Back to Journals » Journal of Inflammation Research » Volume 14
FNDC1 Polymorphism (rs3003174 C > T) Increased the Incidence of Coronary Artery Aneurysm in Patients with Kawasaki Disease in a Southern Chinese Population
Authors Lin K, Zhang L, Wang Y, Li J, Xu Y, Che D, Mai H , Yu H, Fu L, Wei B, Jiang Z, Pi L, Gu X
Received 20 March 2021
Accepted for publication 26 May 2021
Published 21 June 2021 Volume 2021:14 Pages 2633—2640
DOI https://doi.org/10.2147/JIR.S311956
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Kun Lin,1,2,* Linyuan Zhang,1,2,* Yishuai Wang,1– 3,* Jinqing Li,1 Yufen Xu,1 Di Che,1 Hanran Mai,4 Hongyan Yu,1 Lanyan Fu,1 Bing Wei,2 Zhiyong Jiang,2 Lei Pi,1 Xiaoqiong Gu1,2
1Department of Clinical Biological Resource Bank, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, Guangdong, 510623, People’s Republic of China; 2Department of Blood Transfusion and Clinical Lab, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, Guangdong, 510623, People’s Republic of China; 3School of Medicine, South China University of Technology, Guangzhou, Guangdong, 510006, People’s Republic of China; 4Department of Andrology, Guangzhou Women and Children’s Medical Center. Guangzhou Medical University, Guangzhou, Guangdong, 510623, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoqiong Gu; Lei Pi Email [email protected]; [email protected]
Background: A large number of studies demonstrated that the key to the occurrence and development of Kawasaki disease (KD) is the over-activation of immune cells and the generation of various inflammatory factors, leading to the imbalance of the immune system. Recently, mutations in the FNDC1 gene have been shown to be associated with inflammatory responses. However, there have been no reports on the relationship between FNDC1 gene and KD so far.
Methods: We enrolled 1611 controls and 1459 patients with KD, including 372 patients with coronary artery aneurysm (CAA) and 179 patients with coronary artery lesion (CAL). The relationship between FNDC1 rs3003174 polymorphism and KD with CAA or without CAA was investigated.
Results: This study showed no evidence that the association between FNDC1 rs3003174 C>T polymorphism and KD susceptibility was statistically significant (CT versus CC: adjusted odds ratio (OR) =0.897, 95% confidence interval (CI) =0.769– 1.045, P=0.162; TT versus CC: adjusted OR=0.995, 95% CI=0.786– 1.260, P=0.968; dominant model: adjusted OR=0.916, 95% CI=0.792– 1.059, P=0.235; and recessive model: adjusted OR=1.055, 95% CI=0.845– 1.316, P=0.638). However, our further stratified analysis in the control and KD group bore out that the incidence of TT genotype of FNDC1 rs3003174 C > T polymorphism was higher than that of CC/CT genotype in KD patients stratified by CAA (adjusted OR=1.437, 95% CI=1.034– 1.996, P=0.031). Moreover, a stratified analysis of age and gender in KD patients indicated that the rs3003174 TT genotype increased the risk of CAA formation in aged ≦60 months (CC/CT vs TT: adjusted OR=1.580, 95% CI=1.106– 2.259, P=0.012) and male (CC/CT vs TT: adjusted OR=1.653, 95% CI=1.101– 2.481, P=0.015) KD patients.
Conclusion: The results of this study demonstrated that the FNDC1 rs3003174 C>T polymorphism may be a hazard factor in the formation of CAA in KD patients that was not disclosed before.
