Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 8
First-line antiretroviral treatment failure and associated factors in HIV patients at University of Gondar Teaching Hospital, Gondar, Northwest Ethiopia
Authors Biset Ayalew M , Kumilachew D , Belay A , Getu S, Teju D, Endale D, Tsegaye Y, Wale Z
Received 4 May 2016
Accepted for publication 30 July 2016
Published 2 September 2016 Volume 2016:8 Pages 141—146
DOI https://doi.org/10.2147/HIV.S112048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bassel Sawaya
Mohammed Biset Ayalew,1 Dawit Kumilachew,2 Assefa Belay,3 Samson Getu,4 Derso Teju,4 Desalegn Endale,4 Yemisirach Tsegaye,4 Zebiba Wale4
1Department of Clinical Pharmacy, 2Department of Pharmaceutics, 3Department of Pharmacology, 4School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Background: Antiretroviral therapy (ART) restores immune function and reduces HIV-related adverse outcomes. But treatment failure erodes this advantage and leads to an increased morbidity and compromised quality of life in HIV patients. The aim of this study was to determine the prevalence and factors associated with first-line ART failure in HIV patients at the University of Gondar Teaching Hospital.
Patients and methods: A retrospective study was conducted on 340 adults who had started ART during the period of September 2011 to May 2015. Data regarding patients’ sociodemographics, baseline characteristics, and treatment-related information were collected through review of their medical charts. Data were analyzed using SPSS version 21. Descriptive statistics, cross-tabs, and binary and multiple logistic regressions were utilized. P<0.05 was used to declare association.
Results: Among the 340 patients enrolled, 205 were females (60.3%). The mean age at ART initiation was 34.4 years. A total of 14 (4.1%) patients were found to have treatment failure. The median duration of treatment failure from initiation of treatment was 17.5 months (8–36 months). Poor adherence to treatment and low baseline CD4 cell count were found to be significant predictors of treatment failure.
Conclusion: The prevalence of first-line ART failure was 4.1%. Treatment failure was most likely to occur for the patients who had poor drug adherence and those who were delayed to start ART till their CD4 cell count became very low (<100 cells/mm3).
Keywords: treatment failure, antiretroviral drugs, risk factor, adherence
Introduction
Human immunodeficiency virus (HIV) is responsible for a worldwide pandemic, and it is the cause of acquired immune deficiency syndrome (AIDS).1 According to UNAIDS report on the Global AIDS Epidemic in 2012, there were 35.3 million people living with HIV. In addition, 2.3 million people became newly infected with HIV and 1.6 million people died from AIDS-related illnesses in the same year.2,3
Over the past decade, there has been an unparalleled effort to provide access to antiretroviral therapy (ART) for HIV-infected individuals in sub-Saharan Africa, the region with the highest HIV burden.4 ART restores immune function and reduces HIV-related adverse outcomes. Since the beginning of highly active antiretroviral therapy (HAART) in 1996, there have been dramatic declines in morbidity and mortality due to HIV.5 This advantage is eroded when treatment failure develops. Despite the significant reduction in morbidity and mortality among the HIV-infected patients receiving combination ART, a considerable number of patients fail to achieve a sustained virological and immunological response to therapy.6
Treatment failure can be defined as progression of disease after initiation of HAART. Failure can be assessed by clinical (the appearance of new opportunistic infections, ongoing weight loss, etc), immunologic (a decline in CD4 count), or virologic (a viral rebound above a set threshold of 200 copies/mL) criteria.7
Viral load monitoring has become the standard of care for monitoring the success of and diagnosing the failure of ART and has been explicitly recommended, when available, by the World Health Organization (WHO) since 2010.8,9 In settings in which there is no access to viral load testing, clinical monitoring alone or a combination of clinical and immunologic monitoring is used to assess response to ART and determine treatment failure.10
The patients who had failed for first-line drug are 46% more likely to fail again for second-line drugs and are attributed to the higher number of side effects and have greater likelihood of experiencing drug resistance and treatment fatigue as a result of being on treatment longer.11 The development of drug-resistant virus strains can be another threat if this virus starts to transmit in the population.12,13 Early detection of treatment failure is crucial to sustain the effectiveness of the first-line therapy.14,15
Studies in East Africa have shown a high prevalence of immunologic failure ranging from 8% to 57% among clients on first-line HAART, and furthermore, the magnitude increases as the time of follow-up increases.6,16,17
The immunological failure rate in Ethiopia was found to be high. The study conducted at Debremarkos Hospital showed that 21% of the HIV patients had developed immunological failure with a failure rate of eight per 100 patient-years of follow-up.18
The timing and accuracy of identifying treatment failure in resource-limited settings are fundamental but challenging. Delayed detection of treatment failure may increase drug toxicity, may lead to the accumulation of drug resistance-associated mutations (further limiting treatment options), and may result in increased morbidity and mortality.
