Back to Journals » Journal of Pain Research » Volume 16
Fire Needling Therapy of Different Frequencies versus External Diclofenac Diethylamine Emulgel for Knee Osteoarthritis: Study Protocol for a Pilot Randomized Controlled Trial
Authors Wang M , Xu X, Zhao B, Liu L , Zhao L , Zhang F, Ji X, Yuan F, Xia Q, Wang S, Tian W, Wang L, Li B
Received 27 February 2023
Accepted for publication 13 April 2023
Published 24 April 2023 Volume 2023:16 Pages 1381—1390
DOI https://doi.org/10.2147/JPR.S408084
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Houman Danesh
Mina Wang,1,2,* Xiaobai Xu,1,* Bingcong Zhao,1 Lu Liu,1 Luopeng Zhao,1 Fan Zhang,1 Xu Ji,1 Fang Yuan,1 Qiuyu Xia,1 Shaosong Wang,1 Wei Tian,1 Linpeng Wang,1 Bin Li1
1Department of Acupuncture and Moxibustion, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing, People’s Republic of China; 2Graduate School, Beijing University of Chinese Medicine, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Li; Xiaobai Xu, Email [email protected]; [email protected]
Purpose: Knee osteoarthritis (KOA) is regarded as one of the leading musculoskeletal diseases. Although the efficacy is under exploration, fire needling therapy is considered an effective alternative for KOA. This trial aims to investigate the effectiveness of different frequencies of fire needling therapy in attenuating pain and promoting function in KOA patients.
Methods: This is a study protocol for a pilot, three-arm, single-center, randomized controlled trial. A total of 90 participants with KOA will be recruited and randomly assigned to the high-frequency fire needling group (3 sessions per week, for 6 weeks), the low-frequency fire needling group (1 session per week, for 6 weeks) or the positive control group (Diclofenac Diethylamine Emulgel, 3 times per day, for 6 weeks) in a 1:1:1 ratio. Participants will accomplish the trial at Week 14 after a follow-up evaluation. The response rate will be set as the primary outcome that the proportion of participants obtaining a minimal clinically important difference, which is identified as ≥ 2 units on the numerical rating scale (NRS) and ≥ 6 units on the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) function score at Week 6 compared with Week 0. Secondary outcomes are NRS, WOMAC, Brief Pain Inventory, Short-Form Health Survey-12, Timed Up and Go Test, and pain threshold.
Discussion: This is the first standardized protocol comparing fire needling therapy and positive control drugs. This trial may provide reliable evidence for the effectiveness of fire needling therapy and dose–effect property of it in KOA. Trial registration: The trial has been registered on Chinese Clinical Trial Registry (Registered number: ChiCTR2100043041), registered on 4 February 2021.
Keywords: knee osteoarthritis, fire needling therapy, different frequencies, dose–effect property
Introduction
Osteoarthritis (OA) is one of the most popular musculoskeletal diseases, characterized by pain and impaired physical function, which causes disability, decreased social participation, and reduced quality of life.1 The pathogenesis of OA includes mechanical, inflammatory, and metabolic factors, which lead to structural alterations in the whole joint tissues including the hyaline articular cartilage, subchondral bone, ligaments, capsule, synovium, and periarticular muscles.2 Structural destruction causes pain through peripheral nociceptive mechanisms and neuropathic mechanisms. Peripheral nociceptive pain arises from increased responsiveness of peripheral nociceptors by ongoing tissue injury or inflammation in the joint, and neuropathic pain results from structural changes in joint innervation or from nerve changes in the peripheral nervous system or spinal cord. Another origin of OA pain is central pain, which is caused by enhanced activity of descending pain facilitation pathways and loss of descending antinociceptive pathways.3,4 OA affects 1 in 3 older adults, women more so than men.5 The knee is the most affected joint, age, female sex, obesity, previous knee injury, and heavy work activities are risk factors for knee OA (KOA), and symptomatic KOA is highly prevalent in older people, affecting more than 250 million people globally.6,7 The medical cost of OA in various high-income countries accounts for 1% to 2.5% of the gross domestic product,8 and KOA accounts for approximately 85% of the burden of OA worldwide.9 The current therapies for KOA include education, surgery, and pharmacological intervention,10 while they are not effective with a high risk of adverse events. For example, total knee replacement is generally used in the end-stage of KOA, but about 15% of patients have side effects such as continuous pain, mobility problems and recurrence.11
Generally, non-steroidal anti-inflammatory drugs (NSAIDs) are useful for relieving symptoms of OA but useless for preventing cartilage damage, and long-term use may increase the risk of gastrointestinal and cardiovascular diseases.12 Topically administered NSAIDs such as Diclofenac Diethylamine Emulgel are strongly recommended (level 1a) in the guideline to reduce the pain in KOA patients, and topically administered NSAIDs have fewer adverse events compared with oral NSAIDs.13 However, the modest effectiveness and adverse events of NSAIDs require alternative therapies to compensate.
