Back to Journals » International Journal of General Medicine » Volume 15
FMNL3 is Overexpressed in Tumor Tissues and Predicts an Immuno-Hot Phenotype in Pancreatic Cancer
Authors Zhang Q, Nie H, Pan J, Xu H , Zhan Q
Received 29 July 2022
Accepted for publication 10 November 2022
Published 22 November 2022 Volume 2022:15 Pages 8285—8298
DOI https://doi.org/10.2147/IJGM.S384195
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Qinglin Zhang,* He Nie,* Jiadong Pan, Haoran Xu, Qiang Zhan
Department of Gastroenterology, The Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi, Jiangsu, 214023, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qinglin Zhang; Qiang Zhan, Email [email protected]; [email protected]
Background: FMNL3 (Formin-like protein 3) is involved in the tumorigenesis of multiple cancers. The critical role of FMNL3 in malignancies has been preliminarily explored, but its immunological correlation is not clear.
Methods: A pan-cancer analysis was performed to investigate the expressions, prognostic values, and immunological roles of FMNL3 across cancer types in The Cancer Genome Atlas (TCGA) database. Next, the correlations between FMNL3 and immunological features in the tumor microenvironment (TME) of pancreatic cancer (PAAD) were assessed. Besides, the role of FMNL3 in predicting the clinical characteristics and the responses to various therapies in PAAD was evaluated as well. Besides, the correlations between FMNL3 and the emerging immune-related biomarkers were also evaluated.
Results: The pan-cancer analysis uncovered inconsistent expression status and prognostic values in several cancers. Besides, FMNL3 exhibited positive correlations with a majority of immunomodulators and tumor-infiltrating immune cells (TIICs) in several cancer types, including PAAD. In addition, FMNL3 was associated with an inflamed phenotype in the TME and predicted significantly higher responses to multiple anti-cancer therapies. In addition, FMNL3 was notably correlated with immune-related microbiota and N6-methyladenosine (m6A) genes.
Conclusion: In summary, FMNL3 predicts an immuno-hot phenotype, which could be a promising indicator for identifying high immunogenicity in PAAD.
Keywords: FMNL3, PAAD, tumor microenvironment, bioinformatics analysis, formin
Introduction
Pancreatic cancer (PAAD) is a cancerous disease that features with lethal aggressiveness, and the five-year overall survival (OS) rate for which is the lowest among all cancers in the United States.1 Even with surgical resection, the five-year OS rate for PAAD could be improved to 10–25% merely.2 Therefore, it is urgent to seek more promising therapeutic options. In recent years, with the application of immune checkpoint inhibitors (ICIs), immunotherapy has developed rapidly and has gradually changed the therapeutic standard.3 However, the single therapies targeting programmed death 1 or programmed death 1 ligand 1 (PD-1/PD-L1) showed poor therapeutic effects for PAAD patients who are known to have low immunogenicity and an immunosuppressive tumor microenvironment (TME).4,5
Formin-like protein 3 (FMNL3) is a member of the formin family, which interacts with filamentous actin and microtubules.6 FMNL3 might be implicated in polarity control, invasion, and migration through the regulation of the Rho-related signaling pathway.7 Wu et al found that FMNL3 participated in TGF-β1-induced epithelial-to-mesenchymal transition (EMT) and thus promoted nasopharyngeal carcinoma cell metastasis.8 In tongue squamous cell carcinoma, high expression of FMNL3 promoted cancer cell migration and invasion, and also predicted poor prognosis.9 In addition, FMNL3 acted as a cytoskeletal regulator in angiogenesis.10 In colorectal carcinoma, FMNL3 regulated RhoC/FAK pathway and actin assembly to promote cell migration and invasion.11 Overall, existing evidence suggests that FMNL3 plays an oncogenic role in multiple cancers by the function in the actin and microtubule, and could be a promising therapeutic target. However, a systematic pan-cancer analysis of the role of FMNL3 in prognoses and TME has not been conducted.
