Back to Journals » International Journal of General Medicine » Volume 15
FLAIR Vascular Hyperintensity: An Important MRI Marker in Patients with Transient Ischemic Attack
Authors Zeng L , Wang Q, Liao H, Ren F, Zhang Y, Du J, Liao H, Xie M, Wu W
Received 6 May 2022
Accepted for publication 11 July 2022
Published 14 July 2022 Volume 2022:15 Pages 6165—6171
DOI https://doi.org/10.2147/IJGM.S371894
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Lichuan Zeng,1,* Qu Wang,2,* Haodong Liao,1,* Fengchun Ren,1 Yudong Zhang,1 Jian Du,3 Huaqiang Liao,1 Mingguo Xie,1 Wenbin Wu3
1Department of Radiology, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 2Department of Ultrasound, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 3Department of Geriatrics, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingguo Xie, Department of Radiology, Hospital of Chengdu University of Traditional Chinese Medicine, No. 39 Shi-er-qiao Road, Chengdu, People’s Republic of China, Email [email protected] Wenbin Wu, Department of Geriatrics, Hospital of Chengdu University of Traditional Chinese Medicine, No. 39 Shi-er-qiao Road, Chengdu, People’s Republic of China, Email [email protected]
Purpose: We aimed to investigate the prevalence of fluid-attenuated inversion recovery (FLAIR) vascular hyperintensities (FVHs) and the clinical–radiological correlation in transient ischemic attack (TIA) patients.
Materials and Methods: We performed a retrospective analysis of TIA patients who had undergone magnetic resonance imaging (MRI) within 24 h of symptom onset. Two independent neuroradiologists investigated the presence of FVHs, large-artery severe stenosis or occlusion (LASO) in magnetic resonance angiograms, and the nature of factors associated with FVH.
Results: A total of 207 patients were enrolled in this study. FVHs were detected in 42 (20.3%) patients, in whom atrial fibrillation (AF) was confirmed in 25 (59.5%) cases and LASO was confirmed in 30 (71.4%) cases. The corresponding figures were 33 (20.0%) and 10 (6.1%), respectively, for the 165 FVH-negative patients. Logistic regression analysis showed that time from symptom onset to MRI (odds ratio [OR] = 0.82, 95% CI 0.76– 0.97, p = 0.042), previous stroke (OR = 2.95, 95% CI 1.58– 6.74, p = 0.002), AF (OR = 5.83, 95% CI 2.24– 9.46, p < 0.001), and LASO (OR = 4.28, 95% CI 2.96– 10.28, p < 0.001) were independently associated with FVH. Overall, the sensitivity and specificity of FVH for predicting LASO were 0.75 and 0.93, respectively, and the positive predictive value, negative predictive value, and accuracy were 0.71, 0.94, and 0.89, respectively. The area under the receiver operating characteristic curve was 0.839. FVH-positive TIA patients with LASO had less AF (14 [46.7%] versus 11 [91.7%], p = 0.019) and longer times from symptom onset to MRI (6.8 ± 2.8 h versus 4.8 ± 1.3 h, p = 0.004) than those without LASO.
Conclusion: The presence of FVH could be an important marker in TIA patients. Many factors, including LASO, AF, and time from symptom onset to MRI, are associated with the detection of FVH.
Keywords: FLAIR vascular hyperintensity, hyperintense vessel, transient ischemic attack, magnetic resonance imaging
Background
Transient ischemic attack (TIA) is traditionally defined as the sudden onset of a focal neurological deficit of presumed vascular origin that resolves completely within 24 h.1–3 Several neuroimaging-based evaluation techniques, including computed tomography, digital subtraction angiography, magnetic resonance imaging (MRI), and nuclear medical imaging, have been proposed to help identify patients at high risk of true ischemic events.1,4,5 MRI is a relatively sensitive imaging modality that has high predictive value for diagnosing ischemic insults. However, if the severity or duration of ischemia is not sufficient to cause permanent damage, conventional magnetic resonance (MR) findings may be negative, which makes early diagnosis of true TIA or nonischemic etiologies difficult.6,7
Large-artery severe stenosis or occlusion (LASO) is considered to involve a remarkable reduction in flow velocity because of vascular stenosis or occlusion and the occurrence of collateral blood flow.8 It has been identified that the presence of LASO has the highest predictive value, and LASO has long been recognized as a major risk factor for future stroke after TIA.9 Fluid-attenuated inversion recovery (FLAIR) vascular hyperintensities (FVHs) are serpentine or linear hyperintensities that are best visualized within the Sylvian fissure ipsilateral to acute ischemic stroke.10,11 FVH has demonstrated good diagnostic performance for the identification of LASO in stroke, but the relationship between FVH and TIA is still undetermined. The purpose of this study was to investigate the prevalence of FVH and the clinical–radiological correlation in patients with TIA.
