Back to Journals » Clinical Ophthalmology » Volume 11
Fixed combination of travoprost and timolol maleate reduces intraocular pressure in Japanese patients with primary open-angle glaucoma or ocular hypertension: analysis by prostaglandin analogue
Authors Nakano T , Mizoue S , Fuse N, Iwase A, Matsumoto S, Yoshikawa K
Received 25 August 2016
Accepted for publication 27 September 2016
Published 20 December 2016 Volume 2017:11 Pages 55—61
DOI https://doi.org/10.2147/OPTH.S120639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Tadashi Nakano,1 Shiro Mizoue,2 Nobuo Fuse,3 Aiko Iwase,4 Shun Matsumoto,5 Keiji Yoshikawa6
On behalf of the I.CHANGE2 study group
1Department of Ophthalmology, The Jikei University School of Medicine, Nishi-Shimbashi, Minato-ku, Tokyo, 2Department of Ophthalmology, Ehime University Graduate School of Medicine, Shitsukawa, Toon-city, Ehime, 3Department of Integrative Genomics, Tohoku Medical Megabank Organization, Seiryo-machi, Sendai, Miyagi, 4Tajimi Iwase Eye Clinic, Hon-machi, Tajimi, Gifu, 5Department of Ophthalmology, Tokyo Teishin Hospital, Fujimi, Chiyoda-ku, 6Yoshikawa Eye Clinic, Naka-machi, Machida, Tokyo, Japan
Background: We have shown a decrease in mean intraocular pressure (IOP) by switching to travoprost/timolol fixed combination (TTFC) in subjects receiving prostaglandin analogue (PGA) monotherapy and requiring additional medication in a previous report. For analyzing factors affecting IOP reduction, baseline IOP and preceding PGA were selected as statistically and clinically significant factors. In this report, we examine IOP-lowering effect and adverse drug reactions by preceding PGA.
Methods: Patients with primary open angle glaucoma or ocular hypertension who received monotherapy with one of four PGAs (travoprost, latanoprost, tafluprost, or bimatoprost) for at least 3 months at 26 institutions and were determined to require additional medication by their primary physician were included. IOP reduction and adverse events were examined at 4, 8, and 12 weeks for each of four PGAs after switching to TTFC.
Results: In total, 157 patients who could be followed up for at least 4 weeks after switching to TTFC were included in the efficacy analysis. Multiple regression analysis was performed, and baseline IOP and PGA were found to be significant factors to IOP reduction. IOP reduction at week 12, adjusted with the regression model, was -3.5, -1.8, and -1.4 mmHg in the tafluprost, latanoprost, and travoprost groups, whereas it was -0.5 mmHg in the bimatoprost group. Along with differences in baseline IOP between groups, an IOP-lowering effect of >1 mmHg was noted in the tafluprost, latanoprost, and travoprost groups after the switch. IOP was maintained at 13.8–14.8 mmHg throughout the follow-up period. No serious adverse events or noteworthy issues were observed in any group after the switch.
Conclusion: Clinically significant IOP-reducing effects of TTFC were observed in the latanoprost, travoprost, and tafluprost groups when switching from each PGA monotherapy, while there were some differences in effects between groups, with minimal safety concerns.
Keywords: adverse event intraocular pressure, prostaglandin analogue, switching therapy, travoprost/timolol fixed combination
Introduction
Prostaglandin analogue (PGA) is currently the first-line therapy with ophthalmic solution for glaucoma and is considered a potent agent for lowering intraocular pressure (IOP).1,2 Over 40% of glaucoma patients require further reduction of IOP owing to the chronic progressive nature of the disease.2,3
The fixed combination (FC) drug containing PGA and a β-blocker has additional IOP-lowering effects and no major safety issues when switching from PGA.4–8 The additional administration of an ophthalmic solution has been shown to reduce medication adherence,9 a key factor in the success or failure of treatment.10 A recently developed FC drug containing PGA and a β-blocker did not increase the frequency of instillation and therefore exhibited additional IOP-lowering effects as well as a favorable effect on medication adherence.11,12
In the previous report, we examined the efficacy of travoprost/timolol fixed combination (TTFC: DuoTrav®, Alcon Laboratories, Inc., Fort Worth, TX, USA) in patients who had switched from one of four PGAs approved for use in Japan (travoprost, latanoprost, tafluprost, and bimatoprost), including those with IOP values ≤19 mmHg before the switch. Linear multiple regression analysis was performed to investigate the factors affecting IOP reduction in patients who could be followed up for a minimum of 4 weeks after the instillation of TTFC. Preceding PGA and baseline IOPs were found to be statistically and clinically significant factors affecting IOP reduction. Subgroup analysis of IOP reduction by baseline IOP was performed, and significant reductions were observed in proportion to the baseline IOP.13
This report aimed to examine the IOP-lowering effect by preceding PGA in patients with glaucoma.