In the current research, we carried out a pan-cancer analysis to investigate the expression patterns and immunological roles of FMNL3. We found that FMNL3 was closely related to immunological factors in multiple cancers, especially in PAAD. Our further analysis revealed that high FMNL3 expression predicted an inflammatory TME and more therapeutic options in PAAD. Overall, these results suggest that FMNL3 could be a novel immuno-biomarker in PAAD.
Methods
Transcriptome Data Acquisition
The pan-cancer normalized RNA sequencing (RNA-seq) datasets and clinical annotations of The Cancer Genome Atlas (TCGA) were downloaded from the UCSC Xena portal (https://xenabrowser.net/datapages/). The abbreviations for various cancer types are given in Table S1. To explore microorganisms associated with anti-tumor immunity in PAAD, we downloaded the relative abundance data of ~1400 microorganism from the online data portal, ciboPortal (http://www.cbioportal.org/).12
GEPIA Database Analysis
The GEPIA database (http://gepia.cancer-pku.cn/) is an interactive web resource that is based on the TCGA dataset, which utilizes RNA-seq to conduct gene expression analyses.13 In the present study, the GEPIA website was used to investigate the expression levels of FMNL3 in tumor and para-tumor tissues across cancer types.
Association Between FMNL3 Expression and the Immunological Characteristics
Given that the tumor tissues which were subjected to RNA-seq included tumor cells and other cells, such as immune cells, we assessed the immunological characteristics of the TME for each patient from the TCGA dataset. ESTIMATE was used to estimate the Tumor Purity, ESTIMATE Score, Immune Score and Stromal Score,14 while five independent algorithms, including TIMER,15 EPIC,16 MCP-counter,17 quanTIseq18 and TISIDB,19 were utilized to compute the relative abundance of TIICs comprehensively. Besides, the information on 122 immunomodulators, well-known effector genes of tumor-infiltrating immune cells (TIICs), and 18 specific genes correlated with T cell inflammation and their weighting coefficients were also collected from previous studies.20,21 Furthermore, considering that each stage of the cancer-immune cycle plays a crucial role in reflecting the anti-cancer immune response and deciding the destiny of tumor cells, we next calculated the activities of each stage by single-sample gene set enrichment analysis (ssGSEA) according to the expression level of stage-specific signatures.22 To investigate the role of FMNL3 in mediating cancer immunity in PAAD, we divided the patients into the high and low FMNL3 groups at the 50% cut-off criterion according to the expression levels of FMNL3, and then compared the differences in immunological features of TME between the high and low FMNL3 subtypes.
Calculations of the Enrichment Scores of Various Gene Signatures
Referring to a previous study,23 we collected several gene sets positively correlated with anti-tumor immunity, such as genes involved in DNA replication and hypoxia, and gene sets that predicted therapeutic responses, such as EGFR ligands. The enrichment scores of these signatures were calculated using the ssGSEA algorithm.24 Next, the correlations between FMNL3 and these enrichment scores were evaluated.
Prediction of Anti-Tumor Therapeutic Response
Subsequently, we assessed the role of FMNL3 in predicting the responses to anti-tumor therapies. The drug-target genes were derived from the DrugBank database (https://www.drugbank.com/) and then the difference of their expression was compared between the high and low FMNL3 groups. Furthermore, the R package “pRRophetic” was utilized to predict the response scores of several common chemotherapeutic drugs for each patient according to the Cancer Genome Project database (https://www.sciencedirect.com/topics/neuroscience/cancer-genome-project).25 In the prediction process, the sample’s half-maximal inhibitory concentration (IC50) was estimated by ridge regression and the prediction accuracy was evaluated by 10-fold cross-validation based on the CGP training set. Default options were used for all parameters.
Discovery of Immune-Related Tumor Microorganisms
To explore microorganisms that were associated with anti-tumor immunity in PAAD, we downloaded the relative abundance data of ~1400 microorganisms from the online data portal, ciboPortal (http://www.cbioportal.org/),12 and selected the microorganism whose absolute Pearson’s correlation with the T cell inflamed score ≥ 0.3 or ≤ −0.3 and P-value ≤ 0.05. Then, the associations between FMNL3 and these immune-related tumor microorganisms were evaluated.