Materials and Methods
Subjects
We retrospectively investigated the records of patients with TIA in our hospital from January 2015 to December 2021. TIA was defined as a sudden focal neurological deficit with a duration of less than 24 h without acute infarction on imaging. Each diagnosis of TIA was made jointly by at least two neurologists. Enrollment criteria included: (a) transient neurological symptoms thought by a clinical neurologist at the end of evaluation to have a possible vascular etiology; (b) MR scans performed within 24 h of symptom onset; (c) without prior history of stroke, and nonspecific findings on general MR and diffusion-weighted exams; and (d) the patient underwent a magnetic resonance angiography (MRA) study. Exclusion criteria were as follows: (a) a history of stroke, cranial trauma, brain tumors, mental illness, or other known brain abnormality; (b) the patient had severe coexisting or terminal systemic disease; and (c) the image quality was insufficient to assess FVH. The clinical background characteristics of patients, including cardiovascular risk factors, time from symptom onset to MRI, and clinical symptoms of TIA, were collected. Atrial fibrillation (AF) was diagnosed on the basis of either an electrocardiogram performed within 24 h or a confirmed history. ABCD2 scores were generated according to the presence of pre-existing conditions in TIA patients. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Hospital of Chengdu University of Traditional Chinese Medicine Research Ethics Committee.
MRI Protocol
All patients underwent MRI with a Discovery MR750 3.0 T system (GE Healthcare, Milwaukee, WI, USA) and an eight-channel phased-array head coil. The imaging protocol consisted of T2 weighting (repetition time/echo time, 3480/110 ms; field of view [FOV], 240 mm × 240 mm; matrix, 256 × 256; section thickness, 6 mm; gap, 2 mm), T1 weighting (repetition time/echo time/inversion time, 1780/20/860 ms; FOV, 240 mm × 240 mm; matrix, 256 × 256; section thickness, 6 mm; gap, 2 mm), T2-FLAIR (repetition time/echo time/inversion time, 8400/140/2370 ms; FOV, 260 mm × 260 mm; matrix, 256 × 256; section thickness, 5 mm; gap, 1.5 mm), diffusion-weighted imaging (repetition time/echo time, 3000/74 ms; FOV, 240 mm × 240 mm; matrix, 260 × 260; section thickness, 5 mm with no gap; b = 0 and 1000 s/mm2), and MRA (repetition time/echo time, 8.2/3.2 ms; FOV, 240 mm × 240 mm; matrix, 256 × 256; slice thickness, 1 mm).
Image Evaluation
FVHs were defined as linear or serpentine hyperintensities with respect to gray matter in the subarachnoid space that corresponded to typical arterial courses on the T2-FLAIR image. Two independent neuroradiologists who were blinded to clinical information reviewed FLAIR images to identify FVHs and MRA images to locate large-vessel occlusions when present. Patients were divided into FVH-positive and FVH-negative groups on the basis of the presence of an FVH sign. Large vessels were defined as the internal carotid artery, middle cerebral artery (MCA) M1, and MCA M2. Patients who were FVH-positive were divided into two groups, namely, those with LASO and those without large-vessel occlusion, according to the occlusion sites identified by MRA.
Statistical Analysis
Interobserver agreement was assessed using the kappa (κ) statistic. A value of κ > 0.6 was considered to represent good agreement, while κ > 0.8 was considered to represent excellent agreement. Continuous variables with a normal distribution were described as the mean ± SD, and categorical variables were described in terms of frequency (%). The difference in each categorical variable between patients with and without FVH was analyzed using the Mann–Whitney U-test, Student’s t-test, chi-squared tests, and Fisher’s exact test (when the expected cell frequency was <5). Logistic regression analysis was used to test for independence among predictors for the presence of FVH. Diagnostic parameters (sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rate) were also calculated for FVH in predicting LASO tested against MRA as the gold standard. The predictive values were determined by receiver operating characteristic (ROC) analysis. A two-tailed p-value of <0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using SPSS software version 20.0 (SPSS, Inc., Chicago, IL, USA).
