Back to Journals » Infection and Drug Resistance » Volume 15
Five-Year Trend of Malaria Prevalence in Mojo Town, Central Ethiopia: Shifting Burden of the Disease and Its Implication for Malaria Elimination: A Retrospective Study
Authors Abate A , Assefa M , Golassa L
Received 11 November 2021
Accepted for publication 5 February 2022
Published 11 February 2022 Volume 2022:15 Pages 455—464
DOI https://doi.org/10.2147/IDR.S348203
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Andargie Abate,1,2 Mesfin Assefa,1,3 Lemu Golassa1
1Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa, Ethiopia; 2College of Medicine and Health Sciences, Bahir Dar University, Bahir Dar, Ethiopia; 3College of Medicine and Health Sciences, Wachamo University, Hossaena, Ethiopia
Correspondence: Andargie Abate, Email [email protected]
Introduction: Malaria continues to strike hardest against the health and economic development in Ethiopia. The peak of malaria incidence follows the main rainfall season in each year; however, its transmission tends to be highly heterogeneous within or between years and from area to area. Thus, this study was aimed to determine the trend prevalence of malaria in Mojo town, central Ethiopia.
Methods: A retrospective study was conducted in Mojo town, East Shoa zone, Ethiopia from February to March 2021. Malaria cases and related data documented between 2016 and 2020 were carefully reviewed from laboratory registration logbooks. The collected data were analyzed using descriptive statistics.
Results: A total of 19,106 blood films were examined from malaria-suspected patients. The overall microscopically confirmed prevalence of malaria was 4.2% (793/19,106). Plasmodium vivax was the predominant species accounting for 76.2% (604/793) of positive samples. Malaria cases declined from 259 in 2016 to 77 in 2020. The proportion of malaria was higher among males (64.8%, 514/793) than females (35.2%, 279/793) (P < 0.0001). Higher malaria cases were observed from the age group 15– 24 years followed by the age group of 25– 34 (P < 0.0001). The number of malaria cases was at a high level from September to November and lowest from December to February.
Conclusion: Despite a declining trend of malaria prevalence, it remains a public health burden in the area. P. vivax, the predominant species, should get attention during prevention and control strategies for the successful progress of the malaria elimination program.
Keywords: malaria, trend, Mojo, retrospective, Ethiopia
Introduction
Despite being preventable and treatable, malaria continues to be one of the global deadly diseases contributing a significant impact on public health and economic development. An estimated 241 million cases and 627,000 deaths were reported in 2020.1 The disease is more common, particularly in Africa composing 95% of the global cases and 96% of the deaths.1,2
In over a decade, remarkable achievements in malaria control were gained. Nevertheless, the control programs in the past had prioritized to focus on P. falciparum malaria due to its consideration of more pathogenicity. This priority dangerously minimized the clinical and public health importance of P. vivax impacting the progress towards malaria elimination.3,4
Ethiopia has also shared the global burden of malaria and is placed 15th from the highly malaria-affected countries in Africa.1 Thus, malaria continues to strike hardest against the health of the population in the country. The malaria incidence may reach its peak following the main rainfall season (July to September) each year. However, its transmission tends to be highly heterogeneous within or between years, and from area to area.5–8 P. falciparum and P. vivax are the most dominant malaria parasites accounting for nearly 70%, and 30% of all malaria cases, respectively.9 Artemisinin-based combination therapies (ACTs) and chloroquine are the nationally recommended treatments for uncomplicated malaria caused by P. falciparum and P. vivax, respectively. These drugs are efficacious.10–14
Although Ethiopia has targeted malaria elimination, several factors such emergence of resistance of the parasite to antimalarials and vectors to insecticides, the complexity of the control process in P. vivax, seasonal transmission with diverse micro-climates and low population immunity challenged malaria elimination in the country.15 There was, in the country, a fluctuating trend of malaria prevalence within the last decade showing a significant reduction of malaria cases and deaths.5,15–19 However, the report from the country’s public health emergency management (PHEM) has shown an increased malaria prevalence.15 The other study has not clearly defined the situation8 indicating not conclusive trend of malaria in the country.