Keywords: coronary artery aneurysm, Kawasaki disease, fibronectin type III domain containing 1, polymorphisms
Introduction
Kawasaki disease (KD) is an immune-mediated acute vasculitis syndrome with unknown etiology that mostly occurs in infants and children under 5 years old.1 The incidence of KD is higher in boys than in girls, compared to about 1.5 ~ 1.7:1.2 According to reports, the principal cause of pediatric acquired heart disease in developed countries is KD, with 25% of untreated cases leading to coronary artery aneurysm (CAA), which can increase the risk of sudden cardiac death and is a long-term potential life threat.1 So far, despite extensive investigations and studies, the cause of KD remains unknown, which limits its treatment options.3 However, there is growing evidence to support that hereditary factors may play a pivotal role in its occurrence and development.3,4 Studies declared that the incidence of KD varies from race to race, with children in Asian countries having a much higher rate than those in western countries.5 Furthermore, there is familial clustering. Siblings of children with KD have 10-times higher risk than general population, and children of parents who have suffered from KD are twice as likely to develop the disease.6 Some genes which were essential involved in the pathogenesis of cardiovascular diseases or inflammation have been confirmed to work on the onset and development of KD. As shown in our previous studies, there was a connection between P2RY12 rs7637803 genotype,7 PEAR1 Polymorphism (rs12041331)8 or Angiotensinogen Gene Polymorphism (rs5050)9 and the occurrence of CAA in children with KD. Simultaneously, a meta-analysis indicated that SLC8A1 gene polymorphism was not only associated with KD susceptibility but also with coronary artery abnormalities.10 ITPKC single-nucleotide polymorphism was also reported to be related to coronary artery lesion (CAL) and CAA formation in KD.11,12 These prior studies implied that KD was highly correlated with genetic background, thus providing a basis for further research on genetic variation that may be related to KD.
Fibronectin type III domain containing 1(FNDC1), located in human chromosome 6p25.3 region, encodes the conserved fibronectin (FN1) type III domain.13 As an important extracellular matrix protein, FN1 played a part in cell proliferation, migration and apoptosis as a regulator in tumorigenesis. It also participated in the pathogenesis of several blood and cardiovascular systems diseases.14,15 Related studies revealed that expression of FN1 in distant metastatic breast cancer cells was closely related to metalloproteinases in matrix mononuclear inflammatory cells.16 At present, there are few studies on the biological function of FNDC1, but the published reports indicated that FNDC1 might be critical in the functioning of the human inflammatory response. FNDC1, which initially came into existence as a differentially expressed mRNA in human skin fibroblasts, was deemed to be associated with the progression of skin tumors. It was induced by IL-1α, IL-1β, TGF-β1 and TNF-α in vitro, which together constituted the inflammatory microenvironment of tumor cells to help tumor metastasis and spread.17 Meanwhile, FNDC1 was found to be involved in VEGF-mediated angiogenesis in endothelial cells, especially in the distribution and transport of VEGF receptor 2 (VEFR-2), which had a strong tyrosine kinase activity and was a direct factor affecting angiogenesis.18,19 By binding to VEGFR-2, VEGF dimerized the receptor to directly inhibit its activity and interfere with the transmission of downstream signal molecules, subsequently inhibiting angiogenesis.18 The existing studies also displayed that FNDC1 was a pathogenic gene of acute otitis media in children,20 and had a certain correlation with hypertension.21 In summary, the literature on FNDC1 indicated that FNDC1 may be related to inflammation in the body.
Although the pathogenic mechanism of KD is still a mystery, a large number of studies have observed that KD is a kind of vasculitis syndrome with immune response imbalance and abnormal expression of various cytokines, such as VEGF, MMP-3, MMP-9, and CD40L.3,22 Moreover, CAA after KD was formed by the gradual development of coronary vascular inflammation. Therefore, it is reasonable to speculate that FNDC1 gene may truly work in the pathogenesis of KD by altering immune or inflammatory responses. However, as far as we know, the relationship between FNDC1 gene and KD has not been reported. Therefore, in this study, we will focus on whether there is a correlation between the polymorphism of FNDC1 rs3003174 C>T gene and the events of KD.
Materials and Methods
Ethics Statement
The study was approved by the Ethics Review Committee of Guangzhou Women and Children Medical Center (2014073009). All the Kawasaki disease patients and the control group were given detailed information about the purpose of the study and signed an informed consent form. The study was conducted in accordance with the Declaration of Helsinki.
