Back to Journals » OncoTargets and Therapy » Volume 12
FKBP14 Promotes The Proliferation And Migration Of Colon Carcinoma Cells Through Targeting IL-6/STAT3 Signaling Pathway
Authors Yang L, Zhang R, Yang J, Bi T, Zhou S
Received 9 July 2019
Accepted for publication 16 October 2019
Published 1 November 2019 Volume 2019:12 Pages 9069—9076
DOI https://doi.org/10.2147/OTT.S222555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr XuYu Yang
Leilei Yang, Ruili Zhang, Jie Yang, Tienan Bi, Shenkang Zhou
Department of Gastrointestinal Surgery, Taizhou Hospital of Zhejiang Province, Wenzhou Medical University, Linhai, Zhejiang 317000, People’s Republic of China
Correspondence: Shenkang Zhou
Department of Gastrointestinal Surgery, Taizhou Hospital of Zhejiang Province, Wenzhou Medical University, Ximen Street No.150, Linhai, Zhejiang 317000, People’s Republic of China
Tel +86-0576-85199876
Email [email protected]
Purpose: FK506-binding proteins 14 (FKBP14), a highly conserved protein, is identified as an oncogene in certain human tumors. However, the detailed biological function of FKBP14 in colon carcinoma remains unclear. The purpose of the present research is to examine the role of FKBP14 in human colon carcinoma cells.
Methods: In the present study, FKBP14 induced silencing and overexpression in colon carcinoma cells by using RNA interference (RNAi) and lentiviral vector, respectively. A specific JAK/STAT inhibitor AG490 was used to explore the relationship between FKBP14 and STAT3 in colon carcinoma cells. Moreover, quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot were used to examine the level of FKBP14 in colon carcinoma cells. Cell counting kit-8 (CCK-8) assay was used to determine the proliferation rate of colon carcinoma cells. Further, the migration rate of colon carcinoma cells was analyzed by performing a migration assay.
Results: Our results demonstrated that FKBP14 was upregulated in human colon carcinoma tissues. Moreover, high level of FKBP14 was associated with poor prognosis of colon carcinoma patients. Further, our findings firstly elucidated that FKBP14 was a pro-proliferation and migration factor in colon carcinoma cells. More importantly, FKBP14 might be a novel component in IL-6/JAK/STAT3 pathway and targeted STAT3 in colon carcinoma cells.
Conclusion: Our research not only indicated the potential signaling pathway of FKBP14 in colon carcinoma cells but also provided novel insight into the treatment for colon carcinoma.
Keywords: colon carcinoma, proliferation, migration, FKBP14, JAK/STAT, IL-6
Introduction
Colon carcinoma is one of the common tumors all over the world, which has ranked the third place in death-related cancers.1 Although the traditional methods (such as surgical operation and chemotherapy) partly contribute to its treatment, the overall survival rate is still limited within 5 years.2 Due to the high morbidity and mortality, much attention has been paid to investigate the novel therapy in colon carcinoma treatment. However, the molecule pathogenesis of colon carcinoma is far from being fully understood.
FK506-binding proteins (FKBP) are multifunctional proteins that are highly conserved across the species, which play a key role in immunosuppression and cancer biology.3 Moreover, FKBP family members are identified as promising new biomarkers for cancer.4 FK506-binding protein 14 (FKBP14), a member of FKBP, is identified as an oncogene in certain human tumors.5 A previous report has elucidated that FKBP14 overexpression promotes the growth of osteosarcoma cells.6 Moreover, FKBP14 silencing has reduced the proliferation, adhesion and invasion of gastric and ovarian cancer cells, respectively.7,8 Nevertheless, the biological role of FKBP14 is still unclear in colon carcinoma cells.
The signal transducer and activator of transcription 3 (STAT3) is a critical transcription factor, which is activated downstream of the gp130 receptor.9 It has been reported that knockdown of STAT3 suppresses angiogenesis in colon carcinoma.10,11 Moreover, STAT3 pathway is closely associated with the apoptosis and metastasis of colon carcinoma cells.12–14 Growing evidence have demonstrated that IL-6/STAT3 pathway is a potential therapeutic strategy in treating human cancers, including colorectal cancer,15 gastric cancer16 and liver cancer17 Targeting IL-6/STAT3 has provided therapeutic benefit for human cancers.18 However, the detailed molecule network of STAT3 is not clear in colon carcinoma cells.