The fact that the prevalence of malaria, is expected to shift over time, informs an understanding of the contextual diversity of its prevalence within each site. This is important to identify the optimal intervention, and to deliver it according to the site-specific situation of the disease. Therefore, determining epidemiological trends of malaria cases from specific health facilities is valuable to measure progress towards malaria elimination. Thus, this study was aimed to determine the trend prevalence of malaria in Mojo town, East Shoa Zone, Ethiopia. The findings from this study will be helpful in improving the interventions, and providing the information for the development of new strategies for burden reduction and elimination of malaria in the country.
Materials and Methods
Study Setting, and Design
An institution-based retrospective cross-sectional study was conducted in Mojo town from February to March 2021. The town is located in the East Shoa zone, Oromia regional state at a distance of 70 km from Addis Ababa (capital city of Ethiopia), and 25 km from Adama town (Figure 1). Mojo town has an altitude range of 1730–1890m above sea level and is characterized by its mean annual temperature of 16−17°C, and mean annual rainfall of 896mm. It has a latitude and longitude of 08° 37ʹ North and 39° 07ʹ East, respectively. According to the projection of Central Statistical Agency of Ethiopia carried out in 2015, the estimated population of the town was 43,500. The town has two health centers and one hospital, of which Mojo Health Center was selected for this study since the other health center and hospital are newly established health institutions and most of the town’s population visited Mojo Health Center due to its long duration of service provision than the two health institutions (Key informants). Malaria is the most prevalent and seasonal disease in the area where both P. vivax and P. falciparum exist.
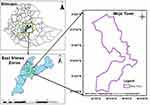 |
Figure 1 Physical location of Mojo town. |
Study Population
The study populations were all malaria-suspected individuals who had visited Mojo Health Center, and gotten blood film test from January 2016 to December 2020.
Eligibility Criteria
Data such as malaria cases diagnosed in months and years, identified types of Plasmodium species, and socio-demographic data (age and sex) were included in the analysis regardless of age, pregnancy, and other infection status. Data missing any one of the variables listed in the inclusion criteria were excluded from the analysis.
Data Collection and Quality Control
Five years (2016–2020) of malaria cases and related data were collected from Mojo Health Center laboratory logbooks using a well-developed checklist. The checklist consisted of year and month of visit, sex, age, blood film status (±), and type of Plasmodium species. The data were collected by experienced medical laboratory technicians. In the health center, microscopic blood film examination was used as a gold standard to confirm the presence of Plasmodium parasite and identification of the species as recommended by WHO’s protocol. The blood film examination for malaria parasite detection was performed according to the national standard operating procedure. The laboratory technologists or technicians who performed the microscopic examination were well trained in malaria microscopy. Microscopy examination was used exclusively for Plasmodium species detection throughout the study period (2016–2020). The data collection process was supervised, and data were checked for completeness before analysis. Incompletely registered data were excluded from the analysis.
Data Processing and Analysis
The collected data were entered into EpiData version 3.1 and exported to Statistical Package for Social Sciences (SPSS) version 25 for analysis. Microsoft office Excel was also used to construct some figures. The findings were presented using frequency, tables, and figures. Descriptive statistics were performed to determine the frequencies and percentages of overall, and trend prevalence of malaria in terms of years, seasons, Plasmodium species, sex, and age. A chi-square test was employed to determine the association of malaria burden by sex and age groups. A P-value <0.05 was considered statistically significant in this study.
Ethical Approval
Ethical clearance was obtained from the Institutional Review Board (IRB) of Addis Ababa University, Aklilu Lemma Institute of Pathobiology (Ref. No. ALIPB IRB/18/2012/20). Support and permission letters were given from Mojo Health Center to use the data for research purposes. No names or identifying information were indicated on the checklists, and confidentiality was assured throughout the study. The study was conducted in accordance with the Declaration of Helsinki.
Results
Prevalence and Annual Trend of Malaria
During the last 5 years (January 2016 to December 2020), a total of 19,106 blood films, from malaria-suspected patients, were examined to detect the presence of Plasmodium parasite. Off whom, 10,407 (54.5%) were females, and 5747 (30.1) were in the age group of 15–24 years. The overall microscopically confirmed prevalence of malaria was 4.2% (793/19,106). P. vivax was the predominant species in each sex, age and year accounting for 76.2% (604/793) followed by P. falciparum (22.8%, 181/793) and mixed infections (1.0%, 8/793) of the total malaria cases. Malaria cases had shown a substantial decline from 2016 to 2020. The relatively highest number of malaria cases (32.7%, 259/793) was observed in 2016, while the least malaria cases (9.7%, 77/793) were determined in 2020 (Table 1). When the proportion was considered, the prevalence was declined from 6.5% (259/3965) in 2016 to 2.3% (104/4550) in 2019, however it was progressively increased to 5.2% (77/1477) in 2020 (Figure 2), and the variation was statistically significant (P<0.0001).