Results
Demographic and Clinical Information
A total of 272 patients with TIA were retrospectively identified, of whom 65 were excluded. Thirty-six patients were excluded as there were no available angiography findings; 20 patients were excluded as a result of delays in MRI or angiography; and nine patients were excluded because of severe artifacts on FLAIR. Finally, the remaining 207 patients (mean age, 60.8 ± 9.0 years; 120 female/87 male) met the inclusion criteria and were retrospectively enrolled in this study. Agreement between two readers on the presence of FVHs (κ = 0.84) and the location of occlusions (κ = 0.89) was good. Characteristics of TIA patients with and without FVH are listed in Table 1.
 |
Table 1 Characteristics of TIA Patients with and without FLAIR Vascular Hyperintensity |
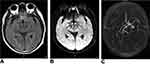
FVHs were detected in 42 (20.3%) patients. The commonest presenting symptoms were focal weakness, speech disturbance, dizziness, and vertigo. The median time between symptom onset and imaging was 8.1 ± 3.5 h. The majority of FVH-positive patients (32/42, 76.2%) had symptoms thought to represent hemispheric TIA. Among these 42 patients, AF was confirmed in 25 (59.5%) cases, and LASO was confirmed in 30 (71.4%) cases. The corresponding figures were 33 (20.0%) and 10 (6.1%), respectively, for the 165 FVH-negative patients. The FVH-positive patients and FVH-negative patients exhibited significant differences in history of TIA, time from symptom onset to MRI, history of previous stroke, AF, and LASO. A representative case of an FVH-positive patient with LASO is shown in Figure 1. Univariate analyses revealed that FVH-positive patients were significantly more likely to have a history of TIA, previous stroke, AF, and LASO and shorter times from symptom onset to MRI than patients without FVH. Logistic regression analysis showed that time from symptom onset to MRI (odds ratio [OR] = 0.82, 95% CI 0.76–0.97, p = 0.042), previous stroke (OR = 2.95, 95% CI 1.58–6.74, p = 0.002), AF (OR = 5.83, 95% CI 2.24–9.46, p < 0.001), and LASO (OR = 4.28, 95% CI 2.96–10.28, p < 0.001) were independently associated with FVH positivity (Table 2).
 |
Table 2 Multivariate Logistic Regression Analysis of Parameters Associated with FVH |
Performance of FVH in Predicting Occlusion/Stenosis
Results for the performance of FVH in predicting LASO are listed in Table 3. Overall, the sensitivity and specificity of FVH for predicting LASO were 0.75 and 0.93, respectively, while the positive predictive value, negative predictive value, and accuracy were 0.71, 0.94, and 0.89, respectively. The ROC curve is shown in Figure 2. The area under the curve was 0.839.
 |
Table 3 Results of the Performances of FVH in Predicting LASO |
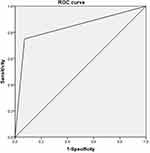 |
Figure 2 Receiver operating characteristic analysis of FVH for the prediction of large-artery severe stenosis or occlusion. The area under the curve was 0.839. |
Results for FVH-Positive Patients with and without LASO
Table 4 lists detailed characteristics of FVH-positive TIA patients with and without LASO. Among the 42 FVH-positive TIA patients, LASO was confirmed in 30 cases and was absent in the remaining 12 cases. FVH-positive TIA patients with LASO had less AF (14 [46.7%] versus 11 [91.7%], p = 0.019), longer times from symptom onset to MRI (6.8 ± 2.8 h versus 4.8 ± 1.3 h, p = 0.004), and a higher percentage rate of previous stroke (53.3% [16/30] versus 8.3% [1/12], p = 0.019) than those without LASO.
 |
Table 4 Characteristics of the FVH-Positive TIA Patients with and without LASO |
Discussion
There are three main findings from the current study. Firstly, the presence of FVH was observed in 20.3% of TIA patients within 24 h, which is consistent with previous studies. Secondly, FVH demonstrated excellent diagnostic performance for the identification of LASO, and FVH-positive patients were significantly more likely to have severe stenosis or occlusion, higher percentage rates of AF, and shorter times from symptom onset to MRI than those without FVH. Thirdly, FVH-positive TIA patients with LASO had less AF and longer times from symptom onset to MRI than those without LASO.