Identification of Immune-Related m6A Genes
According to a recent publication,26 we extracted 64 m6A regulators and m6A interactive protein-coding genes with high coexpression tendencies, and defined as immune-related m6A genes with the criterion of Pearson’s correlation with the T cell inflamed score ≥ 0.3 or ≤ −0.3 and P-value ≤ 0.05. Subsequently, the associations between FMNL3 and these immune-related m6A genes were assessed.
Clinical Samples
To validate the expression of FMNL3 in PAAD and its correlations with immunological factors, a PAAD tissue microarray (TMA, HPanA150CS02) was obtained from Outdo Biotech (Shanghai, China) and next was submitted for immunohistochemistry (IHC) staining. The HPanA150CS02 microarray contained 78 PAAD and 72 para-tumor samples. Ethical approval (YBM-0502) for the study of TMA was granted by the Clinical Research Ethics Committee, Outdo Biotech.
IHC Staining
IHC staining was conducted directly on the TMA with standard procedures. The primary antibodies used in the current studies were as follows: anti-FMNL3 (1:2000 dilution, Cat. ab222797, Abcam, Cambridge, UK), anti-PD-L1 (1:100 dilution, Cat.ab237726, Abcam, Cambridge, UK), and anti-CD8 (Ready-to-use, Cat. PA067, Abcarta, Suzhou, China). Antibody staining was visualized using diaminobenzidine (DAB) and hematoxylin counterstain, and the stained TMA was scanned using Aperio Digital Pathology Slide Scanners. For the semiquantitative assessment of FMNL3 and PD-L1 staining, the immunoreactivity score (IRS) was used according to the previous description.27,28 For semiquantitative evaluation of CD8 staining, the proportion of CD8+ cells was assessed by estimating the percentage of cells with strong intensity of membrane staining in the stroma cells.29
Cell Culture and Transfection
The human PAAD cell line PANC-1 verified mycoplasma negative was obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China) and cultured with DMEM (Cat. KGM31800, KeyGEN, Jiangsu, China) medium containing 10% FBS (Cat. 10099141C, Gibco, New York, USA) at 37 ° C with 5% carbon dioxide. For FMNL3 gene knockdown, we transfected cells with Lipo2000 and siRNA according to the manufacturer’s instructions. The sequence was as follows: sense 2, 5′-GCAUCAAGGAGACAUAUGATT-3′, antisense 2, 5′-UCAUAUGUCUCCUUGAUGCTT-3′. Transfection efficiency was evaluated by Western blot.
Western Blot
At 48 hours after transfection, negative control and FMNL3 konckdown cells were lysed to extract total protein. Western blot analysis were then performed according to the standard protocols. The primary antibodies for anti-FMNL3 (1:1000 dilution, Cat. ab224185, Abcam, Cambridge, UK), anti-PD-L1 ((1:1000 dilution, Cat.ab237726, Abcam, Cambridge, UK), and Tubulin (1:2000 dilution, Cat. 11224-1-AP, ProteinTech, Rosemont, USA) were applied.
Statistical Analysis
Statistical analysis and figure exhibition were performed using R language 3.6.0 and Graphpad Prism 6.0. Pearson’s correlation coefficients were used for correlation analysis. Wilcoxon rank-sum test or unpaired t-test were performed to measure the difference in continuous variables between groups, while Fisher’s exact test was utilized for categorical variables. All tests were two-sides applied and P-value ≤ 0.05 was considered as the statistical significance and labeled with * P-value ≤ 0.05, ** P-value ≤ 0.01, *** P-value ≤ 0.001, and **** P-value ≤ 0.0001.