 |
Table 1 Prevalence and Annual Trends of Malaria Cases in Mojo Health Center from 2016 to 2020 |
 |
Figure 2 Annual trends of malaria prevalence in Mojo Health Center from 2016 to 2020. |
Malaria Distribution with Age and Sex
Out of the confirmed 793 malaria cases in 5 years (2016 to 2020), 514 (64.8%) and 279 (35.2%) were males and females, respectively (Table 1). The difference was statistically significant (P<0.0001). The number of malaria cases among males was higher than females for each year (Figure 3). Malaria cases were reported in all age groups with higher number (37.2%, 296/793) occurred among individuals aged 15–24 years followed by the age group of 25–34 (26.6%, 211/793) (Table 1 and Figure 4). The difference in the prevalence of malaria among age difference was statistically significant (P<0.0001).
 |
Figure 3 Annual trend of malaria cases by sex in Mojo Health Center from 2016 to 2020. |
 |
Figure 4 Annual trend of malaria cases by age in Mojo Health Center from 2016 to 2020. |
Seasonal Distribution of Malaria
The seasonal distribution of malaria cases for the four seasons was analyzed. Malaria cases were reported in all months and seasons with some fluctuating trends. The peak in malaria cases occurred in July followed by November, while the least one was reported in February (Figure 5). The maximum number of malaria cases (33.5%, 266/793) was observed during autumn (September–November), while the least cases (16.9%, 134/793) were during winter (December–February). P. vivax infection was high in the summer and low in winter, while P. falciparum infection was high in the autumn and low in spring (Figure 6).
 |
Figure 5 Trend of malaria prevalence with Plasmodium species and month in Mojo Health Center from 2016 to 2020. |
 |
Figure 6 Seasonal profile of P. vivax, P. falciparum and mixed infections in Mojo Health Center from 2016 to 2020. |
Discussion
Malaria affects the lives of almost all people living in sub-Saharan African countries.1,2 In Ethiopia, malaria continues a major public health problem despite relatively low prevalence compared to some African countries. Unstable malaria transmission patterns make the country prone to epidemics.5,20 The nation-wide elimination plan needs sub-national elimination. This is because the decrease in malaria burden is not uniform in the country9 which is imperative to understand the contextual diversity of malaria prevalence within each site. Thus, this study determined epidemiological trends of malaria cases in a specific health facility.
The current study found that the 5 years (2016–2020) microscopically confirmed malaria cases were 793 with a proportion of 4.2%. The maximum and minimum numbers of malaria cases were reported in 2016 and 2020, respectively. Here, the prevalence was markedly lower than previous studies conducted in several parts of the country such as in Dembia (21.8%),8 Dembecha (16.3%),21 Guba (51.0%),19 Sibu Sire (20.1%),22 Arjo-Didessa sugar development site (33.4%),23 Harari (46.9%),24 Chalia district (7.6%),25 Abeshge (33.8%),26 and Bale Zone (66.7%).27 The prevalence of the present study was also relatively lower than the findings from other parts of Ethiopia like Northwest Tigray (7.0%),6 Wereta (5.4%),28 Kombolcha (7.5%),18 Ataye (8.4%),29 Halaba (9.5%),7 Wolkite (8.6%)30 and Arsi Negelle (11.5%).31
These differences might be due to laboratory personnel performance difference in malaria parasite detection because the quality of microscopic diagnosis is highly influenced by slide preparation and staining methodology, microscopist skill, and quality of microscope.32 The variations in the time of the studies might also bring the difference in the overall prevalence of malaria since malaria is being fluctuated from time to time as supported by the global,2 and national report.9 Moreover, it could also be due to geographical difference because populations’ awareness about malaria and application of intervention activities vary from area to area. Variation in socio-demographic status and utilization of intervention in the same locality also influenced the prevalence as documented by previous studies elsewhere.33–37
In addition, the low prevalence of malaria in the current study might be due to the variation in species showing that P. vivax was the predominant species in this study than P. falciparum in other studies listed above. This is because the parasite density in P. vivax is typically lower than P. falciparum, increasing the risk of false-negative microscopy diagnosis with acute P. vivax malaria.32,38,39 The thought was also supported by the review stating that microscopists correctly identified only 63–86% of confirmed P. vivax-positive blood samples.38 Another review also summarized that an average of 67% of P. vivax infections were reported as not detected by microscopic examination40 masking the actual burden of P. vivax-related malaria.