Subjects
From 2014 to 2019, 1459 children with Kawasaki disease that were diagnosed according to the criteria from the American Heart Association (AHA)23 were recruited from Guangzhou Women and Children’s Medical Center. We tracked the coronary artery results of all patients with echocardiography for 3 months or more, and took the most severe degree of coronary artery damage within three months to group under the guidance of AHA standards.23 All children were Han Chinese without blood relationship. The control group consisted of 1611 healthy children matched with the gender and age of children with KD were randomly selected from children who underwent routine physical examination at the same time.
DNA Extraction
Two-milliliter peripheral blood samples of each participant were collected, and then Genomic DNA Extraction Kit (Tiangen, Beijing, China) was used to extract genomic DNA in accordance with the manufacturer’s instructions. Genomic DNA was measured for concentration and quality using nucleic acid quantifier, and then stored at −80°C for SNP genotyping later.
SNP Genotyping
GeneAmp PCR System 9700 (Thermo Fisher Scientific) was applied to genotype high-quality genomic DNA samples in 384-well plates. The genotyping kit was TIANexact genotyping qPCR premix (probe) produced by TIANGEN in Beijing, China. The PCR amplification procedure was performed as follows: denaturing at 95°C for 5 min, followed by 45 cycles of denaturing at 95°C for 15s and annealing and extension at 60°C for 1 min. The primers and probes were purchased from Thermo Fisher Scientific.
Statistical Analysis
We first tested hardy Weinberg equilibrium (HWE) in the control group by chi-squared goodness-of-fit test. Pearson chi square test was used to evaluate the distribution of demographic variables and genotype frequency in case and control groups. Unconditional logistic regression analyses, odds ratio (ORS) and 95% confidence interval (CI) were used to analyze the relationship between rs3003174 and KD susceptibility. In order to avoid the bias of genetic model, the analysis was put into practice under dominant, recessive and additive models, respectively. Simultaneously, we conducted a stratified analysis of subgroup data, such as gender, age and coronary artery results. SAS software (Version 9.1; SAS Institute, Cary, NC, USA) was adopted for statistical analysis of all data and P value <0.05 was considered statistically significant.
Results
Characteristics of the Subjects
As shown in Table 1, we included 1459 children with KD and 1611 controls in our study. The average age of children with KD was 26.97±22.42 months (range 1.00–156.00), while it was 33.57±24.07 months (range 1.00–168.00) for controls. Meanwhile, males and females accounted for 63.26% (923 cases) and 36.74% (536 cases) of the KD group, respectively. There were no statistical differences in age (P=0.864) and gender (P=0.285) between KD patients and controls. Among the KD cases, CAA was present in 372 cases (25.50%) and CAL was present in 196 cases (13.43%).
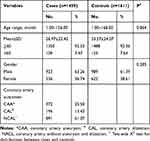 |
Table 1 Characteristics of the Subjects |
Analysis of Association Between FNDC1 rs3003174 C>T Polymorphism and the Risk of KD
The genotype distributions of FNDC1 rs3003174 C>T polymorphism in the KD and control group were described in detail in Table 2. We analyzed all the data under the condition that the distribution of FNDC1 rs3003174C>T polymorphism in the control group met Hardy–Weinberg equilibrium (P=0.051). However, it could be concluded that no significant correlation was observed between FNDC1 rs3003174 C>T polymorphism and KD susceptibility (CT vs CC: adjusted OR=0.897, 95% CI=0.769–1.045, P=0.162; TT vs CC: adjusted OR=0.995, 95% CI=0.786–1.260, P=0.968; dominant model: adjusted OR=0.916, 95% CI=0.792–1.059, P=0.235; and recessive model: adjusted OR=1.055, 95% CI=0.845–1.316, P=0.638).