The matrix metalloproteinases 2 (MMP2) belongs to the MMPs family, which plays a key role in the regulation of cell proliferation, migration, and differentiation.19–21 A previous report has demonstrated that MMP2 promotes the growth and invasion of colon carcinoma cells.22 Moreover, MMP2 is the downstream factor of STAT3 in esophageal squamous cells.23 However, the precise connection between MMP2 and STAT3 remains unclear in colon carcinoma cells.
To further analyze the function of FKBP14 in colon carcinoma cells, we induced silencing and overexpression of FKBP14 in colon carcinoma cells by using RNA interference (RNAi) and lentiviral vector, respectively. Our research not only enhanced the understanding of FKBP14 but also indicated its potential signaling pathway in colon carcinoma cells. Importantly, this investigation provided novel insight into the treatment for human colon carcinoma.
Methods
Tissue Specimens And Cell Culture
Human colon carcinoma and non-cancerous samples (n=38) were supported by Taizhou Hospital of Zhejiang Province, China. Moreover, the colon carcinoma cell lines used in this study were purchased from the cell bank of the Shanghai Biology Institute (Shanghai, China) including SW1116, SW480, RKO, HCT116, LOVO, HT29, CACO2 and FHC cells were grown in DMEM (Trueline, USA) and cultured in a 5% CO2 atmosphere at 37°C. The JAK/STAT inhibitor AG490 (Selleck, USA) and recombinant protein IL-6 (R&D, USA) were dissolved in DMSO and used for cell culture.
Patient And Public Involvement Statement
A total of 80 colon carcinoma patients were involved in a retrospective study. All patients were diagnosed and treated at the Taizhou Hospital of Zhejiang Province between (Zhejiang, China) between May 2007 and April 2008. There were 43 men and 37 women enrolled in the present study, age ranging from 33 to 71 years. The patients were followed up every 3 months after curative resection by an experienced nurse on clinic or by telephone interview. All patients provided written informed consent. Our research was approved by the independent Ethics Committee of Taizhou Hospital of Zhejiang Province and was in accordance with the Declaration of Helsinki.
RNA Isolation And Real-Time PCR
TRIzol Reagent (Invitrogen, USA) was used to extract total RNA. Then, RNA was reverse transcribed into complementary DNA (cDNA) by using the cDNA synthesis kit (Fermentas, Canada). The level of the target gene was normalized by the GAPDH, and relative gene expression was calculated by using the 2−ΔΔCt method. Three replicates were needed for each analysis. Primers that were used in this study are provided in Supplementary File 1.
Lentiviral-Mediated RNA Interference And Overexpression Of FKBP14
Three short interference RNA (siRNA) that target human gene FKBP14 (NM_017946.3) were synthesized (Major, Shanghai, China). Then, all of them were transfected into RKO and HCT116 cells, respectively. Moreover, the full-length of human FKBP14 cDNA was inserted into the lentiviral vector (pLVX-puro) and the recombined vector was transiently transfected into LOVO cells by using Lipofectamine 2000 (Invitrogen, USA). Analyses were conducted at 48 h after the transfection. The sequence of siFKBP14s was provided in Supplementary Table 1.
Western Blot
Protein was extracted by using RIPA lysis buffer (JRDUN, Shanghai, China) from different samples as indicated with an EDTA-free protease inhibitor cocktail (Roche, Germany) and quantified by an Enhanced BCA protein assay kit (Thermo Fisher, USA). Equal amounts of protein (25 μg) were separated by using 10% SDS-PAGE system, transferred onto a nitrocellulose membrane (Millipore, USA) and blotted with the primary antibodies followed by the secondary antibody, respectively. The target protein bands were scanned by an enhanced chemiluminescence system (Tanon, China). Each examination was tested in triplicate, and GAPDH was served as the internal reference. Primary antibodies that were used in this study are provided in Supplementary Table 2.
Cell Proliferation Assay
Cell counting kit-8 (CCK-8) assay (SAB, USA) was utilized to examine the cell proliferation profile according to the protocol of the manufacturer. The OD450nm value was quantified by a microplate reader (Pulangxin, China). Each time point was tested in triplicate.
Migration Assay
Briefly, each indicated siFKBP14 or oeFKBP14 cell was serum starved for 24 hrs followed by seeded in the upper chamber, while the medium supplemented with 30% fetal bovine serum (GIBCO, USA) was placed in the lower chamber. Cells on the upper side of the filters were removed. Then, the remaining cells were fixed in 4% formaldehyde and stained with 0.01% crystal violet (Solarbio, China) after 24 hrs of incubation. Then, the lower surface cells were stained with crystal violet and counted under a microscope, magnification, 200× (Caikon, China). All the procedures were maintained in Boyden chambers (Costar, China).
