Back to Journals » International Medical Case Reports Journal » Volume 14
Fluocinolone Acetonide 0.19 mg Implant in Patients with Cystoid Macular Edema Due To Irvine–Gass Syndrome
Authors Marques JH , Abreu AC , Silva N , Meireles A , Pessoa B , Melo Beirão J
Received 1 December 2020
Accepted for publication 13 January 2021
Published 26 February 2021 Volume 2021:14 Pages 127—132
DOI https://doi.org/10.2147/IMCRJ.S295045
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
João Heitor Marques,1 Ana Carolina Abreu,1 Nisa Silva,1 Angelina Meireles,1,2 Bernardete Pessoa,1,2 João Melo Beirão1,2
1Serviço de Oftalmologia, Centro Hospitalar e Universitário do Porto, Porto, Portugal; 2Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, Porto, Portugal
Correspondence: João Heitor Marques
Centro Hospitalar Universitário do Porto, Serviço de Oftalmologia, Largo do Prof. Abel Salazar, Porto, 4099-001, Portugal
Tel +351913680726
Email [email protected]
Background: Cystoid macular edema (CME) due to Irvine–Gass syndrome (IGS) is one of the common causes of painless visual impairment post-cataract extraction. The treatment of recurrent cases remains unstandardized.
Objective: To evaluate the effectiveness and safety of fluocinolone acetonide intravitreal implant (0.2 μg/day; ILUVIEN®) in the off-label treatment of recurrent CME due to IGS.
Methods: Retrospective 36-month case series in the Ophthalmology Department of Centro Hospitalar Universitário do Porto, Portugal. Consecutive eyes of patients with recurrent cystoid macular edema due to Irvine–Gass syndrome who underwent a single intravitreal injection of fluocinolone acetonide intravitreal implant were included. Best-corrected visual acuity (logMAR), central macular thickness (μm) and safety (intraocular pressure, mmHg) at baseline and at 6, 12, 24 and 36 months post-administration of the fluocinolone acetonide intravitreal implant were recorded.
Results: Five eyes from three patients were included. The duration of cystoid macular edema was 67.8± 25.9 months and all five eyes received more than 2 intravitreal injections of a corticosteroid (triamcinolone and/or dexamethasone implant) prior to fluocinolone acetonide intravitreal implantation. At baseline (median – interquartile range), best-corrected visual acuity was 0.3– 0.3; central macular thickness was 492.0– 38.0; and intraocular pressure was 16.0– 0. By Month 36, best-corrected visual acuity was 0.4 − 0.3; central macular thickness was reduced to 369.0– 324.0 and intraocular pressure was 17.0– 3.0. Four of five eyes had increased intraocular pressure and were managed with intraocular pressure-lowering eye drops.
Conclusion: We report improved functional and anatomical outcomes after treatment with fluocinolone acetonide intravitreal implant, indicating its use as a therapeutic alternative in recurrent cases of cystoid macular edema due to Irvine–Gass syndrome. Additionally, in eyes with suboptimal response to intravitreal therapies, fluocinolone acetonide intravitreal implant may provide longer recurrence-free periods with reduced treatment burden.
Keywords: cataract surgery, Irvine–Gass syndrome, cystoid macular edema, pseudophakic cystoid macular edema, fluocinolone acetonide
Introduction
Cataract surgery is known to be the most frequent surgical procedure performed worldwide. Irvine–Gass syndrome (IGS), also known as pseudophakic cystoid macular edema (CME), is one of the common causes of painless visual impairment post-cataract extraction, occurring even after uneventful surgeries.1
Despite recent surgical improvements, occurrence of CME is reported to be between 1% and 30% with different cataract extraction techniques, of which 1–2% appear clinically significant.2,3 Its pathophysiology remains unclear but various inflammatory factors have been involved, such as vascular instability, vitreomacular tractions, and light toxicity.4 Therefore, most treatment options employed target inflammatory pathways. Currently, there are no guidelines or accepted consensus on the use of different treatment drugs, which include nonsteroidal inflammatory drugs (NSAIDs), topical and intravitreal corticosteroids, systemic acetazolamide and intravitreal anti-vascular endothelial growth factors (anti-VEGF). Regardless of the treatment options preferred in clinical practice, cases of recurrent macular edema still persist.5 New drug delivery systems to the posterior segment of the eye offer a new option for the treatment of CME.6 Sustained-release intravitreal implants of dexamethasone (Ozurdex®) or fluocinolone acetonide (Iluvien®) offer a potentially more predictable and longer drug-release profile in comparison with conventional intravitreal therapies, as well as reducing treatment frequency. Iluvien® use has been reported in both diabetic7 and non-diabetic8 macular edema.