Results
FMNL3 Expression, Prognostics Values, and Immunological Roles in Pan-Cancer
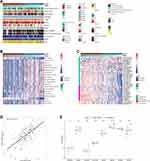
First of all, we utilized the GEPIA database to analyze the expression of FMNL3 in pan-cancers. The results showed that FMNL3 was highly expressed in several tumors, such as DLBC, ESCA, GBM, HNSC, etc., while lowly expressed in LUAD, LUSC, OV, PRAD, etc. (Figure 1A). Next, we also analyzed the prognostic values of FMNL3 in pan-cancers. Low FMNL3 expression was significantly associated with better prognosis in LGG, COAD, and so on, but associated with poor prognosis in several cancers, such as LUAD, HNSC, and ACC (Figure 1B). The pan-cancer analysis about the immunological role of FMNL3 was performed as well. FMNL3 was positively correlated with a majority of TIICs in most cancers (Figure 1C). Similarly, FMNL3 was also positively related to most immunomodulators (Figure 1D). Together, FMNL3 was highly expressed and positively related to most TIICs as well as immunomodulators in PAAD. Considering the inconsistent expression statuses and prognostic values, along with immunological correlations, we selected PAAD for further research to reveal the clinical value of FMNL3.
FMNL3 Shapes an Inflamed TME and Predicts an Immuno-Hot Phenotype in PAAD
Having observated the positive correlation between FMNL3 and a huge number of immunomodulators in PAAD, we next explored the immunological effects of FMNL3. As shown in Figure 2A and B, most MHC molecules, including MHC I and MHC II class genes, were upregulated in the high FMNL3 group, indicating the enhanced capacity of antigen presentation and processing in these patients. Moreover, PAAD patients with high FMNL3 expression had an enhanced transcription of chemokines and paired receptors such as CCL1 and CXCL1 (Figure 2A and B), which can increase the recruitment of effector TIICs. Besides, a majority of immunostimulators such as CD28 and CD80 also increased in the high FMNL3 group (Figure 2A and B). Furthermore, the ESTIMATE analysis showed that compared with the low FMNL3 group, the FMNL3 group exhibited a higher ESTIMATE Score, Immune Score, and Stromal Score, but lower Tumor Purity (Figure 2C). All findings above revealed that tumors with high FMNL3 expression were accompanied by increased TIICs.
Since previous studies demonstrated that the expression of inhibitory immune checkpoints, such as PD-1/PD-L1 was uncovered to be enhanced in the inflamed TME,30 we investigated the correlation between immune checkpoints and FMNL3. Result showed that FMNL3 was highly related to most immune checkpoints in PAAD (Figure 3A). We subsequently compared the signatures of common immune cells such as CD8A for CD8+ T cells and MPP8 for macrophages between the high and low FMNL3 groups, and found that these markers were overexpressed in PAAD patients with high FMNL3 (Figure 3B). Then, we calculated the infiltration levels of TIICs utilizing five independent algorithms (Figure 3C). In line with the previous results, PAAD patients with high FMNL3 expression were accompanied by increased infiltration levels of TIICs such as T cells and macrophages in different algorithms (Figure 3C). In addition, in the high FMNL3 group, the activities of the majority of the steps in the cycle were remarkably enhanced, especially the phases of recruiting immune cells, such as T cell recruiting, CD4 T cell recruiting, and so on (Figure 3D). Collectively, our analysis suggested that PAAD patients with high FMNL3 had a more activated immune response, indicating that FMNL3 was highly correlated with an inflamed TME in PAAD.
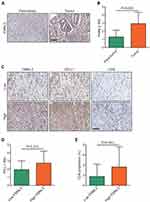
FMNL3 is Associated with Immuno-Hot Phenotypes in the TMA Cohort
We used the IHC staining to validate the expression and immunological role of FMNL3 in PAAD. As revealed, the expression of FMNL3 was upregulated in tumor tissues compared to para-tumor tissues (Figure 4A and B). We also evaluated the expression of PD-L1 and the infiltration level of CD8+ T cells using IHC staining in PAAD tissues (Figure 4C). In addition, when FMNL3 was knocked down, the expression of PD-L1 protein also inhibited (Figure S1). Next, the current PAAD cohort was divided into low (IRS≤2) and high FMNL3 expression (IRS≥2) groups based on the approximate median level of FMNL3 expression. Notably, the expression of PD-L1 and the infiltration level of CD8+ T cells were both higher in the high FMNL3 group than the low FMNL3 group (Figure 4D–E). Overall, the overexpression of FMNL3 and its immunological role in PAAD were validated in an independent cohort.