Recent guidelines emphasize the importance of nonpharmacologic therapies, such as acupuncture.14 Acupuncture involving fire needling, electro-acupuncture, manual acupuncture and other types has been considered the most common complementary and alternative nonpharmacological therapy for KOA.15 Also, acupuncture is better than sham as well as no acupuncture in relieving knee pain and dysfunction.16 Studies have shown that intensive acupuncture (3 sessions per week) immediately promoted knee pain and dysfunction compared with the occasional acupuncture (1 session per week).17 Additionally, the benefit of intensive acupuncture persists throughout follow-up period.18 Therefore, the frequency of acupuncture is strongly related to acupuncture effectiveness.
As one of the most common acupuncture forms, the history of fire-needling therapy in treating KOA can be dated back thousands of years. A red-hot needle is inserted into acupoints to cure diseases, with the extra heat stimuli, fire needling therapy initiates a stronger response in human body.19 Recently, a meta-analysis has demonstrated that the effect of fire needling therapy on knee joint stiffness and function is superior to that of the control group.20 Like drugs, acupuncture has a dose–effect property, which can be measured by the number of needles, retention time, needle manipulation and treatment sessions.21,22 Previous studies compared the effectiveness of fire needling therapy and filiform needles, in which, the frequency of fire needling therapy was set 3 times per week in both studies.23,24 However, there is limited evidence related to the dose–effect property of fire needling therapy. Therefore, our study aims to detect the effect of high-frequency fire needling group (3 sessions per week, for 6 weeks) compared with low-frequency fire needling group (1 session per week, for 6 weeks) and positive control group (Diclofenac Diethylamine Emulgel, 3 times per day, for 6 weeks) in attenuating pain and promoting function in patients with KOA.
Materials and Methods
Study Design and Setting
This study proposes a three-arm, randomized controlled trial at Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University. The trial has been registered on Chinese Clinical Trial Registry (Registered number: ChiCTR2100043041) and approved by the research ethic committee. This study will be conducted in accordance with the Declaration of Helsinki and reported following the Consolidated Standards of Reporting Trials (CONSORT) statement. This protocol has been presented according to the SPIRIT guidelines. Recruitment strategies include online and print advertisements through Wechat (a common social media platform in China), hospital websites, and flyers at outpatient units. The intervention includes 6 weeks of high-frequency fire needling therapy or low-frequency fire needling therapy or Diclofenac Diethylamine Emulgel and 8 weeks of follow-up (Figures 1 and Figure 2).
Participants
Participants will be recruited from the outpatient units of acupuncture and moxibustion department. The enrollment started on February 2021 and is expected to end in December 2023. Each eligible and consented patient will be randomly allocated in a 1:1:1 ratio to receive 6-week high-frequency or low-frequency fire needling therapy or Diclofenac Diethylamine Emulgel.
Inclusion Criteria
- Aged 45–75 years, male or female
- Diagnosed with KOA according to National Institute for Health and Clinical Excellence (NICE) Guidelines 2014 Edition (including the following two situations)25
- Chronic knee pain for the last 1 month
- Morning stiffness ≤30 min
- X-ray confirmation of KOA (Kellgren-Lawrence grade II and above within 6 months)26
- An average pain intensity ≥4 on an 11-point numerical rating scale (NRS) within last week27
- Agreed to refrain from the routine use of analgesics; If participants need rescue medication, they will be provided ibuprofen (SK&F Co, Ltd, Tianjin, China; 0.4 g/8 hours).