Here, for the first time, we report a case series from three patients (five eyes) with recurrent CME and the outcomes achieved when treated off-label with a single FAc implant, following the lack of stability and effectiveness with prior therapies (ie short-acting intravitreal corticosteroids).
The primary objective of this study was to evaluate the effectiveness and safety of an intravitreal FAc implant over 36 months in patients with recurrent CME due to IGS.
Methods
Patient Examination, Diagnosis and Treatment History
This was a retrospective clinical data review of eyes with refractory CME due to IGS treated with a single intravitreal injection of FAc implant, between February 2016 and February 2019, at the ophthalmology department of Centro Hospitalar Universitário do Porto, Hospital de Santo António, Portugal.
Patients with diabetic retinopathy, retinal vein occlusion, exudative age-related macular degeneration and clinical uveitis (secondary causes of CME) were excluded.
All patients underwent a complete ophthalmic evaluation and a spectral-domain optical coherence tomography (SD-OCT) scan with Heidelberg Spectralis®, both at baseline and during the follow-up period (Months 6, 12, 24, and 36). Patient demographic data and history of previous treatments and recurrences of CME were recorded. The variables measured included best-corrected visual acuity (BCVA), using standardized Snellen charts and converted to logMAR, and central macular thickness (CMT) in the 1 mm circle centered in the fovea, accessed with an SD-OCT scan, safety (intraocular pressure [IOP], mmHg), measured with Goldmann applanation tonometer, and the presence of CME at baseline and at 6-, 12-, 24-, and 36-month post-administration of the FAc implant.
Statistical Analysis
Descriptive analysis was performed using the SPSS software (version 24, SPSS, Inc.). Numeric values are reported as median with 25% and 75% quartile values shown in parenthesis, unless otherwise stated.
Ethics Statement
This work was approved by our department (Serviço de Oftalmologia, Centro Hospitalar Universitário do Porto), it complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All included patients were informed about the risks and benefits of this off-label treatment and all patients were incorporated in the decision to treat. All patients signed a written informed consent form for the treatment and also for the publication of the clinical data used for this work. All cases are presented anonymously in this manuscript. Due to its retrospective observational design and to the small number of patients included (3), formal ethics committee approval was not required.
Results
Five eyes from three patients with IGS were reviewed. Of the three patients, two were male and the age ranged between 64 and 76 years. All five eyes had performed an uneventful age-related cataract surgery and were routinely medicated post-operatively with topical NSAID (for 4 weeks), corticosteroid (for 4 weeks) and antibiotic (for 2 weeks). The mean time between surgery and detection of CME was 71.6±30.8 days (range, 18–92 days). Secondary causes of macular edema such as uveitis or retained lens material were excluded by the ophthalmologic examination. All eyes then received intravitreal injections of shorter-acting corticosteroids (triamcinolone [n=2/5] and/or dexamethasone implants [n=4/5]). The mean number of intravitreal corticosteroids received was 3.2 (range: 2–4) and all patients showed recurrent CME. The mean duration of CME was 67.8±25.9 months (range, 26.3 to 88.7 months) before treatment with the FAc implant. At baseline (considered as the last visit before FAc implant), median BCVA was 0.3 logMAR (25% and 75% quartile values: 0.3 and 0.6 logMAR, respectively), CMT was 492 µm (467, 505 µm) and IOP was 16 mmHg (16, 16 mmHg).
Effectiveness Outcomes
Post-FAc implantation, an improved/maintenance of BCVA was observed in all eyes (n=5/5) from baseline (0.3 logMAR; 0.3, 0.6 logMAR (25%, 75% quartile values) to Month 1 (0.3 logMAR; 0.2, 0.3 logMAR) and sustained at 24 months (0.2 logMAR; 0.2, 0.3 logMAR) (Figure 1). By Month 36, BCVA was still improved (0.4 logMAR; 0.2, 0.5 logMAR) versus baseline but was slightly lower than Month 1 values presumably due to the fact that effects in three eyes had started to decline by Month 36.