The documented factors associated with treatment failure include, but are not limited to: sociodemographic factors (eg, age, sex, being an orphan), baseline clinical factors (eg, high pretreatment viral load, low pretreatment CD4 count, prior WHO stage), drug–drug interactions (between the ART and concomitantly administered drugs), drug side effects, drug toxicity or inadequate adherence to treatment, poor adherence, certain ART regimen combinations, primary infection with drug-resistant strains of HIV, prior exposure to antiretroviral monotherapy, high baseline plasma viral load, and low baseline CD4 count.19–25
The objective of this study was to determine the prevalence of first-line ART failure and to identify those risk factors that contribute to treatment failure in the Ethiopian HIV patients. Identification of risk factors helps to define early predictors of treatment efficacy that permit better use of these potent drugs, avoid unnecessary side effects of second-line drug, prevent drug resistance, and decrease economic burden, especially in a resource-limited setting like Ethiopia due to the expensiveness of the second-line drug. It will also help as a guide for health professionals and higher officials to alleviate the problem and to develop strategies to decrease the rate of treatment failure.
Patients and methods
This retrospective record review study was conducted on 340 HIV patients who started ART in the period of September 2011–May 2015 at the University of Gondar Teaching Hospital, Northwest Ethiopia. The hospital serves for ~4 million people in the region. It has >400 beds. The ART Clinic was established in 2003, and a total of 9,390 clients started ART since its establishment. There were 5,138 active patients on ART at the time of this study. This study was conducted from April 20, 2015 to May 19, 2015. Those patients who were younger than 18 years old and those who took antiretroviral drugs for <6 months were excluded from this study. The patients were included in this study using simple random sampling method.
The data were collected by reviewing patients’ medical charts. Data abstraction format was developed after reviewing studies conducted on the topic, and it was pretested on 5% of the sample population on one of the private hospital found in the Gondar town. The data abstraction format contained patient sociodemographics, baseline characteristics, treatment-related data, and treatment failure-related information. Data were collected by four pharmacists who were trained for 2 days on the documentation and techniques of data collection.
Data were checked for its completeness everyday. They were edited, cleaned, and analyzed. The collected data were entered into Epi Info 7 and analyzed using SPSS version 21 (IBM Corporation, Armonk, NY, USA). Cross-tabulation was used in bivariate analysis. A test of association was done using binary logistic regression. P-value <0.05 was considered significant. The results of this study were organized in the form of frequencies and percentages. The data were summarized and described using text and tables.
Ethical clearance was obtained from the research ethical review board of the School of Pharmacy, University of Gondar. A formal letter of permission was also obtained from the University of Gondar Teaching Hospital Administration. Patient information obtained from the charts was kept confidential. Thus, patient identifiers such as name and address of the patient were not recorded in the data abstraction format. The ethics committee of the School of Pharmacy, University of Gondar did not require written informed consent be obtained from all patients, as this was a retrospective study, and all data was anonymous.
Results
Sociodemographic characteristics of patients
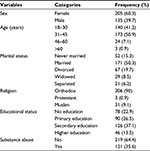
As indicated in Table 1, out of 340 patients included in this study, nearly three out of five (60.3%) patients were females. The mean age of patients at the start of ART was 34.4 years (range 19–67 years). Seventy-eight (22.9%) of the study participants had no formal education, and 121 (35.6%) of them had a history of substance abuse, of which the majority of them used alcohol (68%) and tobacco (30%).