- Written informed consent
Exclusion Criteria
- History of knee surgery or waiting for surgery for either knee (knee replacement or knee arthroscopy)
- Knee pain caused by other diseases (such as joint mobilization, severe articular effusion, malignant tumor, autoimmune disease, trauma, and infection)
- Having undergone arthroscopy in the past 1 year or intra-articular injection in the past 4 months
- Having undergone acupuncture therapy in the past 3 months
- Having serious acute or chronic organic or mental disorders
- Pregnant or breastfeeding women
- Coagulation diseases (such as hemophilia)
- Having participated in other clinical trials in the recent 3 months
- Cardiac pacemaker, metal allergy or fire needle phobia
Randomization and Allocation Concealment
Participants will be randomly allocated to the high-frequency group (group H), low-frequency group (group L), or positive control group in a ratio of 1:1:1. The randomization sequence will be generated with the R software (4.2.1), using block randomization with a block size of 6 by an independent statistician who is not involved in the later implementation or statistical analysis. The randomization sequence will be put into an opaque envelope which is marked with enrollment number on the cover. The generation of randomization sequence and opaque envelopes will be provided by Research Center of Clinical Epidemiology (Peking University Third Hospital). Researchers will be required to open the envelope based on the order of patient’s enrollment and implement intervention based on the group information inside the envelope.
Blinding
The participants and acupuncturists in our study will not be blinded due to the property of the intervention. However, data analysts and outcome assessors who do not participate in allocation and intervention will be blinded to group assignment. The allocation of all participants will be concealed until the statistical analysis is completed.
Interventions
Fire needling therapy is semi-standardized, based on traditional Chinese medicine meridian theory of Bi syndrome, and the therapy prescription was developed from literature reviews as well as the clinical practice. The acupuncturists are required to have the Chinese medicine practitioner license. The acupuncture points in this study are divided into obligatory ones which will be used in each treatment session on the affected knee, and customized ones which will be chosen by experienced acupuncturists according to syndrome differentiation, which requires checking the tongue, pulse, and other concomitant symptoms of participants. Six compulsory local points include ST 35, EX-LE5, ST 34, SP 10, GB 34 and Ahshi point, other selective distal points involve GB39 (liver and kidney deficiency syndrome), RN 4 (the blockage of cold-dampness syndrome), ST 36 and LI 4 (Qi stagnation and blood stasis syndrome). The number of points chosen in this study will not exceed eight (Table 1 and Figure 3).
 |
Table 1 Locations of Acupuncture Points Used in Group H and Group L |
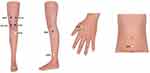 |
Figure 3 Locations of acupuncture points used in group H and group L. Blue circles: Obligatory acupuncture points. Yellow circles: Customized acupuncture points. |
The intervention will be performed with a needle made of manganese and tungsten alloy which will sustain hardness at high temperatures (He’s fire needling instrument, size 0.5mm*25mm). Nail scratch marks will be given at chosen points where the acupuncturists will insert the red-hot needle burnt over a spirit lamp and will withdraw the needle swiftly without retention, each chosen point will be pricked twice. The depth of needling should be 5 to 10mm, depending on the shape of participants. The needle hole will be pressed with a sterilized dry cotton ball for 30 seconds.
High-Frequency Group (Group H)
Participants in group H will receive fire needling therapy 3 times per week (every Monday, Wednesday, and Friday) for 6 weeks.
Low-Frequency Group (Group L)
Participants in group L will receive fire needling therapy once per week (every Monday) for 6 weeks.
Positive Control Group
Participants in positive control group will use a topical ointment Diclofenac Diethylamine Emulgel (Tianjin Smith Kline & French Laboratories Ltd., Tianjin, China, 20g in each tube; H20181225)28 3 times per day, for 6 weeks. Each participant will be shown how to apply the emulgel for the first time under the supervision of a professional researcher who provides detailed instructions for the application of the emulgel and dosing pads to standardize the application amount. The emulgel will be gently rubbed over the affected knee joints (two joints will be treated if both are affected, but the more painful one will be used to analyze). All participants will be required not to wash their knees and reduce joint movement for 1 hour after application.
Rescue Medication
In case of intolerable knee pain, participants will be suggested to take Ibuprofen sustained-release capsules (0.3g/T, Tianjin Smith Kline & French Laboratories Ltd., Tianjin, China) as a rescue medication, the dose should not exceed 1200mg per day. The dose and timing of usage will be documented in a case report form (CRF). Assessor will not evaluate outcomes for participants who take rescue medication within 48h before the baseline and outcome measurements, the outcome measurement visit will be postponed.