FMNL3 Predicts Therapeutic Opportunities in PAAD
To figure out the association between FMNL3 level and clinical characteristics of PAAD patients, we compared several clinicopathological features such as grade and primary therapy outcome between the high and low FMNL3 groups, and found that FMNL3 expression was not associated with any clinicopathological features (Figure 5A, Table S2). Since FMNL3 shapes an inflamed TME, patients with higher FMNL3 should have improved responses to anti-tumor therapy theoretically, including chemotherapy and immunotherapy. Consistent with theory, PAAD patients with high FMNL3 had higher enrichment scores of most immunotherapy-positive gene signatures in the TCGA cohort, such as the IFN-γ and hypoxia signatures (Figure 5B). On the other hand, other signatures, including the cell cycle, EGFR signal, and PPARG signal, were enhanced in the low FMNL3 group, suggesting that potential targeted therapies blocking these pathways could be used to treat PAAD patients with low FMNL3 expression. Moreover, compared with the low FMNL3 group, PAAD patients with high FMNL3 had a stronger response to most chemotherapy, anti-ERBB therapy, anti-angiogenic therapy, and immunotherapy (Figure 5C). Consistent with previous results, FMNL3 expression was also positively related to T cell inflamed score (Figure 5D), an index established for use as an alternative for estimating clinical response to anti-PD-1 therapy,21 indicating the enhanced response in the high FMNL3 group. Furthermore, the IC50 value of anti-cancer drugs in patients selected from the TCGA database according to the pRRophetic algorithm was performed. As shown in Figure 5E, patients with high FMNL3 expression were sensitive to vinblastine, cisplatin, doxorubicin, and sunitinib, while patients in the low FMNL3 group were more sensitive to lapatinib and sorafenib. In a word, these results suggest that patients with different FMNL3 expression levels may respond to various therapeutic opportunities.
FMNL3 is Correlated with Emerging Immune-Related Biomarkers in PAAD
Since the intratumor microbiota play important roles in affecting immunity in human cancers,31 we next systematically screened for the microbiota which was significantly associated with the T cell inflamed score. The results showed that Terrabacter, Candidatus Stoquefichus, Lachnoclostridium, Collimonas, Candidatus Nitrosopelagicus, and Campylobacter were positively correlated with T cell inflamed score (Figure 6A). To further prove these microbiota were immune-related in PAAD, we next calculated the relationships between these microbiota abundances and infiltration levels of immune cells and expression of immune checkpoints, respectively. In line with the previous observation, these microbiotas were positively correlated with immune-related biomarkers (Figure 6B and C). Collectively, results above indicated that high abundances of immune-related microbiotas were associated with enhanced immunotherapy response. In the high FMNL3 group, the abundances of Terrabacter, Candidatus Stoquefichus, Collimonas, Candidatus Nitrosopelagicus, and Campylobacter were consistently higher (Figure 6D).
Previous studies have demonstrated that N6-methyladenosine (m6A), which is methylated at the N6 position of adenosine, plays a crucial role in anti-tumor immunity and can be used as an indicator to identify tumor immunogenicity.32,33 Therefore, we identified and validated a cluster of immune-related m6A regulators and m6A interactive protein-coding genes in PAAD (Figure 7A–C) based on the correlation among m6A genes and immune biomarkers. Encouragingly, CXCR4, BCL2, ETS1, ASB2, ADAM19, LEF1, PTEN, WTAP, NOTCH1, HIF1A and PDCD1 were observably upregulated in the high FMNL3 group, while MZF1 and YTHDF1 were downregulated (Figure 7D). In brief, these findings provide evidence that FMNL3 is associated with immunotherapy response from the perspective of emerging immune-related biomarkers.