According to previous reports,12–15 the prevalence of FVH in TIA patients varied remarkably from 16% to 39.6% and was significantly lower than in acute ischemic stroke. When analyzing factors pertaining to FVH in our study, we found that a history of TIA, AF, and LASO were commoner in instances of FVH positivity, which is consistent with previous studies. Nam et al12 reported that FVH was associated with early recurrence of ischemic lesions in patients with lesion-negative TIA. In our study, the percentages of patients with histories of TIA and previous stroke in the FVH-positive group were significantly higher in comparison with the FVH-negative group. The detection of FVH could be important for such patients.
The mechanism underlying FVH is still controversial, but the association of FVH in acute ischemic stroke with large-artery occlusion or stenosis is well established.16,17 TIA is a common medical emergency and may precede ischemic stroke that causes permanent neurological deficits.3,18 Patients presenting with transient neurological symptoms with evidence of LASO are at a higher risk of subsequent stroke. The early diagnosis and management of TIA with LASO is therefore a matter of clinical urgency to prevent possible stroke. In our study, 71.4% (30/42) of FVH-positive patients were identified as having LASO. Ding et al19 reported that FVH was observed in up to 81.6% (31/38) of hospitalized TIA patients who had severe stenosis or occlusion. In the present study, FVH was detected in 75.0% (30/40) of patients with LASO. FVH may have utility in the evaluation of patients experiencing TIA, especially in patients in whom MRA is not performed.
Some FVHs in TIA patients were proved to be transient and were correlated with symptom resolution.20 Two independent factors, namely, AF and arterial occlusion/stenosis, had significant and independent associations with FVH.17,21 AF was thought to be associated with transient FVH, while arterial stenosis or occlusion was thought to be associated with persistent FVH.15 Transient FVH with normal MRA findings was related to a mechanism of paroxysmal AF and early recanalization of emboli but was not associated with an increased risk of stroke. In contrast, persistent FVH was related to arterial occlusion and was associated with an increased risk of recurrent TIA and ischemic stroke. Kobayashi et al15 reported that AF was commoner than arterial occlusive lesions in FVH-positive patients, but in our study the incidence of AF (59.5%) was lower than that of arterial occlusion (71.4%).
It is noted that the time from symptom onset to MRI in the FVH-positive group was significantly shorter in comparison with the FVH-negative group. On the other hand, in the FVH-positive group the time from symptom onset to MRI in patients without LASO was shorter in comparison with patients with LASO. We believe that the time from symptom onset may significantly influence the presence of FVHs. As previously reported, some FHVs are transient and may disappear when symptoms are relieved. Hence, we believe that some of the patients in the FVH-negative group had originally been FVH-positive. FVH-positive patients with LASO tended to be associated with persistent FVH, which did not resolve despite the longer duration of symptoms.
The current study had several limitations. Firstly, we investigated a relatively small, although relatively uniform, cohort of patients with TIA. The next step will be to repeat this type of investigation with a larger cohort of subjects. Secondly, follow-up MRI is needed to evaluate the persistence and prognostic value of FVH.
Conclusions
The present study demonstrated that the presence of FVHs could be an important and convenient imaging marker for TIA patients. Many factors including LASO, AF, and time of symptoms onset to MRI are associated with the detection of FVHs. We are confident that this radiologic marker will provide a useful tool for future prevention and early intervention.
Acknowledgments
The scientific guarantors of this publication are Mingguo Xie and Wenbin Wu. Authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any commercial funding.
Funding
This research was funded by National Key R&D Program of China (2020YFC2003100, 2020YFC2003104) and the “Xing-lin Scholars” Project of Chengdu University of Traditional Chinese Medicine (Grant No. QNXZ2018004).
Disclosure
The authors have no financial and/or non-financial conflicts of interest.
References
1. Coutts SB. Diagnosis and management of transient ischemic attack. Continuum. 2017;23(1, Cerebrovascular Disease):82–92. doi:10.1212/CON.0000000000000424
2. Silver B, Wulf SR. Stroke: transient ischemic attack. FP Essent. 2014;420:11–15.
3. Okada Y. Transient ischemic attack as a medical emergency. Front Neurol Neurosci. 2014;33:19–29.
4. Tung CE, Olivot JM, Albers GW. Radiological examinations of transient ischemic attack. Front Neurol Neurosci. 2014;33:115–122.