Baseline clinical characteristics of patients
The mean CD4 count at ART initiation was 212.7 cells/mm3 (range 6–704 cells/mm3). Table 2 shows that the majority of the patients start ART when their clinical status was at WHO stage 3. One hundred sixty (47.1%) patients had one or more opportunistic infection at the time of their ART initiation. The majority of the patients, 323 (95.0%) were working by their functional status, and 32 (9.4%) had significant comorbid illness at the start of ART.
Treatment-related information
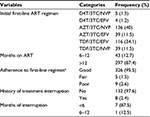
Most of the patients were either on zidovudine–lamivudine–nevirapine (40%) or tenofovir–lamivudine–efavirenz regimen (34.1%). Approximately 87% of the study subjects followed their treatment for >12 months. Majority of the patients, 326 (95.9%), had good adherence to their treatment. The detail on treatment-related information is found in Table 3.
Treatment failure
A total of 14 (4.1%) patients were found to have treatment failure. The average duration between the initiation of treatment and recognition of treatment failure was 17.5 months (8–36 months). As shown in Table 4, majority of treatment failure cases were on a regimen with lamivudine + tenofovir backbone. In most of the cases, the patients had both virologic and immunologic failure.
Factors associated with treatment failure
Table 5 indicates that advanced WHO stage, bedridden baseline functional status, treatment interruption, poor adherence, presence of opportunistic infections, and low baseline CD4 count (<100 cells/mm3) were found to be significant predictors of treatment failure in the binary logistic regression analysis. But in multivariate logistic regression, only poor adherence and low baseline CD4 cell count were found to be significant predictors of the presence of treatment failure.
Drug nonadherence was the strongest risk factor for treatment failure as the patients with poor adherence to treatment were >70 times more likely to have treatment failure when compared to the patients with good adherence to treatment (adjusted odds ratio 70.54, 95% confidence interval 5.57–893.86). Similarly when compared to the patients with baseline CD4 cell count >100 cells/mm3, the patients who had a baseline CD4 cell count <50 cells/mm3 and 50–100 cells/mm3 were 16.7 and 9.1 times more risky to develop treatment failure, respectively, showing that lower baseline CD4 cell counts were associated with higher risk of treatment failure (Table 5).
Discussion
The identification and management of first-line ART failure is a key challenge for HIV programs in resource-limited settings. Staying on a failing first-line therapy is associated with an increased risk of mortality. In addition to this, development of drug resistance limits the ability to construct new, potent, and tolerable regimens in the future. This study was aimed to determine the prevalence of first-line ART failure and factors associated with it.
This study showed that first-line ART failure rate was 4.1%, which is a closer result with the finding in a study conducted in Tanzania in which the failure rate was 7% and a study conducted in South Africa that reported a failure rate of 2.1%.26,27 However, this result was much lower than what was found in the study at Debremarkos Hospital, Ethiopia, in which the failure rate was reported as 21%.6 This larger gap may be due to the fact that most (95%) of the patients in this study had good adherence to their ART than the patients in the study at Debremarkos Hospital.
Even though the binary logistic regression showed many variables to have significant association with ART failure, multivariate logistic regression indicates that only poor adherence and low baseline CD4 cell count have strongest association with treatment failure. Similarly, the study conducted at Chiang Mai University Hospital, Thailand, showed that lower baseline CD4 cell count was one of the main factors significantly associated with ART failure.28
It was found that adherence is the independent predicting factor for treatment failure and the patients with poor drug adherence were found to be 70.5 times at higher risk of developing treatment failure compared to the patients with good adherence to treatment. Although adherence has been demonstrated to be important in many studies, there is discordance of results depending on the types of adherence measurement.22,26,27 In our study, the data on adherence were collected from the chart that was recorded according to the patient’s self-report of the amount of medication taken between visits without standardized set of questions. Therefore, the result may not be completely reliable. However, it is still demonstrated to be an independent factor for treatment failure, as good drug adherence is the major factor to control the viral replication rate and achieve a good improvement in patient’s immunity.