Outcomes
The measurement of outcomes will be conducted on the affected knee for participants with only one side affected. If participants with both knees affected, the more painful one will be assessed.
Primary Outcome Measurement
The primary outcome will be revealed as effective response rate29 that the proportion of participants whose average pain NRS scores decrease at least 2 points and the WOMAC function subscale scores decrease at least 6 points at week 6 compared with Week 0. NRS is an 11-point self-administrated tool, ranging from 0 (no pain at all) to 10 (worst pain).30 Participant’s ability to move and daily activities will be assessed using WOMAC function subscale.31 It scores from 0 to 68 with higher scores suggesting worse function. The Minimal Clinically Important Difference (MCID) of NRS and WOMAC function subscale is 2 units and 6 units, respectively.32
Secondary Outcome Measurement
- Knee-joint pain: The average pain of knee will be assessed using a 5-item WOMAC pain subscale, scored from 0 to 20, and 7-item Brief Pain Inventory (BPI) which ranges from 0 to 10 at Week 0, 4, 6 and 14. WOMAC pain subscale assesses the intensity of pain in five different conditions.31 BPI describes severity and impact of pain in terms of physical sensation and interference with function.33 For both scales, higher scores indicate worse pain.
- Quality of life: Short-Form Health Survey-12 (SF-12) will be used to evaluate the quality of life at Week 0, 4, 6 and 14. SF-12 is comprised of mental part and physical part, each part scores from 0 to 100, and higher scores indicate a better quality of life.34
- Functional mobility: The participant’s functional mobility will be evaluated using Timed Up and Go Test (TUG) at Week 0, 4, and 6. Participants will be instructed to stand up from a chair and walk 3 meters safely with comfortable shoes, then come back and sit in the chair. The time taken to accomplish this test will be recorded using a stopwatch timed to the nearest 1/100 seconds.35
- Pain threshold: Up to six tenderness points will be detected by assessor’s hands and marked with a brown marker. The pressure pain threshold (PPT) of the six points will be measured using a pressure algometer (Somedic AB, Sweden) which consists of a rod with a circular end (1 cm2). The pressure algometer will be placed perpendicularly to the skin and applied at a gradually increasing rate (0.5 kg/cm2/second) until the participant indicates that the pressure sensation changes to slight pain by raising their right hand. Then the algometer will be removed immediately from the skin.36,37 The mechanical withdrawal threshold (MWT) of the selected six points will be measured using the electronic von Frey detector (IITC Life Science). The detector will be applied perpendicularly to the skin surface, with a gradually increasing rate until the participant indicates that stabbing pain first appears by raising their right hand. Then, the detector will be removed immediately from the skin.38,39 Participants will be familiarized with the test by applying gradual pressure to the palm of their right hand before formal assessment. Each point will be assessed three times, and the interval between two points will be two minutes. The lowest value of each point will be recorded by a professional assessor who will implement PPT test and MWT test throughout the trial alone. Lower PPT and MWT values reflect greater pain sensitivity. PPT and MWT will be measured at Week 0, 4, and 6.
- Patient’s general impression of change (PGIC): Participants will score the improvement of their knee condition at Week 6, ranging from 0 (Excellent) to 7 (Terrible).
Compliance of Participants
Participants with scores lower than 80 will be considered as poor compliance ((actual treatment sessions/required treatment sessions) x100) at week 6.
Assessment of Safety
Any AEs happening throughout the study period, whether reported spontaneously by the participants or observed by the researchers, will be recorded and categorized as fire needling-related AEs. Common fire needling-related adverse events involve continuous post-needling pain, dizziness, subcutaneous hematoma, infection, etc., and common adverse events of Diclofenac Diethylamine Emulgel include local anaphylaxis and non-atopic dermatitis.