The results of the present study revealed that a sharply declined fashion of malaria cases was observed during the last 5 years (2016–2020). This finding was consistent with the worldwide declining burden in which 1.5 billion malaria cases have been averted from 2000 to 2019 where most of the averted cases (82%) were in the WHO African regions including Ethiopia.2 A significant decline in malaria has also been reported in Ethiopia.9,30,41
This decline might be due to the scale-up of malaria control interventions such as prompt diagnosis, community awareness creation, increased accessibility of long-lasting insecticide-treated nets (LLITNs), and high coverage of indoor residual spraying (IRS) in Ethiopia although the utilization is not uniform.20 The observed reduction of malaria cases and deaths after implementing intervention supported the conclusion.9,42 The current progressive decline in annual malaria cases is suggestive of good national progress towards achieving 2030 goals to reduce malaria incidence and mortality.9
Contrary to the national profile of Plasmodium species in which P. falciparum is a dominant species,8,9,24,27,43 P. vivax was predominantly reported in the current study which is in line with the previous studies in Arsi Negelle31 and Wolkite.30 It could be because malaria control programs in the past had prioritized P. falciparum malaria because of its consideration as more pathogenicity, and easier to detect and treat.4,44 In addition, it could be due to the emergence of drug resistance,38 and relapse from dormant liver stages.44–46 This increment of P. vivax malaria with the decline of P. falciparum might also be supported by the fact of high risk of P. vivax malaria after clearing of the competing P. falciparum species.47
In the present study, malaria cases were observed in both gender and all age groups of the population throughout the study period. Nevertheless, males were more affected than females, which is consistent with previous studies in Ethiopia.6–8,19,21,23,27 This gender difference in malaria prevalence could be since males are frequently bitten by mosquitoes due to their behavioral differences such as working outdoors, little chance of sleeping under bed nets, and travel to endemic areas for labor work.48 Moreover, hormonal differences might play the role in such a way that testosterone was associated with decreased adaptive immunity against malaria.49 Briggs et al had thought that females cleared their infections at a faster rate than males,50 which might support the lower prevalence of malaria among females in the current study.
This study observed a higher prevalence of malaria among the age group of 15–24 years than younger children and older adults, which is in line with previous studies in Ethiopia.8,19,29 This might be due to the fact that productive age groups frequently engaged in outdoor activities. Surprisingly, a lower prevalence of malaria was observed in children under 5 years of age, which might be because of their less likely exposure to a mosquito bite, and sleeping under bed nets.
The current study observed malaria cases in all months and seasons throughout the study period. Despite this, the peak in malaria cases occurred in July followed by November which is the harvesting season during and after the main rainfall. The highest cases of malaria were detected during autumn (September–November) which was in accordance with the findings in Dembia,8 Dembecha,21 Northwest Tigray,6 Ataye,29 Guba,19 Jimma,41 and Harari.24 It might be related to the formation of breeding sites during and after the heavy rain season, favorable temperature, and high vegetation density for mosquito breeding. The coinciding high prevalence of malaria with these cultivation months has a deleterious socio-economic effect on productivity and development in the country.
Conclusion
The declining trend of the overall malaria prevalence was observed in the study area, suggestive of good progress towards achieving of 2030 goals of malaria elimination. However, malaria remains a public health burden in the area affecting 4.2% of the population. P. vivax is the dominant species in the study area reflecting the shift of P. falciparum to P. vivax malaria challenging the success of the ongoing malaria elimination program. The reproductive age group and males were more affected by the infection, and this was more common during cultivation season impacting public health and economic development. Therefore, malaria control and elimination programs should be strengthened for further reduction of the burden of malaria, particularly among highly affected groups. There is also a need for intensifying the prevention and control strategies on P. vivax.