Methods
Subjects
Patients with primary open angle glaucoma (POAG, broad sense) and ocular hypertension (OH) receiving monotherapy with one of the four PGAs approved for use in Japan were included in this study. The attending physician for various reasons, including progression of visual field disturbance and insufficient IOP control, prescribed additional administration of ophthalmic solution for these patients. The exclusion criteria were as follows: a mean visual field deviation of <−20 dB; history of hypersensitivity to β-blockers or conditions where β-blockers are contraindicated (eg, bronchial asthma, inadequately controlled cardiac failure); concurrent, chronic or recurrent uveal inflammation, scleral inflammation, or corneal herpes; history of ocular trauma, internal eye surgery, or laser surgery within 3 months before the baseline examination; difficulty in undergoing applanation tonometry; use of corticosteroid ophthalmic solution; serious ocular complication; pregnant or lactating women; and severe dementia. In addition, patients with serious ocular complications and those determined to be inappropriate to participate in the study by their primary physician were excluded from the study. Gender and the presence or absence of dry eye were not evaluated at the time of enrollment in the study.
Procedures
This study was approved by the respective institutional ethical review boards of the Jikei University School of Medicine, Tohoku University Hospital, Juntendo University Urayasu Hospital, Nakano General Hospital, Fussa Hospital, Fukui-ken Saiseikai Hospital, and Minami Matsuyama Hospital, as well as by the ethical review board of Kita-machi Clinic for other institutions.
Potential subjects who met the inclusion criteria received sufficient explanation regarding the study and information concerning the treatment according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients included in the study.
A single drop of TTFC was instilled into the conjunctival sac of one or both eyes of the patients once a day in the morning, 1 day after discontinuation of PGA.
During the study period, IOP was measured twice by the same examiner using the Goldmann applanation tonometer, and the mean of the measurement values was adopted. In principle, IOP was measured ~2 hours from the time of initial measurement. We examined safety endpoint by preceding 4 PGAs. This study was conducted between August 2011 and March 2013.
Statistical analysis
IOP values and changes in these values from baseline were considered as the efficacy end points, whereas adverse events and symptoms such as Superficial Punctate Keratitis (SPK and hyperemia were taken as the safety end points.
The eye with a higher eligible baseline IOP, or the right eye in cases where the IOP of both eyes was equivalent, was used as the study eye. IOP reduction by preceding PGA was investigated with mean IOP changes from baseline values adjusted by a regression model, as described in our previous report.13 Furthermore, descriptive statistics including mean and standard deviation (SD) of the IOP value at each visit were provided in order to examine the IOP profile of the patient throughout the study period after switching to TTFC. Missing IOP values after a period of 4 weeks were imputed with preceding measured values (last observation carried forward; LOCF method).
Analysis was conducted by QOL RD Co., Ltd (Nihonbashi, Chuo-ku, Tokyo) and SOC Corporation (Akasaka, Minato-ku, Tokyo). The software used for the analyses was JMP ver. 9.0 and SAS® 9.3 (SAS Institute Inc., Cary, NC, USA).
