen D, Wang S. Notoginsenoside R1 protects against neonatal cerebral hypoxic-ischemic injury through estrogen receptor-dependent activation of endoplasmic reticulum stress pathways. J Pharmacol Exp Ther. 2016;357(3):591–605. doi:10.1124/jpet.115.230359
62. Wang Y, Tu L, Li Y, et al. Notoginsenoside R1 alleviates oxygen-glucose deprivation/reoxygenation injury by suppressing endoplasmic reticulum calcium release via PLC. Sci Rep. 2017;7(1):16226. doi:10.1038/s41598-017-16373-7
63. Tu L, Wang Y, Chen D, et al. Protective effects of notoginsenoside R1 via regulation of the PI3K-Akt-mTOR/JNK pathway in neonatal cerebral hypoxic-ischemic brain injury. Neurochem Res. 2018;43(6):1210–1226. doi:10.1007/s11064-018-2538-3
64. Meng X, Wang M, Wang X, et al. Suppression of NADPH oxidase- and mitochondrion-derived superoxide by notoginsenoside R1 protects against cerebral ischemia-reperfusion injury through estrogen receptor-dependent activation of Akt/Nrf2 pathways. Free Radic Res. 2014;48(7):823–838. doi:10.3109/10715762.2014.911853
65. Meng X, Sun G, Ye J, Xu H, Wang H, Sun X. Notoginsenoside R1-mediated neuroprotection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: a novel mechanism of Nrf2/ARE signaling activation. Free Radic Res. 2014;48(4):445–460. doi:10.3109/10715762.2014.885117
66. Gu B, Nakamichi N, Zhang WS, et al. Possible protection by notoginsenoside R1 against glutamate neurotoxicity mediated by N-methyl-D-aspartate receptors composed of an NR1/NR2B subunit assembly. J Neurosci Res. 2009;87(9):2145–2156. doi:10.1002/jnr.22021
67. Ma B, Meng X, Wang J, et al. Notoginsenoside R1 attenuates amyloid-beta-induced damage in neurons by inhibiting reactive oxygen species and modulating MAPK activation. Int Immunopharmacol. 2014;22(1):151–159. doi:10.1016/j.intimp.2014.06.018
68. Yan S, Li Z, Li H, Arancio O, Zhang W. Notoginsenoside R1 increases neuronal excitability and ameliorates synaptic and memory dysfunction following amyloid elevation. Sci Rep. 2014;4:6352. doi:10.1038/srep06352
69. Chen X, Wei R, Jin T, Du H. Notoginsenoside R1 alleviates TNF-alpha-induced pancreatic beta-cell Min6 apoptosis and dysfunction through up-regulation of miR-29a. Artif Cells Nanomed Biotechnol. 2019;47(1):2379–2388. doi:10.1080/21691401.2019.1624368
70. Zhang B, Zhang X, Zhang C, Shen Q, Sun G, Sun X. Notoginsenoside R1 protects db/db mice against diabetic nephropathy via upregulation of Nrf2-mediated HO-1 expression. Molecules. 2019;24:2.
71. Fan C, Qiao Y, Tang M. Notoginsenoside R1 attenuates high glucose-induced endothelial damage in rat retinal capillary endothelial cells by modulating the intracellular redox state. Drug Des Devel Ther. 2017;11:3343–3354. doi:10.2147/DDDT
72. Zhou P, Xie W, Meng X, et al. Notoginsenoside R1 ameliorates diabetic retinopathy through PINK1-dependent activation of mitophagy. Cells. 2019;8(3):213. doi:10.3390/cells8030213
73. Gui D, Wei L, Jian G, Guo Y, Yang J, Wang N. Notoginsenoside R1 ameliorates podocyte adhesion under diabetic condition through alpha3beta1 integrin upregulation in vitro and in vivo. Cell Physiol Biochem. 2014;34(6):1849–1862. doi:10.1159/000366384
74. Huang G, Lv J, Li T, et al. Notoginsenoside R1 ameliorates podocyte injury in rats with diabetic nephropathy by activating the PI3K/Akt signaling pathway. Int J Mol Med. 2016;38(4):1179–1189. doi:10.3892/ijmm.2016.2713
75. Huang G, Zou B, Lv J, et al. Notoginsenoside R1 attenuates glucose-induced podocyte injury via the inhibition of apoptosis and the activation of autophagy through the PI3K/Akt/mTOR signaling pathway. Int J Mol Med. 2017;39(3):559–568. doi:10.3892/ijmm.2017.2864
76. Chen Z, Li C, Yang C, Zhao R, Mao X, Yu J. Lipid regulation effects of raw and processed notoginseng radix Et rhizome on steatotic hepatocyte L02 cell. Biomed Res Int. 2016;2016:2919034.