Abbreviations
CDC, Center for Diseases Control and Prevention; EPHI, Ethiopian Public Health Institute; FMOH, Federal Ministry of Health; IRS, indoor residual spraying; LLITNs, long-lasting insecticide-treated nets; SPSS, Statistical Package for the Social Science; WHO, World Health Organization; UNDP, United Nations Development Programme; USAID, United States of America Department of State; WWARN, Worldwide Antimalarial Resistance Network.
Data Sharing Statement
All data of findings are available from the corresponding author on reasonable request. All relevant data are within the manuscript.
Acknowledgments
The authors are grateful to Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Bahir Dar University, and Wachamo University for facilities support of the study. We would also like to thank Mojo Health Center laboratory staffs for provision of the necessary information, facilities and technical support. We are indebted to data collectors for their cooperation during the fieldwork.
Author Contributions
All authors made a significant contribution in the conception, study design, execution, acquisition, analysis and interpretation of data to the work reported, and took part in drafting of the manuscript. They critically read and revised for intellectually important content; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. World Health Organization. World malaria report 2021. World Health Organization; 2021.
2. World Health Organization. World malaria report 2020: 20 years of global progress and challenges. World Health Organization; 2020.
3. Beeson JG, Chu CS, Richards JS, Nosten F, Fowkes FJI. Plasmodium vivax malaria: challenges in diagnosis, treatment and elimination. Pediatr Infect Dis J. 2015;34(5):529–531. doi:10.1097/INF.0000000000000671
4. Worldwide Antimalarial Resistance Network. Vivax malaria: the challenges towards malaria elimination. Worldwide Antimalarial Resistance Network (WWARN); 2017.
5. Ethiopian Public Health Institute. Ethiopia national malaria indicator survey 2015. Addis Ababa, Ethiopia: Ethiopian Public Health Institute (EPHI); 2016.
6. Berhe B, Mardu F, Legese H, Negash H. Seasonal distribution and seven year trend of malaria in North West Tigrai: 2012–2018, Ethiopia; 2019. Trop Dis Travel Med Vaccines. 2019;5(1):1–7. doi:10.1186/s40794-019-0091-y
7. Shamebo T, Petros B. Trend analysis of malaria prevalence in Halaba special district, Southern Ethiopia. BMC Res Notes. 2019;12(1):190. doi:10.1186/s13104-019-4215-2
8. Addisu A, Tegegne Y, Mihiret Y, Setegn A, Zeleke AJ. A 7-year trend of malaria at primary health facilities in Northwest Ethiopia. J Parasitol Res. 2020;2020.
9. Federal Ministry of Health. National malaria elimination road map. Federal Ministry of Health (FMOH); 2017.
10. Abamecha A, Yilma D, Addisu W, et al. Therapeutic efficacy of artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Chewaka District, Ethiopia. Malar J. 2020;19(1):1–10. doi:10.1186/s12936-020-03307-4
11. Teklemariam M, Assefa A, Kassa M, Mohammed H, Mamo H. Therapeutic efficacy of artemether-lumefantrine against uncomplicated Plasmodium falciparum malaria in a high-transmission area in northwest Ethiopia. PLoS One. 2017;12(4):e0176004. doi:10.1371/journal.pone.0176004
12. Nega D, Assefa A, Mohamed H, et al. Therapeutic efficacy of artemether-lumefantrine (Coartem®) in treating uncomplicated P. falciparum malaria in Metehara, Eastern Ethiopia: regulatory clinical study. PLoS One. 2016;11(4):e0154618. doi:10.1371/journal.pone.0154618
13. Beyene HB, Beyene MB, Ebstie YA, Desalegn Z. Efficacy of chloroquine for the treatment of vivax malaria in Northwest Ethiopia. PLoS One. 2016;11(8):e0161483. doi:10.1371/journal.pone.0161483
14. Yeshanew S, Dagne A, Taye B. Efficacy study of chloroquine to plasmodium vivax malaria in darimu and Bure Districts, Southwest Ethiopia. Infect Drug Resist. 2021;14:795. doi:10.2147/IDR.S300692
15. United States of America Department of State/Center for Diseases Control and Prevention. President’s malaria initiative Ethiopia-malaria operational plan FY 2017. United States of America Department of State (USAID)/Center for Diseases Control and Prevention (CDC); 2017.