Results
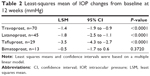
Of 166 patients who provided informed consent, 162 (97.6%) satisfied the inclusion criteria and five dropped out within 4 weeks (three discontinued at their own discretion, two made no hospital visits); thus, a total of 157 patients (96.9%) who could be followed up for more than 4 weeks were included in the final efficacy analysis set. These 157 patients included 54 men and 103 women, with a mean age of 66.6±11.3 years (age range, 39–88 years). As shown in Table 1, of the four preceding PGAs used, travoprost was most commonly administered (n=70, 44.6%), followed by latanoprost (n=45, 28.7%), tafluprost (n=29, 18.5%), and bimatoprost (n=13, 8.3%). Mean IOP changes from baseline at 12 weeks adjusted with a linear regression model were −1.4 mmHg in travoprost, −1.8 mmHg in latanoprost, −3.5 mmHg in the tafluprost group (P<0.0001), and −0.5 mmHg in the bimatoprost group (P=0.3720; Table 2). However, baseline IOPs before switching were different among the four PGA groups: 16.1±2.6 mmHg in the travoprost group, 15.6±2.9 mmHg in the latanoprost group, 17.5±3.2 mmHg in the tafluprost group, and 17.2±5.1 mmHg in the bimatoprost group (Table 3). To examine IOP profile irrespective of baseline IOP after switching to TTFC, descriptive statistics (arithmetic mean and SD) of the IOP values were provided for each PGA group (Table 3). Patients who switched from travoprost, latanoprost, and tafluprost presented with IOP values ranging from 13.8 to 14.8 mmHg from weeks 4 to 12 after switching to TTFC, and showed IOP reductions of 1 mmHg or more when compared to baseline values; whereas the IOP values of patients in the bimatoprost group ranged from 15.4 to 17.5 mmHg (Table 3). Missing IOP values were observed in four patients belonging to the travoprost, latanoprost, and tafluprost groups, and in two patients from the bimatoprost group.
  | Table 1 Demographic data of per protocol set |
  | Table 3 Summary of measured IOP |
SPK was compared against baseline values for each PGA by dividing the cornea into five quadrants. No clinically significant worsening was noticed in any of the groups (Table 4). Similarly, no clinically significant worsening of conjunctival hyperemia was observed in the patients after switching from PGA to TTFC (Table 4).
  | Table 4 Superficial punctate keratopathy scores and conjunctival hyperemia scores |
During the study period, 43 adverse events occurred in 27 (16.3%) of the 166 patients; of these, 26 (15.7%) patients presented with 42 adverse drug reactions whose causal relationship with TTFC could not be ruled out. All events ranged from mild to moderate in intensity. In the case of preceding PGA administration, seven events had occurred in six patients in the travoprost group, 14 events in nine patients in the latanoprost group, 18 events in nine patients in the tafluprost group, and three events in two patients in the bimatoprost group (Table 5).
  | Table 5 Adverse events related to the study drug |
Mean systolic and diastolic blood pressure were maintained at 127.9–138.3 and 72.4–81.9 mmHg, respectively, in all groups during the study period, whereas mean pulse rate was maintained at 63.0–75.6 bpm.
Discussion
In our previous report, we showed the safety and efficacy of switching from PGA to TTFC in Japanese patients with POAG and OH.13 We had performed a multiple linear regression analysis in order to identify the factors that affect the reduction of IOP 12 weeks after switching to TTFC; types of preceding PGA treatments, age, sex, baseline IOP, dosing period of premedication, baseline MD, and baseline PSD were set as independent variables, and changes from baseline in IOP at 12 weeks was used as the dependent variable in that study. Multiple linear regression is ordinarily used as a statistical method to identify the relationship between a dependent variable and one or more independent variables. Baseline IOP and PGA were selected as significant factors (statistical and clinical) that may have an effect on IOP reduction at 12 weeks. In the present report, we analyzed and compared the IOP-lowering effect of four preceding PGAs, since we had already examined the association between baseline IOP values and the changes from baseline values in our previous report; we found that the higher the baseline IOP, the larger the reduction in IOP.13
The mean IOP reductions at 12 weeks after switching from the three PGAs, except for bimatoprost, to TTFC, were −3.5, −1.8, and −1.4 mmHg in the tafluprost, latanoprost, and travoprost groups (P<0.0001), showing additional IOP-lowering effect, respectively. Since only IOP reduction using a regression model was evaluated in the above analysis, the descriptive statistics of the IOP (arithmetic mean and SD) at baseline as well as at observation time points of 4, 8, and 12 weeks after switching to TTFC were also calculated in order to examine the changes in IOP in more detail. There was a maximum difference of ~2 mmHg in baseline IOP between the four groups. However, patients in the tafluprost, latanoprost, and travoprost groups maintained mean IOP values ranging from 13.8 to 14.8 mmHg from weeks 4 to 12 after switching to TTFC, and showed a mean IOP reduction of 1 mmHg or more when compared to the baseline. Patients in bimatoprost group presented with mean IOP values of 15.4 to 17.5 mmHg from weeks 4 to 12. These findings indicate the clinically significant IOP-lowering effect of TTFC when switching from PGA, except for bimatoprost.