77. Ji Z, Cheng Y, Yuan P, Dang X, Guo X, Wang W. Panax notoginseng stimulates alkaline phosphatase activity, collagen synthesis, and mineralization in osteoblastic MC3T3-E1 cells. In Vitro Cell Dev Biol Anim. 2015;51(9):950–957. doi:10.1007/s11626-015-9915-x
78. Wang C, Sun H, Zhong Y. Notoginsenoside R1 promotes MC3T3-E1 differentiation by up-regulating miR-23a via MAPK and JAK1/STAT3 pathways. Artif Cells Nanomed Biotechnol. 2019;47(1):603–609. doi:10.1080/21691401.2019.1573189
79. Liu Y, Lin Z, Guo J, et al. Notoginsenoside R1 significantly promotes in vitro osteoblastogenesis. Int J Mol Med. 2016;38(2):537–544. doi:10.3892/ijmm.2016.2652
80. Wang T, Wan D, Shao L, Dai J, Jiang C. Notoginsenoside R1 stimulates osteogenic function in primary osteoblasts via estrogen receptor signaling. Biochem Biophys Res Commun. 2015;466(2):232–239. doi:10.1016/j.bbrc.2015.09.014
81. Luo C, Sun Z, Li Z, Zheng L, Zhu X. Notoginsenoside R1 (NGR1) attenuates chronic atrophic gastritis in rats. Med Sci Monit. 2019;25:1177–1186. doi:10.12659/MSM.911512
82. Zhang J, Ding L, Wang B, et al. Notoginsenoside R1 attenuates experimental inflammatory bowel disease via pregnane X receptor activation. J Pharmacol Exp Ther. 2015;352(2):315–324. doi:10.1124/jpet.114.218750
83. Cong S, Xiang L, Yuan X, Bai D, Zhang X. Notoginsenoside R1 up-regulates microRNA-132 to protect human lung fibroblast MRC-5 cells from lipopolysaccharide-caused injury. Int Immunopharmacol. 2019;68:137–144. doi:10.1016/j.intimp.2018.12.065
84. Liu J, Hou C, Chen X, Wu L, Wang X. Notoginsenoside R1 protects human renal proximal tubular epithelial cells from lipopolysaccharide-stimulated inflammatory damage by up-regulation of miR-26a. Chem Biol Interact. 2019;308:364–371. doi:10.1016/j.cbi.2019.05.053
85. Wang CZ, Anderson S, Yuan CS. Phytochemistry and anticancer potential of notoginseng. Am J Chin Med. 2016;44(1):23–34. doi:10.1142/S0192415X16500026
86. Yang Q, Wang P, Cui J, Wang W, Chen Y, Zhang T. Panax notoginseng saponins attenuate lung cancer growth in part through modulating the level of Met/miR-222 axis. J Ethnopharmacol. 2016;193:255–265. doi:10.1016/j.jep.2016.08.040
87. Lin YW, Mou YC, Su CC, Chiang BH. Antihepatocarcinoma activity of lactic acid bacteria fermented panax notoginseng. J Agric Food Chem. 2010;58(15):8528–8534. doi:10.1021/jf101543k
88. Li Y, Li Z, Jia Y, Ding B, Yu J. In vitro anti-hepatoma activities of notoginsenoside R1 through downregulation of tumor promoter miR-21. Dig Dis Sci. 2019. doi:10.1007/s10620-019-05856-4
89. Wang CZ, Xie JT, Fishbein A, et al. Antiproliferative effects of different plant parts of panax notoginseng on SW480 human colorectal cancer cells. Phytother Res. 2009;23(1):6–13. doi:10.1002/ptr.2383
90. Yang BR, Hong SJ, Lee SM, et al. Pro-angiogenic activity of notoginsenoside R1 in human umbilical vein endothelial cells in vitro and in a chemical-induced blood vessel loss model of zebrafish in vivo. Chin J Integr Med. 2016;22(6):420–429. doi:10.1007/s11655-014-1954-8