Immunohistochemistry (IHC)
The paraffin-embedded chordoma and adjacent matched tissues were cut into 3 μm slices. Then, the slices were deparaffinized in xylene and subsequently rehydrated in a series of graded alcohol dilutions. After that, antigen retrieval was performed through high pressure in sodium citrate solution (0.01 M) for 15 mins. The slices were incubated in H2O2 (3%) solution to block the activity of endogenous peroxidase. Next, all samples were incubated with rabbit antibody to human FKBP14 antibody (Ab 251703, abcam, UK) overnight at 4°C. Slides were then incubated with HRP at room temperature for 30 mins. Then, all samples were stained by using DAB and re-stained by hematoxylin for 3 mins. The images (magnification: 200 x) were collected by using the microscope (ECLIPSE Ni, NIKON, Japan) and analyzed by the micro-image analysis system (DS-Ri2, NIKON, Japan). The positive area of FKBP14 was determined by the average value from six regions of different tissues.
Statistical Analysis
Statistical analyses were performed with GraphPad Prism software Version 7.0 (CA, USA). Experimental results were expressed as the mean ± SD of at least three samples. Statistical significance was determined by one-way ANOVA for multiple comparisons. P value less than 0.05 was considered significant.
Results
FKBP14 Was Upregulated In Human Colon Carcinoma Tissues
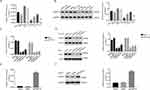
A total of 327 FKBP14 expression profiles were collected from The Cancer Genome Atlas (TCGA) colon carcinoma dataset, including 41 normal tissues and 286 tumor tissues. Our results suggested that the mRNA level of FKBP14 was upregulated in colon carcinoma tumors than that of normal tissues (Figure 1A). Meanwhile, Kaplan-Meier survival curves indicated overexpression of FKBP14 was associated with poor overall survival of colon carcinoma patients (Figure 1B). Data could be recalled at the following website: http://ualcan.path.uab.edu/cgi-bin/TCGA-survival 1.pl? genenam =FKBP14&ctype=COAD. Moreover, we also examined the mRNA level of FKBP14 in 30 pairs of colon carcinoma tissues and para-cancerous samples. As shown in Figure 1C, the relative mRNA level of FKBP14 was upregulated in colon carcinoma tumors than that in normal tissues. Further, results from the immunohistochemistry assay indicated that the protein level of FKBP14 was much higher in colon carcinoma tissues, especially in certain samples (Figure 1D). Overall, these results suggested the expression of FKBP14 showed heterogeneity in different human colon carcinoma tissues.
Knockdown And Overexpression Of FKBP14 In Colon Carcinoma Cells
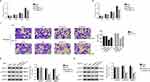
To further assess the function of FKBP14, FKBP14 induced silencing and overexpression in colon carcinoma cells, respectively. First, we examined the relative mRNA and protein level of FKBP14 in 8 colon carcinoma cell lines, including SW1116, SW480, RKO, HCT116, LOVO, HT29, CACO2 and FHC. As presented in Figure 2A and B, the relative mRNA and protein levels of FKBP14 were much higher in RKO and HCT116 cells than that of other cells. Moreover, both the relative mRNA and protein level of FKBP14 were significantly downregulated in LOVO cells compared with that in SW1116 cells. Therefore, RKO, HCT116 and LOVO cells were chosen for the following analyses.
For silencing FKBP14, three short interference RNAs (siRNAs) that targeting human gene FKBP14 (siFKBP14-1, siFKBP14-2 and siFKBP14-3) and a non-specific scrambled siRNA (siNC) were synthesized. Then, all of them were transfected into RKO or HCT116 cells, respectively. The untreated cells were treated as a blank control (BLANK). As shown in Figure 2C and D, all FKBP14 siRNAs were well functioned and strongly reduced the expression of endogenous FKBP14 in colon carcinoma cells. Further, the level of FKBP14 was lower in RNAi1-1 or RNAi1-2 transfected cells than that in RNAi1-3 transfected cells.