16. Deribew A, Dejene T, Kebede B, et al. Incidence, prevalence and mortality rates of malaria in Ethiopia from 1990 to 2015: analysis of the global burden of diseases 2015. Malar J. 2017;16(1):1–7. doi:10.1186/s12936-017-1919-4
17. Girum T, Shumbej T, Shewangizaw M. Burden of malaria in Ethiopia, 2000–2016: findings from the Global Health Estimates 2016. Trop Dis Travel Med Vaccines. 2019;5(1):1–7. doi:10.1186/s40794-019-0090-z
18. Gebretsadik D, Feleke DG, Fiseha M. Eight-year trend analysis of malaria prevalence in Kombolcha, South Wollo, north-central Ethiopia: a retrospective study. Parasit Vectors. 2018;11(1):1–6. doi:10.1186/s13071-018-2654-6
19. Alkadir S, Gelana T, Gebresilassie A. A five year trend analysis of malaria prevalence in Guba district, Benishangul-Gumuz regional state, western Ethiopia: a retrospective study. Trop Dis Travel Med Vaccines. 2020;6(1):1–7. doi:10.1186/s40794-020-0101-0
20. United States of America Department of State/Center for Diseases Control and Prevention. President’s malaria initiative Ethiopia: malaria operational plan FY 2019. United States Agency for International Development (USAID)/Center for Diseases Control and Prevention (CDC); 2019.
21. Haile D, Ferede A, Kassie B, Abebaw A, Million Y. Five-year trend analysis of malaria prevalence in Dembecha Health Center, West Gojjam zone, Northwest Ethiopia: a retrospective study. J Parasitol Res. 2020;2020. doi:10.1155/2020/8828670
22. Gemechu T, Samuel A, Yewhalaw D. Ten years trend analysis of malaria prevalence and its correlation with climatic variables in Sibu Sire District, east Wollega zone, Oromia regional state, Western Ethiopia: a retrospective study. Sci Technol Arts Res J. 2015;4(4):99–105. doi:10.4314/star.v4i4.14
23. Hawaria D, Getachew H, Zhong G, et al. Ten years malaria trend at Arjo-Didessa sugar development site and its vicinity, Southwest Ethiopia: a retrospective study. Malar J. 2019;18(1):1–11. doi:10.1186/s12936-018-2635-4
24. Esayas E, Tufa A, Massebo F, et al. Malaria epidemiology and stratification of incidence in the malaria elimination setting in Harari Region, Eastern Ethiopia. Infect Dis Poverty. 2020;9(1):1–12. doi:10.1186/s40249-020-00773-5
25. Bogale T, Asefa B. Magnitude of malaria infection in Chalia District, West Shoa Zone, Oromia Regional State, Ethiopia. J Health Med Nurs. 2017;35.
26. Yimer F, Animut A, Erko B, Mamo H. Past five-year trend, current prevalence and household knowledge, attitude and practice of malaria in Abeshge, south-central Ethiopia. Malar J. 2015;14(1):1–11. doi:10.1186/s12936-015-0749-5
27. Kalil FS, Bedaso MH, Wario SK. Trends of malaria morbidity and mortality from 2010 to 2017 in Bale Zone, Ethiopia: analysis of surveillance data. Infect Drug Resist. 2020;13:4379. doi:10.2147/IDR.S284281
28. Derbie A, Alemu M. Five years malaria trend analysis in Woreta Health Center, Northwest Ethiopia. Ethiop J Health Sci. 2017;27(5):465–472. doi:10.4314/ejhs.v27i5.4
29. Feleke DG, Gebretsadik D, Gebreweld A. Analysis of the trend of malaria prevalence in Ataye, North Shoa, Ethiopia between 2013 and 2017. Malar J. 2018;17(1):1–6. doi:10.1186/s12936-018-2474-3
30. Solomon A, Kahase D, Alemayehu M. Trend of malaria prevalence in Wolkite health center: an implication towards the elimination of malaria in Ethiopia by 2030. Malar J. 2020;19(1):1–8. doi:10.1186/s12936-020-03182-z
31. Hailemariam M, Gebre S. Trend analysis of malaria prevalence in Arsi Negelle health center, Southern Ethiopia. J Infect Dis Immun. 2015;7(1):1–6. doi:10.5897/JIDI2014.0147
