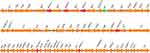Back to Journals » Infection and Drug Resistance » Volume 15
First Identification of a Multidrug-Resistant Pseudomonas putida Co-Carrying Five β-Lactam Resistance Genes Recovered from a Urinary Tract Infection in China
Authors Bao D, Huang L, Yan J, Li Y, Ruan Z , Jiang T
Received 15 March 2022
Accepted for publication 22 April 2022
Published 28 April 2022 Volume 2022:15 Pages 2229—2234
DOI https://doi.org/10.2147/IDR.S366567
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Danni Bao,1,2 Linyao Huang,3 Jianxin Yan,3 Yexuzi Li,4 Zhi Ruan,2 Tian Jiang2,3
1Department of Clinical Laboratory, Sanmen People’s Hospital, Taizhou, Zhejiang, 317100, People’s Republic of China; 2Department of Clinical Laboratory, Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, 310016, People’s Republic of China; 3Department of Clinical Laboratory, The Affiliated Wenling Hospital, Wenzhou Medical University, Wenling, 317500, People’s Republic of China; 4Department of Critical Care Medicine, The Affiliated Wenling Hospital, Wenzhou Medical University, Wenling, 317500, People’s Republic of China
Correspondence: Tian Jiang; Zhi Ruan, Email [email protected]; [email protected]
Abstract: The emergence of multidrug-resistant Pseudomonas spp. in the clinical settings has heightened public awareness. Here, we described the genomic characteristics of a P. putida isolate co-carrying five β-lactam resistance genes recovered from a urinary tract infection in China. Whole-genome sequencing was performed using Illumina NovaSeq 6000 and Oxford Nanopore MinION platforms. The genome sequence was annotated and further subjected to identify the sequence type (ST), antibiotic resistance and virulence genes. Phylogenetic analysis of 193 P. putida strains stored in NCBI public database based on core genome single nucleotide polymorphism (cgSNP) strategy were also performed and visualized. Our study indicated that P. putida PP_2463 was resistant to a wide range of antimicrobial agents tested, including aminoglycosides, carbapenems and fluoroquinolones. The complete genome sequence of P. putida PP_2463 is made up of one chromosome and two plasmids, which could be assigned to a new sequence type (ST) 148. The co-occurrence of β-lactam resistance genes blaIPM-15, blaPME-1, blaCARB-2, and blaNDM-1 were first identified in P. putida, and a novel β-lactamase gene located in the chromosome were among the antimicrobial resistance genes discovered. The closest relative of P. putida PP_2463 was identified in 2012 from a urine sample in China, with a difference of 143 SNPs. Along with the presence of multiple β-lactamase genes and mobile genetic elements, the multidrug-resistant phenotype suggests a significant potential as an antibiotic resistance reservoir for Pseudomonas spp.
Keywords: Pseudomonas putida, whole genome sequencing, multidrug-resistance, blaPME-1, blaNDM-5, blaIPM-15, blaCARB-2, urinary tract infection
Introduction
In light of the high mortality rates associated with severe infections and the limited therapeutic treatment options available, the dissemination of carbapenem-resistant Gram-negative bacteria has been a significant concern in hospital settings worldwide. Pseudomonas putida is a non-fermenting Gram-negative bacillus that causes bacteremia and sepsis in newborns, neutropenic patients, patients with cancer, as well as urinary tract infections (UTIs). P. putida can cause nosocomial infections in hospitalized patients and its high adaptability to challenging environmental conditions enables it to survive in different ecological environments.1 To date, several P. putida outbreaks have occurred in clinical settings, with some outbreaks being linked to the spread of contaminated water outlets.2–4 Although most P. putida are susceptible to a vast majority of antimicrobial agents, multidrug-resistant P. putida isolates harboring metallo-β-lactamase (MBL) genes and transposons have been identified already.5,6 Additionally, the horizontal transmission of antimicrobial resistance determinants between P. putida and P. aeruginosa constitutes a serious concern in clinical settings, although the definitive mechanisms remain largely undefined. In this study, we characterized the genomic features of a multidrug-resistant P. putida isolate co-carrying several β-lactamase gene, including blaPME-1, blaIMP-15, blaNDM-1, blaCARB-2 and a novel β-lactamase gene obtained from a urinary tract infection in China.