91. Chen G, Li Q, Luo Y, et al. Effect of notoginsenoside R1 on autologous adipose graft in rats. Mol Med Rep. 2018;17(4):5928–5933. doi:10.3892/mmr.2018.8596
92. Sun HX, Chen Y, Ye Y. Ginsenoside Re and notoginsenoside R1: immunologic adjuvants with low haemolytic effect. Chem Biodivers. 2006;3(7):718–726. doi:10.1002/cbdv.200690074
93. Zhang J, Zheng Q, Lu H, et al. Notoginsenoside R1 protects human keratinocytes HaCaT from LPS-induced inflammatory injury by downregulation of Myd88. Int J Immunopathol Pharmacol. 2019;33:2058738419857550. doi:10.1177/2058738419857550
94. Pang HH, Li MY, Wang Y, Tang MK, Ma CH, Huang JM. Effect of compatible herbs on the pharmacokinetics of effective components of panax notoginseng in fufang xueshuantong capsule. J Zhejiang Univ Sci B. 2017;18(4):343–352. doi:10.1631/jzus.B1600235
95. Yang Z, Shao Q, Ge Z, Ai N, Zhao X, Fan X. A bioactive chemical markers based strategy for quality assessment of botanical drugs: xuesaitong injection as a case study. Sci Rep. 2017;7(1):2410. doi:10.1038/s41598-017-02305-y
96. Luo L, Kang J, He Q, et al. A NMR-based metabonomics approach to determine protective effect of a combination of multiple components derived from naodesheng on ischemic stroke rats. Molecules. 2019;24(9):1831. doi:10.3390/molecules24091831
97. Pintusophon S, Niu W, Duan XN, et al. Intravenous formulation of panax notoginseng root extract: human pharmacokinetics of ginsenosides and potential for perpetrating drug interactions. Acta Pharmacol Sin. 2019;40(10):1351–1363. doi:10.1038/s41401-019-0273-1
98. Li Y, Wang H, Wang R, et al. Pharmacokinetics, tissue distribution and excretion of saponins after intravenous administration of ShenMai injection in rats. J Chromatogr B Analyt Technol Biomed Life Sci. 2019;1128:121777. doi:10.1016/j.jchromb.2019.121777
99. Chen YY, Li Q, Pan CS, et al. QiShenYiQi Pills, a compound in Chinese medicine, protects against pressure overload-induced cardiac hypertrophy through a multi-component and multi-target mode. Sci Rep. 2015;5:11802. doi:10.1038/srep11802
100. Zheng QN, Wei XH, Pan CS, et al. QiShenYiQi Pills((R)) ameliorates ischemia/reperfusion-induced myocardial fibrosis involving RP S19-mediated TGFbeta1/Smads signaling pathway. Pharmacol Res. 2019;146:104272. doi:10.1016/j.phrs.2019.104272
101. Zhang C, Tong X, Qi B, et al. Components of panax notoginseng saponins enhance the cytotoxicity of cisplatin via their effects on gap junctions. Mol Med Rep. 2013;8(3):897–902. doi:10.3892/mmr.2013.1597
102. Limsuwanchote S, Wungsintaweekul J, Yusakul G, et al. Preparation of a monoclonal antibody against notoginsenoside R1, a distinctive saponin from panax notoginseng, and its application to indirect competitive ELISA. Planta Med. 2014;80(4):337–342. doi:10.1055/s-00000058
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.