For overexpression assay, the full length of FKBP14 cDNA was inserted into the lentiviral vector. Then, the recombine vector was transfected into LOVO cells for overexpressing FKBP14 (oeFKBP14). Meanwhile, a mock plasmid was served as a negative control (oeNC). Both the mRNA and protein level of FKBP14 were remarkably upregulated in oeFKBP14-transfected cells (Figure 2E and F). Therefore, RNAi1-1-, RNAi1-2- and oeFKBP14-transfected cells were chosen in the following analysis.
FKBP14 Silencing Suppressed The Proliferation And Migration Of Colon Carcinoma Cells
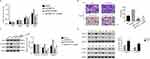
CCK-8 assay was performed to quantify the proliferation rate of different transfected cells as indicated. As shown in Figure 3A and B, the proliferation rate showed no significant difference between BLANK and siNC cells. However, the proliferation of siFKBP14 transfected cells was deeply decreased compared with that in siNC-transfected cells. Therefore, these results suggested that FKBP14 was a pro-proliferation regulator in colon carcinoma cells. Moreover, knockdown of FKBP14 significantly reduced the migration rate of colon carcinoma cells (Figure 3C). Therefore, FKBP14 promoted the migration of colon carcinoma cells.
Then, Western blot was used to examine the protein content of STAT3, the phosphorylation of STAT3 (p-STAT3) and MMP2 in siFKBP14 transfected cells. As presented in Figure 3D and E, knockdown of FKBP14 significantly inhibited the expression of MMP2 in colon carcinoma cells. Interestingly, our findings indicated that FKBP14 silencing deeply suppressed. These results were in agreement in two cell lines. Overall, these results indicated that FKBP14 was positively correlated with p-STAT3 and MMP2 in colon carcinoma cells.
The JAK-STAT Inhibitor AG490 Abolished The Function Of FKBP14 In Colon Carcinoma Cells
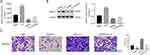
To further analyze the connection between FKBP14 and STAT3 in colon carcinoma cells, the JAK-STAT inhibitor AG490 (50μM) was used to suppress the activity of STAT3 in oeNC or oeFKBP14-transfected cells, respectively. As shown in Figure 4A, the proliferation rate of oeFKBP14-transfected cells was much higher than that in oeNC transfected cell, whereas this effect was deeply abolished by the JAK-STAT inhibitor AG490. Moreover, FKBP14 overexpression significantly promoted the migration of colon carcinoma cells. Furthermore, the JAK-STAT inhibitor AG490 deeply inhibited the role of oeFKBP14 in colon carcinoma cell migration (Figure 4B). Further, the JAK-STAT inhibitor AG490 also significantly suppressed the expression of MMP2 and p-STAT3 in oeFKBP14-transfected cells (Figure 4C). Much importantly, both the related protein content of FKBP14 and p-STAT3 were significantly upregulated in colon carcinoma tissues (n=8) than that of normal samples (Figure 4D). Taken together, all these results demonstrated that FKBP14 was positively correlated with the phosphorylation of STAT3. FKBP14 might promote the proliferation and migration of colon carcinoma cells through improving the phosphorylation of STAT3.
The Recombinant Protein Interleukin-6 Could Rescue The Function Of FKBP14 In Colon Carcinoma Cells
To investigate the relationship between FKBP14 and IL-6 in colon carcinoma cells, the human recombinant protein IL-6 (10ng/mL) was used to culture LOVO cells. As shown in Figure 5A and B, both the mRNA and protein level of FKBP14 were significantly upregulated in LOVO cells in the presence of recombinant protein IL-6. Meanwhile, the JAK-STAT inhibitor AG490 deeply suppressed the expression of FKBP14 in colon carcinoma cells. Interestingly, the level of FKBP14 in the recombinant protein IL-6 cultured cells was remarkably inhibited in the presence of the inhibitor AG490.
Moreover, our results revealed that the recombinant protein IL-6 increased the migration rate of siFKBP14-transfected cells (Figure 5C). Taken together, all these results suggested that the recombinant protein IL-6 could rescue the function of FKBP14 in colon carcinoma cells.
Discussion
Colon carcinoma is one of the common death-related cancer all over the world, which has high metastasis.24 Most of colon carcinoma deaths are closely associated with the metastasis. However, the underlying mechanism of colon carcinoma metastasis is still not clear. In this research, we aimed to examine the role of FBKP14 in the migration of colon carcinoma cells. It was the first time to indicate that FKBP14 was a pro-proliferation migration factor in colon carcinoma cells. Therefore, our analyses indicated the potential value of FKBP14 as a therapy target in the treatment of colon carcinoma.