Discussion
Formin proteins are well known as regulators of microfilament assembly and cell migration.34 However, whether these proteins are related to anti-tumor immunity and the TME has not been concerned. In the current study, we first analyzed the pan-cancer expression patterns and immuno-biological correlations of FMNL3 using the TCGA database. We found that FMNL3 was highly expressed in most cancers, but had conflicting prognostic values. According to the previous research, FMNL3 plays an oncogenic role in multiple cancers, including nasopharyngeal carcinoma cell metastasis,8 tongue squamous cell carcinoma,9 and colorectal carcinoma.11 However, high expression of FMNL3 predicted a better prognosis in several cancers, including LUAD, HNSC, BRCA, CESC and PAAD. Interestingly, we found that FMNL3 had tight immuno-correlations in specific cancers. We next selected PAAD for further investigation and found that the expression of FMNL3 in PAAD was related to immune factors, which could predict an inflammatory TME and more therapeutic options. Furthermore, we also validated the expression and immunobiological correlations of FMNL3 in PAAD using IHC staining, which was consistent with our conclusions obtained from the database.
Specifically, our study showed that FMNL3 was positively related to the expression values of critical immune biomarkers, including MHC II molecules, chemokines and paired receptors, as well as to the activities of the phases of recruiting immune cells and infiltration levels of TIICs. In contrast, several reported immune-suppressive oncogenic pathways, such as the β-catenin and PPAR-γ pathways, have been shown to inhibit the infiltration of TIICs by decreasing the expression of immunomodulators, shaping a non-inflamed TME.35,36 FMNL3 plays an important immunological role in recruiting TIICs and promotes the formation of inflamed TME. Our study showed that immuno-promotive pathways, such as the IFN-γ signature, EGFR ligands, and hypoxia scores were observably enriched in high FMNL3 patients, whereas oncogenic pathways were not, indicating that PAAD patients with high FMNL3 had a stronger response to immunotherapy. The microbiome may not only be involved in the occurrence and development of cancer but also may have potential diagnostic and therapeutic effects.37 Evidence shows that microbes may increase the susceptibility to certain cancers and may influence the responses to therapeutics including immunotherapy.38 For example, Riquelme et al found that the microbiomes of long-term survival PAAD patients were characterized by Pseudoxanthomonas-Streptomyces-Saccharopolyspora-Bacillus clausii and had higher alpha-diversity.39 In this research, we found that Terrabacter, Candidatus Stoquefichus, Lachnoclostridium, Collimonas, Candidatus Nitrosopelagicus, and Campylobacter were significantly correlated with T cell inflamed score and were defined as immune-related tumor microorganisms. In addition, we found that FMNL3 expression was significantly associated with the abundance of five immune-related tumor microorganisms. Besides, using a similar approach, the immune-associated m6A genes were also defined and tight associations were found between FMNL3 and these genes.
Conclusions
In this study, we revealed that FMNL3 is positively associated with an inflammatory TME in PAAD and has the potential to predict the therapeutic response to multiple therapeutic options. In addition, FMNL3 is associated with emerging immune-related biomarkers in PAAD, including microbiota abundance and m6A gene expression. However, it remains to be clarified whether FMNL3 is merely a biomarker for tumor immunogenicity or has potential regulatory effects on anti-tumor immunity.
Abbreviations
PAAD, pancreatic cancer; OS, overall survival; ICI, immuno-checkpoint inhibitor; PD-1, programmed death 1; PD-L1, programmed death 1 ligand 1; TME, tumor microenvironment; FMNL3, Formin-like protein 3; EMT, epithelia-to-mesenchymal transition; RNA-seq, RNA sequencing; TCGA, the Cancer Genome Atlas; TIIC, tumor-infiltrating immune cell; ssGSEA, single-sample gene set enrichment analysis; IC50, half-maximal inhibitory concentration; TMA, tissue microarray; IHC, immunohistochemistry; DAB, diaminobenzidine; IRS, immunoreactivity score; m6A, N6-methyladenosine.
Data Sharing Statement
All data are included in the article.