Anatomical Outcomes
After initiation of therapy with the FAc implant, the SD-OCT images showed considerable reduction in CMT in all eyes at Month 1 (−198 μm; −188, −237 μm), which was also sustained to Month 24 (−187 μm; −157, −202 μm) (Figure 2). At Month 36, mean CMT was still below baseline (−179 μm; −200, 112 μm) with three of the five eyes still maintaining an improvement versus baseline and two eyes experiencing an increase (+112, +175 μm from baseline levels, respectively). Interestingly, one of the eyes presented with an incomplete response to the FAc implant, which was observed at Month 12 (changes in CMT of −43 μm and BCVA increasing from 0.3 at baseline to 0.7 logMAR at Month 12). This patient was submitted for vitrectomy with internal limiting membrane peeling and an improvement in CMT (−157 μm from baseline) was observed at Month 24 and BCVA stabilized (0.3 logMAR).
Safety Outcomes
Post-FAc implantation, there was a slight increase in the IOP in two eyes by Month 12 (Figure 3). Four eyes (80%) required treatment with IOP-lowering eye drops; two of these eyes (40%) had a history of ocular hypertension pre-administration of the FAc implant. All cases were successfully managed with IOP-lowering eye drops, and no surgeries were required within 36 months following therapy. Indeed, through to Month 36, only one eye had an IOP ≥21 mmHg with IOP lowering eyedrops, which was observed in the same eye at both baseline and Month 1, but at all other time points, IOP remained below 21 mmHg. Table 1 shows demographic informations, IOP and number of IOP-lowering eye drugs used, from baseline to Month 36, by case.
 |
Table 1 Demographic Information, Intraocular Pressure (IOP, mmHg) and Number of IOP-Lowering Eye Drugs Used (ND), from Baseline to Month 36, by Case |
Case Study
A 69-year-old male patient where the right eye underwent cataract extraction in March 2013 and 3 months later developed CME. The patient received four dexamethasone implants and showed good but unstable anatomical response, leading to recurrent CME with time. In February 2016 he received an FAc implant. At baseline CMT was 467 µm, visual acuity was 55 ETDRS letters and IOP was 16 mmHg.
One month after intravitreal injection of the FAc implant, CMT decreased to 279 µm (Figure 4, baseline) VA improved to 70 ETDRS letters and IOP was 14 mmHg.
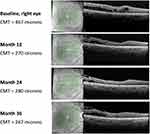 |
Figure 4 OCT foveal B-scan from baseline to Month 36. Abbreviations: CMT, central macular thickness; OCT, optical coherence tomography. |
During the remaining treatment period, CMT remained below 300 µm (Figure 4, month 12 and 24) and by year 3 was 267 µm (Figure 4, month 36). Both VA (75 ETDRS letters) and IOP (20 mmHg) remained stable throughout the period of therapy.
Discussion
The FAc implant is designed to elute FAc for up to 3 years and is likely to control edema following cataract surgery, as well as provide recurrence-free periods with the potential to decrease the need for frequent corticosteroid injections (triamcinolone and dexamethasone implant) in patients with CME. A previous case report has shown the long-acting intravitreal FAc implant to be an effective and potentially safe treatment for non-diabetic patients with persistent CME after undergoing vitreoretinal surgery.8 This case series further demonstrates the beneficial effect of a single injection of an FAc implant in patients with recurrent CME due to IGS that lasts for up to 36 months post-cataract surgery.
To the authors’ knowledge, this is the first case series of patients with CME due to IGS that were treated with FAc implant in a routine clinical setting, after suboptimal response and stability to prior treatment with short-acting corticosteroids. Treatment with FAc implant led to the stabilization of VA and a considerable reduction in CMT in three of five eyes. This was accompanied by a slight increase in IOP, which remained below 21 mmHg with IOP lowering eyedrops for the majority of the follow-up period (ie from Month 1 to Month 36). Given this, the patient profile must also be contemplated, balancing the benefit of less intravitreal injections and the burden of extra medical appointments to manage the IOP.