Materials and Methods
In March 2021, P. putida strain PP_2463 was recovered from the urine sample of a 65-year-old male patient with prostate cancer admitted to a tertiary hospital in Wenling, Zhejiang, China. The specimen was initially incubated overnight at 37°C on Columbia blood agar. Then, a single colony of the target strain was grown in Mueller Hinton broth overnight at 37°C (Oxoid Ltd, Basingstoke, UK). The strain was initially identified by VITEK 2 (bioMérieux, Marcy-l’Étoile, France) and Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF-MS, Bruker, Billerica, MA, USA), and then subjected to 16S rRNA gene sequencing. Antimicrobial agents were purchased from Sigma-Aldrich (St Louis, Missouri, USA) and dissolved according to the manufacturers’ instructions. Antimicrobial susceptibility testing was performed by DxM MicroScan WalkAway ID/AST system for the following antimicrobial agents: amikacin, gentamicin, tobramycin, piperacillin, ticarcillin/clavulanic acid, piperacillin/tazobactam, ceftazidime, cefepime, cefazolin, cefuroxime, cefoxitin, aztreonam, imipenem, meropenem, ciprofloxacin, and levofloxacin. The minimum inhibitory concentrations (MICs) were determined according to the Clinical and Laboratory Standards Institute (CLSI) 2020 guidelines. The MICs of the 17 tested antibacterial agents are shown in Table 1. Escherichia coli ATCC 25922 was used as a quality control strain.
 |
Table 1 Genotype and Phenotypic Resistance Profile of the P. Putida PP_2463 |
A Genomic DNA Purification Kit (QIAGEN, Valencia, CA, USA) was used to extract genomic DNA from the isolate and NanoDropTM spectrophotometer (Thermo Scientific, Waltham, MA, USA) was used to assess the purity and concentration of DNA. Whole genome shotgun sequencing of P. putida PP_2463 was undertaken using both short-read Illumina NovaSeq 6000 (Illumina Inc., San Diego, CA, USA) and long-read Oxford Nanopore MinION (Oxford Nanopore Technologies, Oxford, UK) platforms to study the mechanisms of antimicrobial resistance. Unicycler 0.4.8 was used to accomplish hybrid assembly of Illumina and Nanopore sequence reads.7 The genome sequence was automatically annotated by the NCBI Prokaryotic Genomes Annotation Pipeline (PGAP). In silico multilocus sequence typing (MLST) analysis was performed by PubMLST database.8,9 ABRicate 1.0.1 was used in conjunction with ResFinder 4.1 and CARD 2020 to identify the antimicrobial resistance genes (ARGs) in the genome.10,11 IslandViewer 4, ISfinder 1.0, PHASTER 2016, CRISPRCasFinder 1.0, and antiSMASH 5.2.0 were used to predict genomic islands, insertion sequence (IS) elements, prophage sequences, clustered regularly interspaced short palindromic repeat (CRISPR) sequences, and secondary metabolite gene clusters, respectively.12–16
Genomic sequences and the associated metadata of 192 P. putida strains stored in NCBI GenBank database were obtained. The bacterial core genome single nucleotide polymorphism (cgSNP) analysis between P. putida PP_2463 and 192 complete or draft genomic sequences of P. putida strains were determined to construct a maximum likelihood phylogenetic tree using CSI Phylogeny 1.4.17 This analysis was performed using the default parameters and P. putida NBRC 14164 (RefSeq ID: NC_021505.1) as the reference genome. SNP distance matrixes were calculated using SNP-dists. Phylogenetic tree were visualized and annotated by Interactive Tree of Life (iTOL) V5 web server.18 Easyfig was used to analyse genetic context of the antimicrobial resistance genes identified in P. putida PP_2463.19
Results and Discussion
The complete genome sequence of P. putida PP_2463 is made up of three contigs totaling 5,561,304 bp. The strain’s overall G+C content was 62.5%, and a total of 5129 coding sequences (CDSs), 100 RNAs (74 tRNA, 22 rRNA, and 4 ncRNA) genes were found. Antimicrobial susceptibility testing showed that the isolate was resistant to all tested antimicrobial agents, including aminoglycosides, β-lactams, and fluoroquinolones (Table 1). The resistome of P. putida PP_2463 is made up of several genes that are responsible for resistance to β-lactams (blaPME-1, blaIPM-15, blaCARB-2, blaNDM-1 and a novel β-lactamase gene), tetracyclines [tet(G)], aminoglycosides [aadA2, aadA11, aph(3’)-VIa, aph(6)-Id, aph(3”)-Ib, rmtB and aph(3’)-XV], fluoroquinolones (qnrVc1) and sulphonamide (sul1) (Table 1). The multidrug resistance phenotype to aminoglycosides, third-generation cephalosporins, carbapenems, quinolones and tetracyclines was adequately explained by these findings. Interestingly, all the antimicrobial resistant genes were located in the chromosome (contig 1) and three resistance gene clusters were formed (Figure 1). The first cluster contained the blaNDM-5 gene, two copies of blaPME-1, and a novel β-lactamase gene. Analysis of the genetic environment revealed that IS91 and IS5 exists upstream and downstream of β-lactam resistance genes as a truncated fragment. The genetic environment of the second cluster revealed that intl1 and Tn3 lies downstream of blaCARB-2, while the aadA, folP, and floR2 genes are located upstream of the blaCARB-2 gene. Besides, the second cluster also carried the aph(6)-Id, aph(3”)-Ib, aadA2, sul1 and tet(G) genes. The third cluster carried several β-lactam resistance genes, including blaCARB-2, blaIMP-15 and other resistance genes, including rmtB, tet(G), aph(3’)-XV, dfrA27, aph(6)-Id, aph(3”)-Ib, sul1, aadA11, and qnrVC1. There are at least 65 genomic islands and multiple IS elements in the genome of P. putida PP_2463, and the majority of them belong to the IS3, IS5, and IS66 families. Besides, a total of 10 prophages and just one CRISPR sequence can be predicted. Interestingly, we found a new β-lactamase gene (NCBI accession number OM585599) that has not even been named yet, and to our knowledge the co-occurrence of blaPME-1, blaIPM-15 and blaNDM-1 genes was also firstly identified in P. putida.