Ethics Approval and Consent to Participate
Ethical approval (YBM-0502) for the study of the TMA was granted by the Clinical Research Ethics Committee, Outdo BioTech (Shanghai, China).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Top Talent Support Program for Young and Middle-aged People of Wuxi Health Committee (HB2020009), the Scientific Research Projects of Wuxi Health Commission (Q202109), the Technology Demonstration Project of Wuxi Science and Technology Bureau (N20201004), the High-end Talents of 2020 Taihu Talent Program, and the High-end Medical Expert Team of the 2020 Taihu Talent Plan.
Disclosure
The authors declare no conflict of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
2. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. doi:10.1016/S0140-6736(20)30974-0
3. Tan S, Li D, Zhu X. Cancer immunotherapy: pros, cons and beyond. Biomed Pharmacother. 2020;124:109821. doi:10.1016/j.biopha.2020.109821
4. Feng M, Xiong G, Cao Z, et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Lett. 2017;407:57–65. doi:10.1016/j.canlet.2017.08.006
5. Karamitopoulou E. Tumour microenvironment of pancreatic cancer: immune landscape is dictated by molecular and histopathological features. Br J Cancer. 2019;121(1):5–14. doi:10.1038/s41416-019-0479-5
6. Hegsted A, Yingling CV, Pruyne D. Inverted formins: a subfamily of atypical formins. Cytoskeleton. 2017;74(11):405–419.
7. Katoh M, Katoh M. Identification and characterization of human FMNL1, FMNL2 and FMNL3 genes in silico. Int J Oncol. 2003;22(5):1161–1168.
8. Wu Y, Shen Z, Wang K, et al. High FMNL3 expression promotes nasopharyngeal carcinoma cell metastasis: role in TGF-beta1-induced epithelia-to-mesenchymal transition. Sci Rep. 2017;7:42507.
9. Liu J, Chen S, Chen Y, Geng N, Feng C. High expression of FMNL3 associates with cancer cell migration, invasion, and unfavorable prognosis in tongue squamous cell carcinoma. J Oral Pathol Med. 2019;48(6):459–467.
10. Hetheridge C, Scott AN, Swain RK, et al. The formin FMNL3 is a cytoskeletal regulator of angiogenesis. J Cell Sci. 2012;125(Pt 6):1420–1428. doi:10.1242/jcs.091066
11. Zeng YF, Xiao YS, Liu Y, et al. Formin-like 3 regulates RhoC/FAK pathway and actin assembly to promote cell invasion in colorectal carcinoma. World J Gastroenterol. 2018;24(34):3884–3897. doi:10.1016/j.celrep.2016.12.019
12. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi:10.1158/2159-8290.CD-12-0095
13. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. doi:10.1093/nar/gkx247
14. Yoshihara K, Shahmoradgoli M, Martinez E, et al. Inferring tumour purity and stromal and immune cell admixture from expression data. Nat Commun. 2013;4(1):2612. doi:10.1038/ncomms3612
15. Li T, Fu J, Zeng Z, et al. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020;48(W1):W509–w514. doi:10.1093/nar/gkaa407
16. Racle J, de Jonge K, Baumgaertner P, Speiser DE, Gfeller D. Simultaneous enumeration of cancer and immune cell types from bulk tumor gene expression data. Elife. 2017;6. doi:10.7554/eLife.26476
17. Becht E, Giraldo NA, Lacroix L, et al. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol. 2016;17(1):218. doi:10.1186/s13059-016-1070-5
18. Finotello F, Mayer C, Plattner C, et al. Molecular and pharmacological modulators of the tumor immune contexture revealed by deconvolution of RNA-seq data. Genome Med. 2019;11(1):34. doi:10.1186/s13073-019-0638-6
19. Ru B, Wong CN, Tong Y, et al. TISIDB: an integrated repository portal for tumor-immune system interactions. Bioinformatics. 2019;35(20):4200–4202. doi:10.1093/bioinformatics/btz210
20. Charoentong P, Finotello F, Angelova M, et al. Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell Rep. 2017;18(1):248–262.