 |
Table 2 Genotype and Allele Frequencies of FNDC1 in KD Patients and Controls |
Stratification Analysis of FNDC1 Polymorphisms in KD Patients and Controls
KD is correlated with age and gender, and coronary artery disease is one of its complications. While, FNDC1 has been reported to be associated with angiogenesis. Therefore, with the control group as a reference, we further analyzed the inheritance model of FNDC1 rs3003174 in the stratification of different ages, genders, and severity of coronary artery disease in the KD group. The results in Table 3 manifested that the incidence of TT genotype of FNDC1 rs3003174 C>T polymorphism was higher than that of CC/CT genotype in KD patients stratified by CAA (adjusted OR=1.437, 95% CI=1.034–1.996, P=0.031). We also analyzed subgroups stratified by gender (age-adjusted), subgroups stratified by age (gender-adjusted), but no other significant relationships were suggested in our analysis.
 |
Table 3 Stratification Analysis of FNDC1 Polymorphisms in KD Patients and Controls |
Analysis of Association Between FNDC1 rs3003174 C>T Polymorphism and CAA Susceptibility in KD
KD is a vasculitis disease characterized by a low onset-age and a high male-proportion. As revealed in Table 3, compared with controls, FNDC1 rs3003174 TT genotype was a risk factor for KD with CAA. Hence, we conducted a stratified analysis of FNDC1 rs3003174 C>T polymorphism in KD CAA and NCAA groups (Tables 4 and 5). Table 4 displayed that in KD patients, the FNDC1 rs3003174 TT genotype was associated with an increased risk of CAA (TT vs CC: adjusted OR=1.547, 95% CI=1.067–2.243, P=0.021; dominant model: adjusted OR=1.098, 95% CI=0.861–1.401, P=0.449; and recessive model: adjusted OR=1.551, 95% CI=1.097–2.193, P=0.013). Meanwhile, we also found that the FNDC1 rs3003174 TT genotype increased in aged ≦60 months (CC/CT vs TT: adjusted OR=1.580, 95% CI=1.106–2.259, P=0.012) and male (CC/CT vs TT: adjusted OR=1.653, 95% CI=1.101–2.481, P=0.015) KD patients (Table 5).
 |
Table 4 Genotype and Allele Frequencies of FNDC1 in KD Patients with or without CAA |
 |
Table 5 Stratification Analysis of FNDC1 Polymorphisms in KD Patients with or without CAA |
Discussion
Kawasaki disease, also called mucosal lymph node syndrome, has been reported in most countries or regions in the world since it was first described by Japanese doctor Tomi Kawasaki in 1967.22 With the in-depth study of KD, growing evidence reveals that its pathogenesis and development are inseparable from genetic factors.3,4 In our study, we compared 1459 controls and 1611 cases in the southern Chinese population, and found that the rs3003174 polymorphism of the FNDC1 gene had no relevance to KD susceptibility. However, it was very interesting that in our further stratified analysis, we detected that the FNDC1 rs3003174 C>T polymorphism was bound up with coronary aneurysm complications in KD.
The most serious complication in patients with KD was heart attack caused by coronary artery aneurysm whose underlying molecular mechanism had not been elucidated clearly.1,24 However, coronary artery dysfunction caused by cardiovascular inflammation that was induced by inflammatory factor infiltration was confirmed to be a leading cause of coronary artery lesion.24,25 In the process of angiogenesis and remodeling, there were close and complex interactions among vascular endothelial cells, extracellular matrix, inflammatory cells and related inflammatory factors. Studies had shown that FNDC1, also known as activator of G-protein signaling 8, was an independent helper protein receptor for the G protein β and γ subunits and expressed in different tissue types, including heart, thyroid, kidney and adipose tissue.26–28 Various signaling pathways involved in FNDC1 gene were interrelated, which were closely related to the inflammatory response and immune response. For example, it could activate PI3Kgamma to regulate inflammation by stimulating the production of neutrophils;29,30 in addition, it also activated RhoGef to participate in the process of lymphocyte chemotaxis and actin polymerization.31 FNDC1 isolated from rat hearts undergoing repeated transient ischemia with collateral circulation may be involved in inducing cardiomyocyte apoptosis under inflammatory or hypoxic conditions. Hypoxia induced FNDC1 mRNA