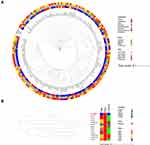
In silico MLST analysis indicated that P. putida PP_2463 represent a novel sequence type, which has been assigned to ST148 after we submitted to the PubMLST database and curated. The phylogenetic connections between P. putida PP_2463 and a total of 192 P. putida strains currently deposited in the NCBI GenBank database were examined to evaluate the genomic epidemiological features of P. putida strains in a global context (Figure 2A). The 193 strains of P. putida in this study were recovered from various hosts, including human (n = 32, 16.5%), plant (n = 21, 10.8%), animal (n = 4, 2.0%) and other unknown resources (n = 136, 70.4%). The isolation dates ranged from 1977 to 2021, but most isolates were obtained between 2011 and 2021 (55.9%, n = 108). Europe (25.3%, n = 49) is the main separation continent, followed by Asia (24.3%, n = 47), North America (20.2%, n = 39), Africa (5.6%, n = 11), Antarctica (1%, n = 2), Oceania (1.0%, n = 2), South America (0.5%, n = 1). According to the results of phylogenetic analysis, the closest relative of P. putida PP_2463 was identified in 2012 from a urine sample in Sanya, China, with just 143 SNPs difference. Interestingly, after we submitted the genome sequence to NCBI for annotation, the curator used P. juntendi as the organism’s name for this genome due to a higher level of similarity according to the current taxonomic nomenclature. We then further performed the average nucleotide identity (ANI) analysis of P. putida PP_2463 with the genome sequence of the type strains of P. putida and P. juntendi. The ANI results revealed that the genome of P. putida PP_2463 is > 99.9% identical to the type strain of both species. P. juntendi was a novel species firstly identified in hospital patients in Japan and Myanmar in 2019,20 which is close to several species in the genus of Pseudomonas, including P. putida. Therefore, we also investigated the genomic epidemiological features of the currently available P. juntendi strains in a global context (Figure 2B). The closest relative of P. putida PP_2463, obtained from a blood sample in Brazil in 2010, with 10,607 SNPs difference. Ribosomal Multilocus Sequence Typing (rMLST) analysis also showed that P. putida PP_2463 could be identified as P. putida or P. juntendi with equal confidence score.
Conclusion
In summary, we firstly reported a ST148 P. putida strain in China that carrying multiple chromosome-borne blaPME-1, blaIPM-15, blaCARB-2, blaNDM-1 and a novel β-lactamase genes. The prevalence of P. putida isolates carrying β-lactamase resistance determinants raises major concerns, emphasizing the critical need for alternate antibiotic therapy and continuing active surveillance. These findings will shed new light on the genomic epidemiological characteristics and the global transmission dynamics of P. putida and demonstrates its superior capacity for acquiring and maintaining foreign resistance determinants.
Data Sharing Statement
The genome sequences of the chromosome and plasmids of the isolate have been deposited in NCBI GenBank under accession numbers CP091088-CP091090.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and obtained approval from Medical Ethics Committee at the Affiliated Wenling Hospital, Wenzhou Medical University, China. Written informed consent was provided by the patient to allow the case details to be published.