This study found that the patients with an initial CD4 cell count of <50 cells/mm3 and 50–100 cells/mm3 have 16.7 and 9.1 times more risk of developing treatment failure than the patients with a higher baseline CD4 count, respectively. This study also found that all the failing patients had a CD4 cell count of <200 cells/mm3 at baseline. This might be due to the reason that the patients with a low baseline CD4 cell count have a lesser immunity that may favor the probability of developing immunological failure. Similarly, a study in Ethiopia at Debremarkos University indicated that baseline CD4 count ≤100 cells/mm3 (adjusted hazard ratio 2.16, 95% confidence interval 1.44–3.25) is a significant predictor of immunological failure.6
Study limitation
This is a retrospective chart review study. The accuracy of analysis depends on the completeness of data and being retrospective may also limit the study to find out more predictors of treatment failure than those recorded in the charts. Being a single-centered study, the result may not also be generalized to all hospitals. Thus, we suggest further prospective and multicentered studies to be done.
Conclusion
We conclude from this study that the first-line ART failure rate was 4.1%. Treatment failure was most likely to occur for the patients who had poor drug adherence and those who were delayed to start ART till their CD4 cell count became very low (<100 cells/mm3).
Acknowledgments
Our appreciation goes to the School of Pharmacy, University of Gondar, for providing us essential materials and the ART Clinic of the University of Gondar Teaching Hospital for their great support in providing information and other material support during the research process.
Disclosure
The authors report no conflicts of interest in this work.
References
UNAIDS/WHO [webpage on the Internet]. Report on AIDS epidemic; 2013. Available from: www.unaids.org/en/resources/campaigns/globalreport2013/globalreport. Accessed August 10, 2015. | ||
AIDS 2014. 20th International AIDS Conference. Melbourne, Australia. Global Fact Sheet: HIV/AIDS. Available from: http://www.unaids.org/en/resources/campaigns/globalreport2013/factsheet. Accessed August 29, 2016. | ||
UNAIDS. Report on the Global AIDS Epidemic. Geneva: Joint United Nations Program on HIV/AIDS (UNAIDS); 2012. | ||
WHO. Towards Universal Access, Scaling up Priority HIV/AIDS Interventions in the Health Sector; Progress Report 2009. Geneva: World Health Organization; 2009. | ||
Meseret W, Andualem T, Segewkal H. Antiretroviral therapy regimen change among HIV/AIDS patients in Nekemt Hospital: a primary care hospital in Oromia regional state, Ethiopia. J Appl Pharm Sci. 2013;3(08):036–040. | ||
Melsew YA, Terefe MW, Tessema GA, Ayele TA. Rate of immunological failure and its predictors among patients on highly active antiretroviral therapy at Debremarkos hospital, Northwest Ethiopia: a retrospective follow up study. J AIDS Clin Res. 2013;4:211. | ||
Aldous JL, Haubrich RH. Defining treatment failure in resource-rich settings. Curr Opin HIV AIDS. 2009;4(6):459–466. | ||
World Health Organization [webpage on the Internet]. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach [2010 Revision]. Geneva: World Health Organization; 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf. Accessed March 20, 2015. | ||
World Health Organization [webpage on the Internet]. Antiretroviral Therapy for HIV Infection in Adults and Adolescents. Recommendations for a Public Health Approach [2006 Revision]. Geneva: World Health Organization; 2006. Available from: http://www.who.int/hiv/pub/guidelines/artadultguidelines.pdf. Accessed February 24, 2015. | ||
World Health Organization (WHO). Towards Universal Access: Scaling Up Priority HIV/AIDS Interventions in the Health Sector. Progress Report 2008. 2008:1–77. Available from: https://www.unicef.es/sites/www.unicef.es/files/200806_TowardsUniversalAccessReport2008_en.pdf. Accessed December 27, 2015. | ||