Sample Size
There is no previous study regarding the effective response rate of fire needling therapy on knee osteoarthritis (the proportion of participants whose average pain NRS decreases at least 2 units, and the WOMAC function subscale score decreases at least 6 units at week 6 compared with baseline). The exploratory nature of the study does not necessarily require a formal sample size calculation. Therefore, a sample size of 90 participants (30 for each group) will be recruited to achieve the practical purpose of the trial based on clinical experience.40,41
Statistical Analysis
A blinded biostatistician will perform the statistical analyses using SAS (SAS Institute, Cary, NC, USA) and R software (4.2.1). Demographic characteristics will be summarized based on treatment arm. Mean (standard deviation) or median (interquartile range) will be used to describe continuous variables according to the normality of the distribution. Categorical variables will be described as a percentage. Analyses will be conducted as intention-to-treat (ITT) that include all the participants who have been randomized and given at least one treatment session. For the dropout analysis, last observation carried forward method will be used. Participants who have a good response to the treatment before Week 6 will discontinue the treatment but continue the outcomes assessment for statistical analysis. For primary outcome, the effective response rate will be compared between group H with group L and positive control group using a chi-squared test. α will be adjusted by Bonferroni and set at 0.017. In terms of secondary outcomes, WOMAC pain, stiffness, and function scores, BPI, NRS, SF-12, TUG, PPT, and MWT will be analyzed based on repeated measurement using a mixed-effects model.α will not be adjusted for multiple comparisons of secondary outcomes. Two-tailed P value <0.05 is considered statistically significant. The compliance scores and adverse events rate will be described using a chi-squared test.
Discussion
KOA is regarded as one of the most common diseases to cause chronic pain and disability in elderly and results in a considerable financial burden for society.8 Current treatment methods such as drugs and surgery cannot meet the patient’s needs in KOA. In China, fire needling therapy is often used to treat KOA patients.42 Growing clinical evidence has suggested that fire needling therapy is effective for KOA. Our research team has found that fire needling therapy decreases pain-related behaviors in KOA mice and improves synovial membrane injury and pathological cartilage damage.43 However, fire needling therapy is rarely compared with the first-line drugs recommended by guidelines.44 At the same time, the relationship between acupuncture dose and effect has been paid more attention by researchers, and it has been found that acupuncture frequency is one of the aspects of acupuncture dose, whereas the study about dose–effect property of fire needling therapy is limited.
In addition, an appropriate control group plays a critical role in a well-designed clinical trial. Diclofenac Diethylamine Emulgel is a first-line topical drug recommended by guidelines for KOA patients. Thus, the comparison between fire needling therapy and the positive control group (Diclofenac Diethylamine Emulgel) will be valid in this trial. All in all, this trial will evaluate the effectiveness of high-frequency fire needling group, low-frequency fire needling group, and the positive control group in alleviating the symptoms of KOA. The methodological demands such as adequate randomization, allocation concealment, and blinding of outcome assessors and statisticians are well established in this trial. All the processes are based on traditional Chinese medicine theory as well as clinical practice. There will be 1 to 3 treatment sessions each week in a 6-week treatment phase, achieving a total of 6 to 18 sessions.
Our study has several limitations. First, the placebo effect is difficult to be ruled out without a sham control. However, our study aims to assess the effectiveness of fire needling therapy compared with the optimal treatment for KOA recommended in the guideline. If all treatments are possibly effective, it is easier to recruit participants with fewer ethical problems, for participants who are seeking a treatment for their discomfort, an active group instead of a sham control is more pragmatic. Therefore, external Diclofenac Diethylamine Emulgel, one of the most effective therapies in KOA, will be chosen as a comparison in our study. Secondly, the participants and acupuncturists will not be blinded. Therefore, it is difficult to exclude the placebo effects from the participants who received high-frequency fire needling therapy compared with those who received low-frequency fire needling therapy and the control group. Thirdly, the fire needling therapy groups will have more study visits [6 visits (Group L) or 18 visits (Group H) in total] compared with the control group (2 visits in total). Longer contact time with close interaction between participants and researchers certainly contributes to the observed effects. However, all the researchers will be advised not to make additional contact with the study participant in fire needling group to avoid extra placebo effect. Moreover, we only use two types of treatment frequency, which seems insufficient to explore the relationship between frequency and effect. Future studies should exploit more than three types of treatment frequency to investigate the optimal frequency of fire needling therapy for KOA. Finally, Diclofenac Diethylamine Emulgel will be used three times daily in our study based on evidence from previous literature45 and clinical experience. However, it may lead to relatively poor compliance for patients and cause a certain compliance bias. At the end of our study, we hope the findings will provide high-quality evidence to demonstrate the value of fire needling therapy and dose–effect property of it in KOA.