The exact pathogenesis for CME due to IGS is not fully understood and it is not surprising there are a limited number of therapies licensed for patients with this condition. Most commonly, topical/periocular corticosteroids and NSAIDs are used as the first-line treatment, either in combination or as a monotherapy, to reduce the CME and bring about normalization of the foveal thickness. The use of intravitreal corticosteroids (including dexamethasone and triamcinolone) have been shown to be effective in treatment of CME due to IGS as a first-line therapy or in persistent or recurrent cases. The effectiveness of dexamethasone implants has been noted in the EPISODIC studies9,10 and yet recurrence of macular edema has been reported in these patients (within the duration of 24 weeks).11
Results from the present study support the effectiveness of intravitreal corticosteroids per se. Furthermore, this is the first case series to report the effectiveness and safety of the FAc implant in controlling the recurrence of cystoid edema, for up to 3 years, in patients with IGS treated in a real-life clinical setting. The case also highlights treatment outcomes where there is currently an unmet patient need due to a lack of therapies providing sustained control of edema.
Limitations include the retrospective nature of the study, and small patient number. Larger and prospective) studies with more patients and longer follow-up are still required to substantiate the current findings and to assess the reproducibility of the current results.
Conclusions
Following therapy with a short-acting corticosteroid, an FAc implant was administered and led to overall improvements in visual acuity and central retinal thickness. The current cases highlight the potential of FAc to provide recurrence-free periods and a reduced need for intravitreal therapies for up to 3 years. Larger studies are now required to evaluate the effectiveness of FAc implant in this disease, the responses following earlier therapy in IGS and the long-term benefit of reducing the number of recurrences of cystoid edema in IGS.
Funding
The authors declare that this work did not receive any funding.
Disclosure
The authors have no financial disclosure. Medical writing and editorial assistance for this study were provided by Rhutika Dessai of Prescript Communications, Letchworth Garden City, UK. Support for this assistance was funded by Alimera Sciences Ltd.
References
1. Kodjikian L, Bellocq D, Bodaghi B. Management of Irvine-Gass syndrome. J Fr Ophtalmol. 2017;40(9):788–792. doi:10.1016/j.jfo.2017.09.001
2. Grzybowski A, Sikorski B, Ascaso F, Huerva V. Pseudophakic cystoid macular edema: update 2016. Clin Interv Aging. 2016;11:1221–1229. doi:10.2147/CIA.S111761
3. Guo S, Patel S, Baumrind B, et al. Management of pseudophakic cystoid macular edema. Surv Ophthalmol. 2015;60(2):123–137. doi:10.1016/j.survophthal.2014.08.005
4. Shelsta H, Jampol L. Pharmacologic therapy of pseudophakic cystoid macular edema: 2010 update. Retina. 2011;31(1):4–12. doi:10.1097/IAE.0b013e3181fd9740
5. Oliveira Dias J, Nunes R, Goldhardt R. New drugs and new posterior delivery methods in CME. Curr Ophthalmol Rep. 2017;5(2):160–168. doi:10.1007/s40135-017-0134-3
6. Pearce W, Hsu J, Yeh S. Advances in drug delivery to the posterior segment. Curr Opin Ophthalmol. 2015;26(3):233–239. doi:10.1097/ICU.0000000000000143
7. Campochiaro P, Brown D, Pearson A, et al., FAME Study Group. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmol. 2012;119(10):2125–2132. doi:10.1016/j.ophtha.2012.04.030
8. Ong S, Walter S, Chen X, Thomas A, Finn A, Fekrat S. Bilateral intravitreal 0.19-mg fluocinolone acetonide implant for persistent nondiabetic cystoid macular edema after vitrectomy. Retin Cases Brief Rep. 2018;12(1):1–5. doi:10.1097/ICB.0000000000000432
9. Bellocq D, Korobelnik J, Burillon C, et al. Effectiveness and safety of dexamethasone implants for post-surgical macular oedema including Irvine-Gass syndrome: the EPISODIC study. Br J Ophthalmol. 2015;99(7):979–983. doi:10.1136/bjophthalmol-2014-306159
10. Bellocq D, Pierre-Kahn V, Matonti F, et al. Effectiveness and safety of dexamethasone implants for postsurgical macular oedema including Irvine-Gass syndrome: the EPISODIC-2 study. Br J Ophthalmol. 2017;101(3):333–341. doi:10.1136/bjophthalmol-2016-308544
11. Ohn MT, Thompson E, Waghmare A, Chandra A, Karia N. Usefulness of Intravitreal dexamethasone implant in treatment of persistent cystoid macular edema due to Irvine–Gass syndrome. J Clin Res Ophthalmol. 2019;6(1):1–6.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