expression in rat aortic smooth muscle cells, endothelial cells or myocardial fibroblasts, suggesting a cardiomyocyte-specific adaptive mechanism including G protein signaling pathway remodeling.28,32,33 A study found that knocking down FNDC1 by small interfering RNA could effectively inhibit the angiogenesis induced by VEGF, and also inhibit cell growth and migration.18 We previously made a hypothesis in the introduction that FNDC1 gene may influence the pathogenesis of KD, and data from this study further supported this supposition. It was seen from our analysis that compared with CC/CT genotype, KD patients with TT genotype of FNDC1 rs3003174 C>T polymorphism had a higher morbidity of CAA. Geng et al34 demonstrated that T allele of rs2392989, the SNP locus of FNDC1, was more prone to acute otitis media, and may be associated with lower methylation level of CG05678571 and higher expression of FNDC1 gene. In 2021, Bouchareb et al35 found that as the degree of calcification of aortic valve became more severe, the expression of FNDC1 in the extracellular matrix was increased. More interestingly, the metabolic enzymes of aortic valve were also down-regulated, leading to significant destruction of metabolic pathways. This observation indicated that the mutations of FNDC1 gene were related to vascular disease of the heart. To a certain degree, our results were consistent with these studies, which proved that the variants of FNDC1 gene may induce systemic or local inflammatory responses in vivo. Recently studies had widely shown that excessive activation of immune cells to produce plenty of inflammatory factors were the key to the onset and development of KD.36,37 Moreover, combined with our present data and the above mentioned studies on function of FNDC1 in inflammation and angiogenesis, we preliminarily inferred that the mechanism of FNDC1 gene mutation affecting KD depends on the loss of the complete function of FNDC1 protein, thereby affecting the signal transduction of the G protein signaling pathway that was critical to the blood vessel development and cellular inflammation. However, this is the first study on the relationship between FNDC1 rs3003174 C>T polymorphism and KD. Therefore, more studies on KD patients with CAA, combined with genomics, proteomics and clinical index analysis, are needed to further reveal the exact role FNDC1 plays in the pathogenesis of CAA in KD.
There are some limitations in this study that need to be improved. First of all, since the selected subjects of this study are only from the southern Chinese population, we lack KD patients and healthy controls from other regions. Second, this study only studied the rs3003174 C>T polymorphism of FNDC1 gene, and did not include other SNPs in FNDC1 gene that may potentially affect KD. Third, because this is a retrospective study, we only considered age and gender in the logistic regression analysis, ignoring the influence of other factors such as familial hereditary factors and birth history. Future research should consider these influencing factors to confirm the results of this study.
In general, 1459 controls and 1611 KD patients were recruited for the study. No data in the study revealed that there was a significant correlation between FNDC1 rs3003174 C>T polymorphism and KD susceptibility. Nevertheless, our results suggested that FNDC1 rs3003174 C>T polymorphism may be involved in the development of CAA. In other words, its genotype could be considered as a predictive biomarker of CAA in southern Chinese KD patients, especially male children younger than 60 months. Follow-up studies are needed to further investigate the mechanisms of FNDC1 rs3003174 C>T on KD CAA.
Acknowledgments
The authors would like to thank the Clinical Biological Resource Bank of Guangzhou Women and Children’s Medical Center for providing all the clinical samples.
Funding
This study was funded by the Guangdong Natural Science Fund, China (grant numbers 2019A1515012061, 2021A1515011207), the Guangzhou Science and Technology Program Project, China (grant numbers 201904010486, 202102010197), and the Guangzhou Institute of Pediatrics/Guangzhou Women and Children’s Medical Center Fund, China (Grant Number: GCP-2019-003, GCP-2019-006, and YIP-2019-050).
Disclosure
The authors report no conflicts of interest in this work.