Abbreviations
BPI, Brief Pain Inventory; ITT, Intention-to-treat; KOA, Knee osteoarthritis; MWT, Mechanical withdrawal threshold; NRS, Numerical rating scale; NSAIDs, Non-steroidal anti-inflammatory drugs; OA, Osteoarthritis; PGIC, Patient’s general impression of change; SF-12, Short-Form Health Survey-12; PPT, Pressure pain threshold; TUG, Timed Up and Go Test; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Data Sharing Statement
The CRFs will be preserved in a locked cabinet at the participating hospitals and can be accessed by the research team only. Patient identifiable data will be used to provide clinical care and follow-up merely, and the trial database will be anonymized. The aggregated research findings will be submitted for publication in a peer-reviewed clinical journal to have widespread dissemination. The original paper files and electronic data will be preserved for at least 5 years after publication, which can be accessible from the corresponding author with appropriate reasons.
Ethical Approval and Consent to Participate
The study followed the ethical standards established in the 1961 Declaration of Helsinki. This protocol has been registered in Chinese Clinical Trial Registry and approved by Beijing Hospital of Traditional Chinese Medicine Affiliated to Capital Medical University Research Ethics Committee (number: 2021BL02-001-01). The Ethics Committee will audit the trial every 3 months to decide on any premature closure of the study. Written informed consent will be obtained from all participants.
Funding
This work was supported by Beijing Traditional Chinese Medicine Science and Technology Development Fund Youth Project (grant number: QN-2020-28); China National Natural Science Foundation (grant number: 82004452; 82074547; 82205246).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Murray CJL, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. doi:10.1016/S0140-6736(12)61689-4
2. Donell S. Subchondral bone remodelling in osteoarthritis. EFORT Open Rev. 2019;4(6):221–229.
3. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759.
4. Belluzzi E, Stocco E, Pozzuoli A, et al. Contribution of Infrapatellar Fat Pad and Synovial Membrane to Knee Osteoarthritis Pain. Biomed Res Int. 2019;2019:6390182.
5. Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800.
6. He J, Ma L, Zhou F, et al. The efficacy and safety of auricular point pressing therapy for knee osteoarthritis: a protocol for systematic review and meta-analysis. Medicine. 2022;101(15):e29098.
7. Cui A, Li H, Wang D, Zhong J, Chen Y, Lu H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29-30:100587.
8. Hunter DJ, Schofield D, Callander E. The individual and socioeconomic impact of osteoarthritis. Nat Rev Rheumatol. 2014;10(7):437–441.
9. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545–1602.
10. Mahler EAM, Minten MJ, Leseman-Hoogenboom MM, et al. Effectiveness of low-dose radiation therapy on symptoms in patients with knee osteoarthritis: a randomised, double-blinded, sham-controlled trial. Ann Rheum Dis. 2019;78(1):83–90. doi:10.1136/annrheumdis-2018-214104
11. Jones CA, Beaupre LA, Johnston DW, Suarez-Almazor ME. Total joint arthroplasties: current concepts of patient outcomes after surgery. Rheum Dis Clin North Am. 2007;33(1):71–86.
12. Mandl LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage. 2019;27(3):359–364.
13. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, Hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589.
14. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020;72(2):220–233.
15. Wang M, Liu L, Zhang CS, et al. Mechanism of Traditional Chinese Medicine in Treating Knee Osteoarthritis. J Pain Res. 2020;13:1421–1429.
16. Tu JF, Yang JW, Shi GX, et al. Efficacy of Intensive Acupuncture Versus Sham Acupuncture in Knee Osteoarthritis: a Randomized Controlled Trial. Arthritis Rheumatol. 2021;73(3):448–458.
17. Lin -L-L, Tu J-F, Wang L-Q, et al. Acupuncture of different treatment frequencies in knee osteoarthritis: a pilot randomised controlled trial. Pain. 2020;161(11):2532–2538.
18. Lin -L-L, Tu J-F, Shao J-K, et al. Acupuncture of different treatment frequency in knee osteoarthritis: a protocol for a pilot randomized clinical trial. Trials. 2019;20(1):55.