5. Souillard-Scemama R, Tisserand M, Calvet D, et al. An update on brain imaging in transient ischemic attack. J Neuroradiol. 2015;42(1):3–11. doi:10.1016/j.neurad.2014.11.001
6. Lee SH, Nah HW, Kim BJ, et al. Role of perfusion-weighted imaging in a diffusion-weighted-imaging-negative transient ischemic attack. J Clin Neurol. 2017;13(2):129–137. doi:10.3988/jcn.2017.13.2.129
7. Verclytte S, Fisch O, Colas L, et al. ASL and susceptibility-weighted imaging contribution to the management of acute ischaemic stroke. Insights Imaging. 2017;8(1):91–100. doi:10.1007/s13244-016-0529-y
8. Li Z, Li N, Qu Y, et al. Application of 3.0T magnetic resonance arterial spin labeling (ASL) technology in mild and moderate intracranial atherosclerotic stenosis. Exp Ther Med. 2016;12(1):297–301. doi:10.3892/etm.2016.3318
9. Patrick L, Halabi C. Inpatient management of acute stroke and transient ischemic attack. Neurol Clin. 2022;40(1):33–43. doi:10.1016/j.ncl.2021.08.003
10. Zeng L, Chen J, Liao H, et al. Fluid-attenuated inversion recovery vascular hyperintensity in cerebrovascular disease: a review for radiologists and clinicians. Front Aging Neurosci. 2021;13:790626. doi:10.3389/fnagi.2021.790626
11. Lee KY, Kim JW, Park M, et al. Interpretation of fluid-attenuated inversion recovery vascular hyperintensity in stroke. J Neuroradiol. 2021;49:258–266. doi:10.1016/j.neurad.2021.01.009
12. Nam KW, Kim CK, Kim TJ, et al. FLAIR vascular hyperintensities predict early ischemic recurrence in TIA. Neurology. 2018;90(9):e738–e744.
13. Dong X, Bai C, Nao J. Influential factors and clinical significance of fluid-attenuated inversion recovery vascular hyperintensities in transient ischemic attacks of carotid arterial system. Neuroradiology. 2017;59(11):1093–1099. doi:10.1007/s00234-017-1906-z
14. Yoshioka K, Ishibashi S, Shiraishi A, et al. Distal hyperintense vessels on FLAIR images predict large-artery stenosis in patients with transient ischemic attack. Neuroradiology. 2013;55(2):165–169. doi:10.1007/s00234-012-1092-y
15. Kobayashi J, Uehara T, Toyoda K, et al. Clinical significance of fluid-attenuated inversion recovery vascular hyperintensities in transient ischemic attack. Stroke. 2013;44(6):1635–1640.
16. Apfaltrer P, Wenz H, Bohme J, et al. FLAIR vascular hyperintensities indicate slow poststenotic blood flow in ICA stenosis. Clin Neuroradiol. 2021;31(3):827–831. doi:10.1007/s00062-020-00941-y
17. Kashiwazaki D, Yamamoto S, Akioka N, et al. High-intensity vessel sign on fluid-attenuated inversion recovery imaging: a novel imaging marker of high-risk carotid stenosis-a MRI and SPECT study. Acta Neurochir. 2020;162(10):2573–2581. doi:10.1007/s00701-020-04408-4
18. Sorensen AG, Ay H. Transient ischemic attack: definition, diagnosis, and risk stratification. Neuroimaging Clin N Am. 2011;21(2):303–313. doi:10.1016/j.nic.2011.01.013
19. Ding B, Chen Y, Jiang H, et al. Fluid-attenuated inversion recovery vascular hyperintensities in transient ischemic attack within the anterior circulation. Biomed Res Int. 2020;18(2020):7056056.
20. Taieb G, Renard D, Macri F. FLAIR vascular hyperintensity resolution in a TIA patient: clinical-radiologic correlation. Neurology. 2014;82(22):2039. doi:10.1212/WNL.0000000000000474
21. Kawashima M, Noguchi T, Takase Y, et al. Decrease in leptomeningeal ivy sign on fluid-attenuated inversion recovery images after cerebral revascularization in patients with Moyamoya disease. AJNR Am J Neuroradiol. 2010;31(9):1713–1718. doi:10.3174/ajnr.A2124
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.