The largest IOP reduction was observed in patients belonging to the tafluprost group, followed by those in the travoprost and latanoprost groups. The IOP-lowering effect of tafluprost cannot be sufficiently evaluated because of the fewer number of reports available when compared to those of other PGAs; some of these reports indicate that the effect of tafluprost might be slightly inferior to those of the other PGAs.14–16 In the present report, it is suggested that the IOP-lowering effect of TTFC may reflect not only the additional effect of the β-blocker but also the changes in PGA when switching from tafluprost.
Stable IOP-lowering effect was also observed after switching from travoprost to TTFC, demonstrating the effect of adding a β component while switching to a compounded drug. TTFC has been reported to have a neutral pH, which allows for the high permeability of the β component into the eye.17 Quaranta et al discussed the IOP-lowering effect of TTFC when switched from travoprost monotherapy in their review report.18,19 From their reports there is a possibility of greater IOP-lowering effect of TTFC when switched from travoprost than other FC when switched from each PGA. This feature may be reflected in the results of the present study.
The mean IOP level was maintained at 13.8–14.2 mmHg throughout the study period, and additional IOP-lowering effects of 1.4–1.8 mmHg were observed in patients who switched from latanoprost to TTFC. Furthermore, the baseline IOP was lower in this group when compared to the other three groups. There are a lot of patients with normal tension glaucoma in Japan,20,21 and several of them often require further IOP reduction due to progression of visual field disturbance.22 The results of the present study suggest that switching to combination drugs such as TTFC is useful for patients with adequately lowered IOP, and that TTFC can be an option while considering a switch from latanoprost to a combination drug containing PGA.
Meanwhile, no IOP reduction was noted in patients switching from bimatoprost to TTFC. Bimatoprost has been reported to have the most superior IOP-lowering effect,14 and there is a possibility that the effect of switching to the FC has not been clearly exhibited in the present report because IOP was already reduced to adequate levels. In addition, among the four types of PGA approved for use in Japan, the number of patients using bimatoprost is small, thus a sufficient number of patients could not be enrolled in this group in the study. Hence, more patients may be needed to examine the efficacy in future studies.
Safety was examined in each group considering the possibility that there might be a difference in the trend of adverse drug reactions by preceding PGA; however, no apparent differences were noted.
The IOP-lowering effect of TTFC had some differences between previous PGA groups. Nevertheless, there was an imbalance in the number of patients in each PGA group in this study. Moreover, owing to the absence of randomization, there were discrepancies in the distributions of the baseline characteristics of the patients resulting in insufficient statistical accuracy, which is a limitation of this study. The usefulness of switching to combination drugs from PGA can be further exhibited by planning a prospective confirmatory study with randomization of the premedication.
Meanwhile, the importance of adherence to instillation therapy is attracting attention as a measure against visual function impairment associated with glaucoma.23 The use of FC drugs requiring no increase in the number of ophthalmic solutions has been reported to actually achieve favorable adherence.24
In conclusion, the findings of this study show that switching from tafluprost, latanoprost, and travoprost to TTFC achieved further clinically significant IOP-lowering effect, revealing no major safety issues, thereby indicating the usefulness of switching to a combination drug in patients receiving PGA.
Acknowledgments
This study was conducted with funding and support from the Japan Association of Health Service and Alcon Japan Ltd. The article processing charges and open access fee for this publication were funded by Alcon Japan Ltd. The assistance service for English language editing was funded by Alcon Japan Ltd.