A previous report has demonstrated that STAT3 is a promising biomarker for colon carcinoma diagnosing.25 Suppressing the activity of STAT3 contributes to inhibiting the growth of colon carcinoma cells.11,26 Moreover, JAK/STAT signaling pathway plays a critical role in the migration of breast cancer cells.27 Activating JAK/STAT3 signaling pathway promotes the development of colon carcinoma cells.28 In the present study, our results indicated that the phosphorylation of STAT3 was positively correlated with FKBP14 in colon carcinoma cells. Therefore, FKBP14 might promote the proliferation and migration through promoting the activity of the JAK/STAT signaling pathway in colon carcinoma cells.
Moreover, a previous report has illustrated that STAT3 targets MMP2 in cell migration and invasion of esophageal squamous carcinoma cells.23,29 In this research, our results suggested that FKBP14 was positively correlated with MMP2 in colon carcinoma cells. Therefore, MMP2 might involve in FKBP14/STAT3 signaling pathway in colon carcinoma cells.
Interleukin-6 (IL-6) is a pleiotropic cytokine, which not only plays an important role in a wide range of various biological activities but also promotes the progression of colon carcinoma.30 Moreover, IL-6/JAK/STAT3 pathway has affected the proliferation and metastasis of colon carcinoma cells.31 In this study, our results indicated that IL-6 rescued the function of FKBP14 in colon carcinoma cells. Therefore, FKBP14 might be the novel component in IL-6/JAK/STAT3 signaling pathway. FKBP14 might promote the proliferation and migration of colon carcinoma cells through regulating the IL-6/JAK/STAT3 pathway.
Conclusion
In brief, we concluded that FKBP14 was a pro-proliferation factor and positively correlated with STAT3 and MMP2 in colon carcinoma cells. This research not only enhanced the understanding of FKBP14 but also indicate its potential signaling pathway in colon carcinoma cells.
Acknowledgments
This study was supported by the Science and Technology Department of Taizhou City (No. 1701KY07) and Zhejiang Medical and Health Youth Talents Project (No. 2019RC087)
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jafri SH, Mills G. Lifestyle modification in colorectal cancer patients_ an integrative oncology approach. Future Oncol. 2013;9(2):207–218. doi:10.2217/fon.12.184
2. Li X, Peng S. Identification of metastasis-associated genes in colorectal cancer through an integrated genomic and transcriptomic analysis. Chin J Cancer Res. 2013;25(6):623–636. doi:10.3978/j.issn.1000-9604.2013.11.01
3. Romano S, D’Angelillo A, Romano MF. Pleiotropic roles in cancer biology for multifaceted proteins FKBPs. Biochim Biophys Acta Gen Subj. 2015;1850(10):2061–2068. doi:10.1016/j.bbagen.2015.01.004
4. Solassol JM, Mange A, Maudelonde T. FKBP family proteins as promising new biomarkers for cancer. Curr Opin Pharmacol. 2011;11(4):320–325. doi:10.1016/j.coph.2011.03.012
5. Ghartey-Kwansah G, Li Z, Feng R, et al. Comparative analysis of FKBP family protein: evaluation, structure, and function in mammals and drosophila melanogaster. BMC Dev Biol. 2018;18(1):7. doi:10.1186/s12861-018-0167-3
6. Huang Z, Xiao L, Li J, Tong P. FKBP14 overexpression contributes to osteosarcoma carcinogenesis and indicates poor survival outcome. Oncotarget. 2015;7(26):39872.
7. Wang R, Fang H, Fang Q. Downregulation of FKBP14 by RNA interference inhibits the proliferation, adhesion and invasion of gastric cancer cells. Oncol Lett. 2017;13(4):2811–2816. doi:10.3892/ol.2017.5781
8. Lu M, Miao Y, Qi L, Bai M, Zhang J, Feng Y. RNAi-mediated downregulation of FKBP14 suppresses the growth of human ovarian cancer cells. Oncol Res. 2016;23(6):267–274. doi:10.3727/096504016X14549667333963