19. Organization WH. WHO International Standard Terminologies on Traditional Medicine in the Western Pacific Region; 2007.
20. Wang Y, Xie X, Zhu X, et al. Fire-Needle Moxibustion for the Treatment of Knee Osteoarthritis: a Meta-Analysis. Evid Based Complement Alternat Med. 2016;2016:1392627.
21. Armour M, Smith CA. Treating primary dysmenorrhoea with acupuncture: a narrative review of the relationship between acupuncture ‘dose’ and menstrual pain outcomes. Acupunct Med. 2016;34(6):416–424.
22. White A, Cummings M, Barlas P, et al. Defining an adequate dose of acupuncture using a neurophysiological approach--A narrative review of the literature. Acupunct Med. 2008;26(2):111–120.
23. Fu YB, Chen JW, Li B, Yuan F, Sun JQ. 火针治疗轻中度膝骨关节炎及对相关血清炎性细胞因子的影响 [Effect of fire needling on mild to moderate knee osteoarthritis and related serum inflammatory cytokines]. Zhongguo Zhen Jiu. 2021;41(5):493–497. Chinese.
24. Wang B, Hu J, Zhang N, Wang J, Chen Z, Wu Z. 火针扬刺治疗膝骨关节炎临床观察 [Clinical study of fire acupuncture with centro-square needles for knee osteoarthritis]. Zhongguo Zhen Jiu. 2017;37(5):463–466. Chinese.
25. NICE. Osteoarthritis: care and management. Guidance and guidelines. Available from: https://www.nice.org.uk/guidance/cg177.
26. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502.
27. Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain. 1986;27(1):117–126.
28. Luo X, Hou XS, Tian ZY, Meng X, Li SM, Bai P. 针刺干预早期膝关节骨性关节炎:随机对照试验 [Randomized controlled clinical trial of acupuncture treatment for knee osteoarthritis in the early stage]. Zhen Ci Yan Jiu. 2019;44(3):211–215. Chinese
29. Tu J-F, Yang J-W, Lin -L-L, et al. Efficacy of electro-acupuncture and manual acupuncture versus sham acupuncture for knee osteoarthritis: study protocol for a randomised controlled trial. Trials. 2019;20(1):65.
30. Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res. 2011;63(Suppl 11):S240–252.
31. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the Hip or knee. J Rheumatol. 1988;15(12):1833–1840.
32. Tubach F, Ravaud P, Baron G, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and Hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64(1):29–33.
33. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318.
34. Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233.
35. Alghadir A, Anwer S, Brismée JM. The reliability and minimal detectable change of Timed Up and Go test in individuals with grade 1-3 knee osteoarthritis. BMC Musculoskelet Disord. 2015;16:174.
36. Fernandes GS, Sarmanova A, Warner S, et al. Knee pain and related health in the community study (KPIC): a cohort study protocol. BMC Musculoskelet Disord. 2017;18(1):404.
37. Stefanik JJ, Frey-Law L, Segal NA, et al. The relation of peripheral and central sensitization to muscle co-contraction: the MOST study. Osteoarthritis Cartilage. 2020;28(9):1214–1219.
38. Tena B, Escobar B, Arguis MJ, Cantero C, Rios J, Gomar C. Reproducibility of Electronic Von Frey and Von Frey monofilaments testing. Clin J Pain. 2012;28(4):318–323.
39. Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10(1):77–88.
40. Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4(4):287–291.
41. Johanson GA, Brooks GP. Initial scale development: sample size for pilot studies. Educ Psychol Meas. 2010;70(3):394–400.
42. Gao Y, Liu L, Li B, et al. Evaluation of the efficacy and safety of fire needle compared to filiform needle on knee osteoarthritis: study protocol for a randomized controlled trial. Trials. 2020;21(1):596.
43. Wei J, Liu L, Li Z, et al. Fire Needling Acupuncture Suppresses Cartilage Damage by Mediating Macrophage Polarization in Mice with Knee Osteoarthritis. J Pain Res. 2022;15:1071–1082.
44. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis & Rheumatology. 2020;72(2):220–233.
45. Wang W, Yu S, Long Z, et al. Electroacupuncture vs topical diclofenac sodium gel for patients with hand osteoarthritis: study protocol for a randomized controlled trial. J Orthop Surg Res. 2022;17(1):56.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.