IOP Checked and Assessed in Normal tension Glaucoma by Exceptional glaucomatologists 2 (I.CHANGE2) study group: Okazaki Eye Clinic, Hokkaido, Japan: Hiroko Okazaki; Department of Ophthalmology, Tohoku University, Miyagi, Japan: Nobuo Fuse; Kido Eye Clinic, Niigata, Japan: Motohiro Shirakashi; Yaoeda Eye Clinic, Niigata, Japan: Kiyoshi Yaoeda; Juntendo University Urayasu Hospital, Chiba, Japan: Itaru Kimura; Department of Ophthalmology, The Jikei University School of Medicine, Tokyo, Japan: Tadashi Nakano; Yoshikawa Eye Clinic, Tokyo, Japan: Keiji Yoshikawa; Ueno Eye Clinic, Tokyo, Japan: Tairo Kimura; Nakano General Hospital, Tokyo, Japan: Hirotaka Suzumura; Department of Ophthalmology, Fussa Hospital, Tokyo, Japan: Toyoaki Tsumura; Nihonmatsu Eye Hospital, Tokyo, Japan: Mami Nanno; Nishikamakura Tanino Eye Clinic, Kanagawa, Japan: Tomihiko Tanino; Department of Ophthalmology, Fukui-ken Saiseikai Hospital, Fukui, Japan: Koji Nitta; Tajimi Iwase Eye Clinic, Gifu, Japan: Aiko Iwase; Yamabayashi Eye Clinic, Aichi, Japan: Shigeki Yamabayashi; Reiko Eye Clinic, Osaka, Japan: Reiko Sugimoto; Hirakata Yamagishi Eye Clinic, Osaka, Japan: Kazuya Yamagishi; Kozaki Eye Clinic, Osaka, Japan: Jun Kozaki; Sagara Eye Clinic, Yamaguchi, Japan: Takeshi Sagara; Department of Ophthalmology, Minami-Matsuyama Hospital, Ehime, Japan: Shiro Mizoue; Mizoguchi Eye Clinic, Nagasaki, Japan: Takanori Mizoguchi; Ozaki Eye Clinic, Miyazaki, Japan: Mineo Ozaki; Unoki Eye Clinic, Kagoshima, Japan: Kazuhiko Unoki; Nakamura Eye Clinic, Okinawa, Japan: Yoshimi Nakamura.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work. All authors take responsibility for the integrity of the work as a whole, and have given final approval for the current version to be published. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Disclosures
Tadashi Nakano has received consultant fees from Topcon Corporation and lecture fees from Alcon Japan Ltd, Santen Pharmaceutical Co., Ltd, Otsuka Pharmaceutical Co., Ltd, R-Tech Ueno Ltd, Senju Pharmaceutical Co., Ltd, Carl Zeiss Co., Ltd, Kaken Pharmaceutical Co., Ltd, MSD K.K., and Pfizer Japan Inc. Shiro Mizoue has received lecture fees from Alcon Japan Ltd, Pfizer Japan Inc., MSD K.K, NIDEK Co., Ltd, Senju Pharmaceutical Co., Ltd, Santen Pharmaceutical Co., Ltd, KOWA Pharmaceutical Co., Ltd, R-Tech Ueno Ltd, and Kaken Pharmaceutical Co., Ltd. Nobuo Fuse has received lecture fees from Alcon Japan Ltd, Santen Pharmaceutical Co., Ltd, Pfizer Japan Inc., R-Tech Ueno Ltd, and MSD K.K. Aiko Iwase has a patent for Topcon Corporation and received payment for clinical trial from Heidelberg Engineering, Inc., Topcon Corporation and Santen Pharmaceutical Co., Ltd, and lecture fees from Alcon Japan Ltd, Santen Pharmaceutical Co., Ltd, Carl Zeiss Co., Ltd, Senju Pharmaceutical Co., Ltd, Otsuka Pharmaceutical Co., Ltd, KOWA Pharmaceutical Co., Ltd, Topcon Corporation and Pfizer Japan Inc. Shun Matsumoto has received lecture fees from Alcon Japan Ltd, Senju Pharmaceutical Co., Ltd, Santen Pharmaceutical Co., Ltd, and Kaken Pharmaceutical Co., Ltd. Keiji Yoshikawa has received fees for expert testimony from Santen Pharmaceutical Co., Ltd, and lecture fees from Alcon Japan Ltd, Santen Pharmaceutical Co., Ltd, Pfizer Japan Inc., Otsuka Pharmaceutical Co., Ltd, R-Tech Ueno Ltd, Kaken Pharmaceutical Co., Ltd, Senju Pharmaceutical Co., Ltd, KOWA Pharmaceutical Co., Ltd, and MSD KK. The authors report no other conflicts of interest related to this work.