Funding
This study was supported by Zhejiang Provincial Medical and Health Science and Technology Plan (2022RC299), Scientific Research Fund of Taizhou Science and Technology Bureau (20ywb152), Scientific Research Fund of Wenling Science and Technology Bureau (2021S00229, 2020S0180116).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Horii T, Muramatsu H, Iinuma Y. Mechanisms of resistance to fluoroquinolones and carbapenems in Pseudomonas putida. J Antimicrob Chemother. 2005;56(4):643–647. doi:10.1093/jac/dki254
2. Aumeran C, Paillard C, Robin F, et al. Pseudomonas aeruginosa and Pseudomonas putida outbreak associated with contaminated water outlets in an oncohaematology paediatric unit. J Hosp Infect. 2007;65(1):47–53. doi:10.1016/j.jhin.2006.08.009
3. Liu Y, Liu K, Yu X, Li B, Cao B. Identification and control of a Pseudomonas spp (P. fulva and P. putida) bloodstream infection outbreak in a teaching hospital in Beijing, China. Int J Infect Dis. 2014;23:105–108. doi:10.1016/j.ijid.2014.02.013
4. van der Zwet WC, Nijsen IEJ, Jamin C, et al. Role of the environment in transmission of Gram-negative bacteria in two consecutive outbreaks in a haematology-oncology department. Infect Prev Pract. 2022;4(2):100209. doi:10.1016/j.infpip.2022.100209
5. Peter S, Oberhettinger P, Schuele L, et al. Genomic characterisation of clinical and environmental Pseudomonas putida group strains and determination of their role in the transfer of antimicrobial resistance genes to Pseudomonas aeruginosa. BMC Genomics. 2017;18(1):859. doi:10.1186/s12864-017-4216-2
6. Rajabal V, Taner F, Sanlidag T, et al. Genetic characterisation of antibiotic resistance transposons Tn6608 and Tn6609 isolated from clinical Pseudomonas strains in Cyprus. J Glob Antimicrob Resist. 2021;26:330–334. doi:10.1016/j.jgar.2021.07.016
7. Wick RR, Judd LM, Gorrie CL, Holt KE. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol. 2017;13(6):e1005595. doi:10.1371/journal.pcbi.1005595
8. Jolley KA, Bray JE, Maiden MCJ. Open-access bacterial population genomics: bIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018;3:124. doi:10.12688/wellcomeopenres.14826.1
9. Feng Y, Zou S, Chen H, Yu Y, Ruan Z. BacWGSTdb 2.0: a one-stop repository for bacterial whole-genome sequence typing and source tracking. Nucleic Acids Res. 2021;49(D1):D644–D650. doi:10.1093/nar/gkaa821
10. Bortolaia V, Kaas RS, Ruppe E, et al. ResFinder 4.0 for predictions of phenotypes from genotypes. J Antimicrob Chemother. 2020;75(12):3491–3500. doi:10.1093/jac/dkaa345
11. Alcock BP, Raphenya AR, Lau TTY, et al. CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020;48(D1):D517–D525. doi:10.1093/nar/gkz935
12. Bertelli C, Laird MR, Williams KP, et al. IslandViewer 4: expanded prediction of genomic islands for larger-scale datasets. Nucleic Acids Res. 2017;45(W1):W30–W35. doi:10.1093/nar/gkx343
13. Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 2006;34:D32–D36. doi:10.1093/nar/gkj014
14. Arndt D, Grant JR, Marcu A, et al. PHASTER: a better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016;44(W1):W16–21. doi:10.1093/nar/gkw387
15. Couvin D, Bernheim A, Toffano-Nioche C, et al. CRISPR CasFinder, an update of CRISRFinder, includes a portable version, enhanced performance and integrates search for cas proteins. Nucleic Acids Res. 2018;46(W1):W246–W251. doi:10.1093/nar/gky425
16. Blin K, Shaw S, Steinke K, et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019;47(W1):W81–W87. doi:10.1093/nar/gkz310
17. Kaas RS, Leekitcharoenphon P, Aarestrup FM, Lund O. Solving the problem of comparing whole bacterial genomes across different sequencing platforms. PLoS One. 2014;9(8):e104984. doi:10.1371/journal.pone.0104984
18. Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–W296. doi:10.1093/nar/gkab301
19. Sullivan MJ, Petty NK, Beatson SA. Easyfig: a genome comparison visualizer. Bioinformatics. 2011;27(7):1009–1010. doi:10.1093/bioinformatics/btr039
20. Tohya M, Watanabe S, Teramoto K, et al. Pseudomonas juntendi sp. nov., isolated from patients in Japan and Myanmar. Int J Syst Evol Microbiol. 2019;69(11):3377–3384. doi:10.1099/ijsem.0.003623
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.