32. Tangpukdee N, Duangdee C, Wilairatana P, Krudsood S. Malaria diagnosis: a brief review. Korean J Parasitol. 2009;47(2):93. doi:10.3347/kjp.2009.47.2.93
33. Chirebvu E, Chimbari MJ, Ngwenya BN. Assessment of risk factors associated with malaria transmission in Tubu village, northern Botswana. Malar Res Treat. 2014;2014:1–10. doi:10.1155/2014/403069
34. Cook J, Owaga C, Marube E, et al. Risk factors for Plasmodium falciparum infection in the Kenyan highlands: a cohort study. Trans R Soc Trop Med Hyg. 2019;113(3):152–159. doi:10.1093/trstmh/try122
35. Essendi WM, Vardo-Zalik AM, Lo E, et al. Epidemiological risk factors for clinical malaria infection in the highlands of Western Kenya. Malar J. 2019;18(1):1–7. doi:10.1186/s12936-019-2845-4
36. Habyarimana F, Ramroop S. Prevalence and risk factors associated with malaria among children aged six months to 14 years old in Rwanda: evidence from 2017 Rwanda malaria indicator survey. Int J Environ Res Public Health. 2020;17(21):7975. doi:10.3390/ijerph17217975
37. Ugwu CLJ, Zewotir T. Spatial distribution and sociodemographic risk factors of malaria in Nigerian children less than 5 years old. Geospat Health. 2020;15(2). doi:10.4081/gh.2020.819
38. Baird JK, Maguire JD, Price RN. Diagnosis and treatment of Plasmodium vivax malaria. Adv Parasitol. 2012;80:203–270. doi:10.1016/B978-0-12-397900-1.00004-9
39. Baird JK, Valecha N, Duparc S, White NJ, Price RN. Diagnosis and treatment of Plasmodium vivax malaria. Am J Trop Med Hyg. 2016;95(6_Suppl):35–51. doi:10.4269/ajtmh.16-0171
40. Cheng Q, Cunningham J, Gatton ML. Systematic review of sub-microscopic P. vivax Infections: prevalence and determining factors. PLoS Negl Trop Dis. 2015;9(1):e3413. doi:10.1371/journal.pntd.0003413
41. Jemal A, Ketema T. A declining pattern of malaria prevalence in Asendabo Health Center Jimma zone, Southwest Ethiopia. BMC Res Notes. 2019;12(1):1–5. doi:10.1186/s13104-019-4329-6
42. Aregawi M, Lynch M, Bekele W, et al. Time series analysis of trends in malaria cases and deaths at hospitals and the effect of antimalarial interventions, 2001–2011, Ethiopia. PLoS One. 2014;9(11):e106359. doi:10.1371/journal.pone.0106359
43. Minwuyelet A, Aschale Y. Analysis of five-year trend of malaria at Bichena primary hospital, Amhara Region, Ethiopia. J Parasitol Res. 2021;2021:1–6. doi:10.1155/2021/6699373
44. Price RN, Commons RJ, Battle KE, Thriemer K, Mendis K. Plasmodium vivax in the era of the Shrinking P. falciparum map. Trends Parasitol. 2020;36(6):560–570. doi:10.1016/j.pt.2020.03.009
45. Baird JK. Chloroquine Resistance inPlasmodium vivax. Antimicrob Agents Chemother. 2004;48(11):4075–4083. doi:10.1128/AAC.48.11.4075-4083.2004
46. Anstey NM, Russell B, Yeo TW, Price RN. The pathophysiology of vivax malaria. Trends Parasitol. 2009;25(5):220–227. doi:10.1016/j.pt.2009.02.003
47. Hossain MS, Commons RJ, Douglas NM, et al. The risk of Plasmodium vivax parasitaemia after P. falciparum malaria: an individual patient data meta-analysis from the world wide antimalarial resistance network. PLoS Med. 2020;17(11):e1003393. doi:10.1371/journal.pmed.1003393
48. United Nations Development Programme. Discussion paper: gender and malaria. United Nations Development Programme (UNDP); 2015.
49. Steeg LG, Flores-Garcia Y, Zavala F, Klein SL. Irradiated sporozoite vaccination induces sex-specific immune responses and protection against malaria in mice. Vaccine. 2019;37:4468–4476. doi:10.1016/j.vaccine.2019.06.075
50. Briggs J, Teyssier N, Nankabirwa JI, et al. Sex-based differences in clearance of chronic Plasmodium falciparum infection. eLife. 2020;9:e59872. doi:10.7554/eLife.59872
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