References
Rahman MQ, Abeysinghe SS, Kelly S, et al. Persistence of glaucoma medical therapy in the Glasgow glaucoma database. Br J Ophthalmol. 2011;95(7):966–970. | ||
Werner EB, Drance SM. Progression of glaucomatous field defects despite successful filtration. Can J Ophthalmol. 1977;12(4):275–280. | ||
Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary-open angle glaucoma. Arch Ophthalmol. 2002;120(6):701–713. | ||
Dunker S, Schmucker A, Maier H; Latanoprost/Timolol Fixed Combination Study Group. Tolerability, quality of life, and persistency of use in patients with glaucoma who are switched to the fixed combination of latanoprost and timolol. Adv Ther. 2007;24(2):376–386. | ||
Costa VP, Moreira H, Paolera MD, de Moraes Silva MR. Efficacy and safety of travoprost 0.004%/timolol 0.5% fixed combination as transition therapy in patients previously on prostaglandin analog monotherapy. Clin Ophthalmol. 2012;6:699–706. | ||
Pfeiffer N, Scherzer ML, Maier H, et al. Safety and efficacy of changing to the travoprost/timolol maleate fixed combination (DuoTrav) from preceding mono- or adjunctive therapy. Clin Ophthalmol. 2010;4:459–466. | ||
Arend KO, Raber T. Observational study results in glaucoma patients undergoing a regimen replacement to fixed combination travoprost 0.004%/timolol 0.5% in Germany. J Ocul Pharmacol Ther. 2008;24(4):414–420. | ||
Paranhos A, Mendonça M, Silva MJ, et al. Hyperemia reduction after administration of a fixed combination of bimatoprost and timolol maleate to patients on prostaglandin or prostamide monotherapy. J Ocul Pharmacol Ther. 2010;26(6):611–615. | ||
Djafari F, Lesk MR, Harasymowycz PJ, Desjardins D, Lachaine J. Determinants of adherence to glaucoma medical therapy in a long-term patient population. J Glaucoma. 2009;18(3):238–243. | ||
Sleath B, Robin AL, Covert D, Byrd JE, Tudor G, Svarstad B. Patient-reported behavior and problems in using glaucoma medications. Ophthalmology. 2006;113(3):431–436. | ||
Chrai SS, Makoid MC, Eriksen SP, Robinson JR. Drop size and initial dosing frequency problems of topically applied ophthalmic drugs. J Pharm Sci. 1974;63(3):333–338. | ||
Higginbotham EJ. Considerations in glaucoma therapy: fixed combinations versus their component medications. Clin Ophthalmol. 2010;4:1–9. | ||
Nakano T, Mizoue S, Fuse N, Iwase A, Matsumoto S, Yoshikawa K. Fixed combination of travoprost and timolol maleate reduces intraocular pressure in Japanese patients with primary open-angle glaucoma or ocular hypertension: a prospective multicenter open-label study. Adv Ther. 2015;32(9):823–837. | ||
Tianjing Li, Lindsley K, Rouse B, et al. Comparative effectiveness of first-line medications for primary open-angle glaucoma: a systematic review and network meta-analysis. Ophthalmology. 2016;123(1):129–140. | ||
Liang Lin, Zhao YJ, Chew PT, et al. Comparative efficacy and tolerability of topical prostaglandin analogues for primary open-angle glaucoma and ocular hypertension. Ann Pharmacother. 2014;48(12):1585–1593. | ||
Konstas AG, Quaranta L, Katsanos A, et al. Twenty-four hour efficacy with preservative free tafluprost compared with latanoprost in patients with primary open angle glaucoma or ocular hypertension. Br J Ophthalmol. 2013;97(12):1510–1515. | ||
Fukuda M, Sasaki H. The transcorneal penetration of commercial ophthalmic formulations containing timolol maleate in rabbit eyes. J Ocul Pharmacol Ther. 2015;31(1):57–60. | ||
Quaranta L, Riva I, Katsanos A, Floriani I, Centofanti M, Konstas AG. Safety and efficacy of travoprost solution for the treatment of elevated intraocular pressure. Clin Ophthalmol. 2015;9:633–643. | ||
Quaranta L, Biagioli E, Riva I, et al. Prostaglandin analogs and timolol-fixed versus unfixed combinations or monotherapy for open-angle glaucoma: a systematic review and meta-analysis. J Ocul Pharmacol Ther. 2013;29(4):382–389. | ||
Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese. The Tajimi Study. Ophthalmology. 2006;113(8):1354–1362. | ||
Yamamoto T, Iwase A, Araie M, et al. The Tajimi Study Report 2 prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology. 2005;112(10):1661–1669. | ||
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Collaborative Normal-Tension Glaucoma Study Group. Am J Ophthalmol. 1998;126(4):498–505. | ||
Dreer LE, Owsley C, Campbell L, Gao L, Wood A, Girkin CA. Feasibility, patient acceptability, and preliminary efficacy of a culturally informed, health promotion program to improve glaucoma medication adherence among African Americans: “Glaucoma management optimism for African Americans living with Glaucoma” (GOAL). Curr Eye Res. 2016;41(1):50–58. | ||
Holló G, Topouzis F, Fechtner RD. Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin Pharmacother. 2014;15(12):1737–1747. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.