9. Rebecca T. Hepatoprotection via the IL-6/Stat3 pathway. J Clin Invest. 1900;10(39):349–369.
10. Qian WF, Guan WX, Gao Y, et al. Inhibition of STAT3 by RNA interference suppresses angiogenesis in colorectal carcinoma. Braz J Med Biol Res. 2011;44(12):1222–1230. doi:10.1590/S0100-879X2011007500143
11. Zhang J, Lu Y, Yue X, et al. MiR-124 suppresses growth of human colorectal cancer by inhibiting STAT3. PLoS One. 2013;8(8):e70300. doi:10.1371/journal.pone.0070300
12. Rokavec M, Öner MG, Li H, Hermeking H. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124(4):1853–1867. doi:10.1172/JCI73531
13. Liu H, Ren G, Wang T, et al. Aberrantly expressed Fra-1 by IL-6/STAT3 transactivation promotes colorectal cancer aggressiveness through epithelial-mesenchymal transition. Carcinogenesis. 2015;36(4):459–468. doi:10.1093/carcin/bgv017
14. Xiong H, Zhang Z-G, Tian X-Q, et al. Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells. Neoplasia. 2008;10(3):287–297. doi:10.1593/neo.07971
15. Shu-Wei W, Yue-Ming S. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (review). Int J Oncol. 2014;44(4):1032–1040. doi:10.3892/ijo.2014.2259
16. Wu X, Tao P, Zhou Q, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8(13):20741–20750. doi:10.18632/oncotarget.15119
17. Wang X, Sun W, Shen W, et al. Long non-coding RNA DILC represses self-renewal of liver cancer stem cells via inhibiting autocrine IL-6/STAT3 axis. J Hepatol. 2016;64(6):1283–1294. doi:10.1016/j.jhep.2016.01.019
18. Johnson DE, OK RA, JR G. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15(4):234. doi:10.1038/nrclinonc.2018.8
19. Rempe RG, Hartz AM, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: versatile breakers and makers. J Cereb Blood Flow Metab. 2016;36(9):1481–1507. doi:10.1177/0271678X16655551
20. Cui N, Hu M, Khalil RA. Biochemical and biological attributes of matrix metalloproteinases. Prog Mol Biol Transl Sci. 2017;147:1–73.
21. Tokito A, Jougasaki M. Matrix metalloproteinases in non-neoplastic disorders. Int J Mol Sci. 2016;17(7):1178. doi:10.3390/ijms17071178
22. Dong W, Li H, Zhang Y, et al. Matrix metalloproteinase 2 promotes cell growth and invasion in colorectal cancer. Acta Biochim Biophys Sin (Shanghai). 2011;43(11):840–848. doi:10.1093/abbs/gmr085
23. Xuan X, Li S, Lou X, et al. Stat3 promotes invasion of esophageal squamous cell carcinoma through up-regulation of MMP2. Mol Biol Rep. 2015;42(5):907–915. doi:10.1007/s11033-014-3828-8
24. Tao YJ, Li YJ, Zheng W, et al. Antisense oligonucleotides against microRNA-21 reduced the proliferation and migration of human colon carcinoma cells. Cancer Cell Int. 2015;15:77. doi:10.1186/s12935-015-0228-7
25. Gordziel C, Bratsch J, Moriggl R, Knosel T, Friedrich K. Both STAT1 and STAT3 are favourable prognostic determinants in colorectal carcinoma. Br J Cancer. 2013;109(1):138–146. doi:10.1038/bjc.2013.274
26. Ren D, Lin B
27. Khanna P, Lee JS, Sereemaspun A, Lee H, Baeg GH. GRAMD1B regulates cell migration in breast cancer cells through JAK/STAT and Akt signaling. Sci Rep. 2018;8(1). doi:10.1038/s41598-018-27864-6
28. Xue X, Jungles K, Onder G, Samhoun J, Győrffy B, Hardiman KM. HIF-3α1 promotes colorectal tumor cell growth by activation of JAK-STAT3 signaling. Oncotarget. 2016;7(10). doi:10.18632/oncotarget.7272
29. Ou Y, Liu L, Xue L, et al. TRAP1 shows clinical significance and promotes cellular migration and invasion through STAT3/MMP2 pathway in human esophageal squamous cell cancer. J Genet Genomics. 2014;41(10):529–537. doi:10.1016/j.jgg.2014.08.004
30. Xu J, Ye Y, Zhang H, et al. Diagnostic and prognostic value of serum interleukin-6 in colorectal cancer. Medicine. 2016;95(2):e2502. doi:10.1097/MD.0000000000002502
31. Wang S, Zhang W. Genetic variants in IL-6/JAK/STAT3 pathway and the risk of colon carcinoma. Tumour Biol. 2016;37(5):6561–6569. doi:10.1007/s13277-015-4529-1
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.