James T, Heiner G, John C, et al. Risk factors influencing HIV infection incidence in a rural African population a nested case-control study. J Infect Dis. 2006;193:458–466. | ||
Petersen ML, van der Laan MJ, Napravnik S, Eron JJ, Moore RD, Deeks SG. Long-term consequences of the delay between virologic failure of highly active antiretroviral therapy and regimen modification. AIDS. 2008;22(16):2097–2106. | ||
Kantor R, Diero L, Delong A, et al. Misclassification of first-line antiretroviral treatment failure based on immunological monitoring of HIV infection in resource limited settings. Clin Infect Dis. 2009;49(3):454–462. | ||
El-Khatib Z, Katzenstein D, Marrone G, et al. Adherence to drug-refill is a useful early warning indicator of virologic and immunologic failure among HIV patients on first-line ART in South Africa. PLoS One. 2011;6(3):e17518. | ||
Mutevedzi PC, Lessells RJ, Rodger AJ, Newell ML. Association of age with mortality and virological and immunological response to antiretroviral therapy in rural South African adults. PLoS One. 2011;6(7):e21795. | ||
Ahoua L, Guenther G, Pinoges L, et al. Risk factors for virological failure and subtherapeutic antiretroviral drug concentrations in HIV-positive adults treated in rural northwestern Uganda. BMC Infect Dis. 2009;9:81. | ||
Jaka HM, Mshana SE, Liwa AC, Peck R, Kalluvya S. Prevalence of immunological failure and durability of first line antiretroviral therapy at Bugando Hospital Mwanza, Tanzania. Tanzan Med J. 2009;24:5–8. | ||
Iroha E, Esezobor CI, Ezeaka C, Temiye EO, Akinsulie A. Adherence to antiretroviral therapy among HIV-infected children attending a donorfunded clinic at a tertiary hospital in Nigeria. Afr J AIDS Res. 2010;9(1):25–30. | ||
Buck WC, Kabue MM, Kazembe PN, Kline MW. Discontinuation of standard first-line antiretroviral therapy in a cohort of 1434 Malawian children. J Int AIDS Soc. 2010;13:31. | ||
Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa: the International epidemiologic databases to evaluate AIDS (IeDEA) Southern Africa collaboration. J Acquir Immune Defic Syndr. 2011;56(3):270–278. | ||
Nitta K, Romanee C, Thira S, Khuanchai S. Incidence and risk factors of antiretroviral treatment failure in treatment-naïve HIV-infected patients at Chiang Mai University Hospital, Thailand. AIDS Res Ther. 2011;8(42):1–7. | ||
Cadosch D, Bonhoeffer S, Kouyos R. Assessing the impact of adherence to anti-retroviral therapy on treatment failure and resistance evolution in HIV. J R Soc Interface. 2012;9(74):2309–2320. | ||
Datay MI, Boulle A, Mant D, Yudkin P. Associations with virologic treatment failure in adults on antiretroviral therapy in South Africa. J Acquir Immune Defic Syndr. 2010;54(5):489–495. | ||
Ma Y, Zhao D, Yu L, et al. Predictors of virologic failure in HIV-1- infected adults receiving first-line antiretroviral therapy in eight provinces in China. Clin Infect Dis. 2010;50(2):264–271. | ||
Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;193(3):339–347. | ||
Vanobberghen FM, Bonita K, Alison W, et al. Immunological failure of first-line and switch to second-line antiretroviral therapy among HIV-infected persons in Tanzania: analysis of routinely collected national data. Trop Med Int Health. 2015;20(7):880–892. | ||
Ncaca L-N, Kranzer K, Orrell C. Treatment interruption and variation in tablet taking behaviour result in viral failure: a case-control study from Cape Town, South Africa. PLoS One. 2011;6(8):e23088. | ||
Khienprasit N, Chaiwarith R, Sirisanthana T, Supparatpinyo K. Incidence and risk factors of antiretroviral treatment failure in treatment-naïve HIV-infected patients at Chiang Mai University Hospital, Thailand. AIDS Res Ther. 2011;8(42):3–4. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.