21. Ayers M, Lunceford J, Nebozhyn M, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest. 2017;127(8):2930–2940. doi:10.1172/JCI91190
22. Xu L, Deng C, Pang B, et al. TIP: a web server for resolving tumor immunophenotype profiling. Cancer Res. 2018;78(23):6575–6580. doi:10.1158/0008-5472.CAN-18-0689
23. Hu J, Yu A, Othmane B, et al. Siglec15 shapes a non-inflamed tumor microenvironment and predicts the molecular subtype in bladder cancer. Theranostics. 2021;11(7):3089–3108. doi:10.7150/thno.53649
24. Hanzelmann S, Castelo R, Guinney J. GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinform. 2013;14(1):7. doi:10.1186/1471-2105-14-7
25. Geeleher P, Cox N, Huang RS. pRRophetic: an R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS One. 2014;9(9):e107468. doi:10.1371/journal.pone.0107468
26. Shen S, Zhang R, Jiang Y, et al. Comprehensive analyses of m6A regulators and interactive coding and non-coding RNAs across 32 cancer types. Mol Cancer. 2021;20(1):67. doi:10.1186/s12943-021-01362-2
27. Mei J, Yan T, Huang Y, et al. A DAAM1 3’-UTR SNP mutation regulates breast cancer metastasis through affecting miR-208a-5p-DAAM1-RhoA axis. Cancer Cell Int. 2019;19(1):55. doi:10.1186/s12935-019-0747-8
28. Mei J, Xu B, Hao L, et al. Overexpressed DAAM1 correlates with metastasis and predicts poor prognosis in breast cancer. Pathol Res Pract. 2020;216(3):152736. doi:10.1016/j.prp.2019.152736
29. Cai Y, Ji W, Sun C, Xu R, Chen X, Deng Y, Pan J, Yang J, Zhu H, Mei J. (2021). Interferon-Induced Transmembrane Protein 3 Shapes an Inflamed Tumor Microenvironment and Identifies Immuno-Hot Tumors. Front Immunol. 12:704965. doi:10.3389/fimmu.2021.704965
30. Gajewski TF, Corrales L, Williams J, Horton B, Sivan A, Spranger S. Cancer immunotherapy targets based on understanding the T cell-inflamed versus non-T cell-inflamed tumor microenvironment. Adv Exp Med Biol. 2017;1036:19–31.
31. Sepich-Poore G, Zitvogel L, Straussman R, Hasty J, Wargo J, Knight R. The microbiome and human cancer. Science. 2021;371(6536). doi:10.1126/science.abc4552
32. Zhu H, Jia X, Wang Y, et al. M6A classification combined with tumor microenvironment immune characteristics analysis of bladder cancer. Front Oncol. 2021;11:714267. doi:10.3389/fonc.2021.714267
33. Liu Z, Zhong J, Zeng J, et al. Characterization of the m6A-associated tumor immune microenvironment in prostate cancer to aid immunotherapy. Front Immunol. 2021;12:735170. doi:10.3389/fimmu.2021.735170
34. Le S, Yu M, Bershadsky A, Yan J. Mechanical regulation of formin-dependent actin polymerization. Semin Cell Dev Biol. 2020;102:73–80. doi:10.1016/j.semcdb.2019.11.016
35. Korpal M, Puyang X, Jeremy Wu Z, et al. Evasion of immunosurveillance by genomic alterations of PPARgamma/RXRalpha in bladder cancer. Nat Commun. 2017;8(1):103. doi:10.1038/s41467-017-00147-w
36. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231–235. doi:10.1038/nature14404
37. Wang Y, Yang G, You L, et al. Role of the microbiome in occurrence, development and treatment of pancreatic cancer. Mol Cancer. 2019;18(1):173. doi:10.1186/s12943-019-1103-2
38. Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy. Nat Med. 2019;25(3):377–388. doi:10.1038/s41591-019-0377-7
39. Riquelme E, Zhang Y, Zhang L, et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 2019;178(4):795–806.e712. doi:10.1016/j.cell.2019.07.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.