References
1. McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. 2017;135(17):e927–e999. doi:10.1161/CIR.0000000000000484
2. Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Child. 2015;100(11):1084–1088. doi:10.1136/archdischild-2014-307536
3. Kumrah R, Vignesh P, Rawat A, et al. Immunogenetics of Kawasaki disease. Clin Rev Allergy Immunol. 2020;59(1):122–139. doi:10.1007/s12016-020-08783-9
4. Rife E, Gedalia A. Kawasaki Disease: an Update. Curr Rheumatol Rep. 2020;22(10):75. doi:10.1007/s11926-020-00941-4
5. Skochko SM, Jain S, Sun X, et al. Kawasaki disease outcomes and response to therapy in a multiethnic community: a 10-year experience. J Pediatr. 2018;203:e3. doi:10.1016/j.jpeds.2018.07.090
6. Shrestha S, Wiener HW, Aissani B, et al. Imputation of class I and II HLA loci using high-density SNPs from ImmunoChip and their associations with Kawasaki disease in family-based study. Int J Immunogenet. 2015;42(3):140–146. doi:10.1111/iji.12190
7. Lu Z, Xu Y, Fu L, et al. P2RY12:rs7637803TT variant genotype increases coronary artery aneurysm risk in Kawasaki disease in a southern Chinese population. J Gene Med. 2019;21(1):e3066. doi:10.1002/jgm.3066
8. Pi L, Xu Y, Fu L, et al. A PEAR1 polymorphism (rs12041331) is associated with risk of coronary artery aneurysm in Kawasaki disease. Ann Hum Genet. 2019;83(1):54–62. doi:10.1111/ahg.12285
9. Liu Y, Fu L, Pi L, et al. An Angiotensinogen Gene Polymorphism (rs5050) Is Associated with the Risk of Coronary Artery Aneurysm in Southern Chinese Children with Kawasaki Disease. Dis Markers. 2019;2019:2849695. doi:10.1155/2019/2849695
10. Shimizu C, Eleftherohorinou H, Wright VJ, et al. Genetic Variation in the SLC8A1 Calcium Signaling Pathway Is Associated with Susceptibility to Kawasaki Disease and Coronary Artery Abnormalities. Circ Cardiovasc Genet. 2016;9(6):559–568. doi:10.1161/CIRCGENETICS.116.001533
11. Onouchi Y, Gunji T, Burns JC, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40(1):35–42. doi:10.1038/ng.2007.59
12. Kuo HC, Hsu Y-W, Lo M-H, et al. Single-nucleotide polymorphism rs7251246 in ITPKC is associated with susceptibility and coronary artery lesions in Kawasaki disease. PLoS One. 2014;9(3):e91118. doi:10.1371/journal.pone.0091118
13. Ren J, Niu G, Wang X, et al. Overexpression of FNDC1 in Gastric Cancer and its Prognostic Significance. J Cancer. 2018;9(24):4586–4595. doi:10.7150/jca.27672
14. Halper J, Kjaer M. Basic Components of Connective Tissues and Extracellular Matrix: elastin, Fibrillin, Fibulins, Fibrinogen, Fibronectin, Laminin, Tenascins and Thrombospondins. Advances in Experimental Medicine and Biology. 2014;802:31–47. doi:10.1007/978-94-007-7893-1_3
15. Marchand M, Monnot C, Muller L, et al. Extracellular matrix scaffolding in angiogenesis and capillary homeostasis. Semin Cell Dev Biol. 2019;89:147–156. doi:10.1016/j.semcdb.2018.08.007
16. Fernandez-Garcia B, Eiró N, Marín L, et al. Expression and prognostic significance of fibronectin and matrix metalloproteases in breast cancer metastasis. Histopathology. 2014;64(4):512–522. doi:10.1111/his.12300
17. Anderegg U, Breitschwerdt K, Kohler MJ, et al. MEL4B3, a novel mRNA is induced in skin tumors and regulated by TGF-b and proinflammatory cytokines. Exp Dermatol. 2005;Sep;14(9(9):709–718. doi:10.1111/j.0906-6705.2005.00349.x
18. Hayashi H, Al Mamun A, Sakima M, et al. Activator of G-protein signaling 8 is involved in VEGF-mediated signal processing during angiogenesis. J Cell Sci. 2016;129(6):1210–1222. doi:10.1242/jcs.181883
19. Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: a Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011;2(12):1097–1105. doi:10.1177/1947601911423031
20. van Ingen G, Li J, Goedegebure A, et al. Genome-wide association study for acute otitis media in children identifies FNDC1 as disease contributing gene. Nat Commun. 2016;7(1):12792. doi:10.1038/ncomms12792
21. Schönbach C, Chauvet C, Ménard A, et al. Alterations in Fibronectin Type III Domain Containing 1 Protein Gene Are Associated with Hypertension. PLoS One. 2016;11(4):e0151399. doi:10.1371/journal.pone.0151399
22. Council on Cardiovascular Disease in the Young, C.o.R.F. Endocarditis, and Kawasaki Disease, American Heart Association, Diagnostic Guidelines for Kawasaki Disease. AHA Sci Statement. 2001.
23. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114(6):1708–1733. doi:10.1542/peds.2004-2182
24. Joshi M, Tulloh R. Kawasaki disease and coronary artery aneurysms: from childhood to adulthood. Future Cardiol. 2017;Sep;13(5:491–501. doi:10.2217/fca-2017-0039
25. Noval Rivas M, Arditi M. Kawasaki disease: pathophysiology and insights from mouse models. Nat Rev Rheumatol. 2020;16(7):391–405.
26. To WS, Midwood KS. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis Tissue Repair. 2011;4:21.
27. Lapointe J, Li C, Higgins JP, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101(3):811–816. doi:10.1073/pnas.0304146101
28. Sato M, Jiao Q, Honda T, et al. Activator of G protein signaling 8 (AGS8) is required for hypoxia-induced apoptosis of cardiomyocytes: role of G betagamma and connexin 43 (CX43). J Biol Chem. 2009;284(45):31431–31440. doi:10.1074/jbc.M109.014068
29. Stephens L, Smrcka A, Cooke FT, et al. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell. 1994;77(1):83–93. doi:10.1016/0092-8674(94)90237-2
30. Hirsch E. Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science. 2000;287(5455):1049–1053. doi:10.1126/science.287.5455.1049
31. Runne C, Chen S. PLEKHG2 promotes heterotrimeric G protein βγ-stimulated lymphocyte migration via Rac and Cdc42 activation and actin polymerization. Mol Cell Biol. 2013;Nov;33(21:4294–4307. doi:10.1128/MCB.00879-13
32. Sato M, Cismowski MJ, Toyota E, et al. Identification of a receptor-independent activator of G protein signaling (AGS8) in ischemic heart and its interaction with Gbetagamma. Proc Natl Acad Sci U S A. 2006;103(3):797–802. doi:10.1073/pnas.0507467103
33. Yuan C, Sato M, Lanier SM, et al. Signaling by a Non-dissociated Complex of G Protein βγ and α Subunits Stimulated by a Receptor-independent Activator of G Protein Signaling, AGS8. J Biol Chem. 2007;282(27):19938–19947. doi:10.1074/jbc.M700396200
34. Geng R, Wang Q, Chen E, et al. Current Understanding of Host Genetics of Otitis Media. Front Genet. 2019;10:1395. doi:10.3389/fgene.2019.01395
35. Bouchareb R, Guauque-Olarte S, Snider J, et al. Proteomic Architecture of Valvular Extracellular Matrix: FNDC1 and MXRA5 Are New Biomarkers of Aortic Stenosis. JACC Basic Transl Sci. 2021;6(1):25–39. doi:10.1016/j.jacbts.2020.11.008
36. Li Y, Zheng Q, Zou L, et al. Kawasaki disease shock syndrome: clinical characteristics and possible use of IL-6, IL-10 and IFN-gamma as biomarkers for early recognition. Pediatr Rheumatol Online J. 2019;17(1):1. doi:10.1186/s12969-018-0303-4
37. Burns JC. History of the worldwide emergence of Kawasaki disease. Int J Rheum Dis. 2017.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
