Back to Journals » Journal of Pain Research » Volume 15
Fire Needling Acupuncture Suppresses Cartilage Damage by Mediating Macrophage Polarization in Mice with Knee Osteoarthritis
Authors Wei J, Liu L , Li Z, Lyu T, Zhao L , Xu X, Song Y, Dai Y, Li B
Received 30 January 2022
Accepted for publication 31 March 2022
Published 13 April 2022 Volume 2022:15 Pages 1071—1082
DOI https://doi.org/10.2147/JPR.S360555
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Jiangyan Wei,1,* Lu Liu,1,* Zhijuan Li,1 Tianli Lyu,1 Luopeng Zhao,2 Xiaobai Xu,1 Yine Song,1 Yidan Dai,1 Bin Li1
1Department of Acupuncture and Moxibustion, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Key Laboratory of Acupuncture Neuromodulation, Beijing, People’s Republic of China; 2Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Institute of Traditional Chinese Medicine, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Li, Department of Acupuncture and Moxibustion, Beijing Hospital of Traditional Chinese Medicine, Capital Medical University, Beijing Key Laboratory of Acupuncture Neuromodulation, 23 Meishuguan Back Street, Dongcheng District, Beijing, 100010, People’s Republic of China, Tel +86-18910781852, Fax +86-87906055, Email [email protected]
Purpose: Macrophage polarization contributes to the mechanisms of treating knee osteoarthritis (KOA). In previous studies, fire needling acupuncture has been shown to affect KOA favorably. However, the mechanism of fire needling acupuncture on macrophage polarization is not well-defined. Thus, this study was conducted to determine that fire needling acupuncture exerts a therapeutic role in KOA by modulating macrophage polarization.
Methods: Thirty mice were allocated at random into three groups of ten. The groups were labeled as “control”, “model”, and “fire needling acupuncture”. Each group consisted of ten mice. From the second day of intra-articular injection MIA, the right “xiyan” (EX-LE5), “dubi” (ST35), “liangqiu” (ST34), and “xuehai” (SP10) acupoints were manipulated once every other day for two weeks in the fire needling acupuncture group. Mechanical withdrawal threshold and weight distribution were evaluated for behavioral testing in each group. The synovial morphology was monitored by HE staining. Pathological morphology was observed by HE staining, Saf-O staining, and toluidine blue staining. The polarization of macrophages in synovial tissue was detected using immunofluorescence (F4/80, CD86, and CD206).
Results: Fire needling acupuncture increased the percentage weight-bearing difference and the mechanical withdrawal threshold, and improved synovial inflammation and cartilage damage in MIA-induced KOA mice. F4/80 and CD86 expression were downregulated by fire needling acupuncture, but CD206 was increased.
Conclusion: Fire needling acupuncture decreases pain behaviors in KOA mice and improves synovial membrane injury and pathological cartilage damage. The macrophage polarization is involved in the mechanism of fire needling acupuncture’s amelioration of articular cartilage damage.
Keywords: fire needling acupuncture, knee osteoarthritis, synovial membrane, cartilage, macrophage polarization
Introduction
Osteoarthritis (OA) is a progressive degenerative multifactorial disease of the whole joint, which weakens the quality of life, characterized by a series of symptoms such as pain, stiffness, dysfunction, and even deformity, most commonly in the knee joints and hip joints.1,2 It has high morbidity and disability among older adults, and 250 million people are currently affected according to worldwide estimates, which brings an enormous burden to individuals and society.3 Now, the clinical treatment of OA focuses on managing symptoms through non-pharmacological, pharmacological, and surgical interventions.4 Non-steroidal anti-inflammatory drugs, commonly used to relieve symptoms of OA, do not prevent cartilage damage, and long-term use may cause a risk of gastrointestinal and cardiovascular diseases.5 Artificial joint replacement is a common method to treat advanced OA. Yet, its effectiveness is hindered due to high costs, loosening of artificial joints, and complications.6 Therefore, it is crucial to systematically understand the pathogenesis of OA and find effective treatments in the early stage of OA.
KOA is characterized by synovitis, cartilage erosion, matrix degradation, osteophyte formation, and abnormal subchondral bone remodeling. The development of KOA includes implicating the cartilage, subchondral bone, meniscus, muscle, tendon, synovium, and infrapatellar fat pad (IFP).7 OA reflects a low-grade inflammatory state. Targeted inflammatory therapy has the potential to suppress the progression of OA by preventing or reducing multiple pathological features.8,9 Recent studies have demonstrated a significant correlation between synovitis and the severity of KOA. Activated synovial macrophages appear to play an essential role in the development and progression of KOA.10,11 Macrophages have plastic phenotypes and would adjust to the local microenvironment under physiological and pathological conditions.12 The activated macrophages exhibit conventionally activated M1 and alternately engaged M2 macrophages under different microenvironments.13 M1 macrophages represent a pro-inflammatory phenotype, secreting pro-inflammatory cytokines and chemokines such as interleukins-(IL-) 1α, IL-6, and tumor necrosis factor-α (TNF-α); M2 macrophages represent an anti-inflammatory phenotype, releasing anti-inflammatory cytokines such as IL-4, IL-10 and transforming growth factor-β (TGF-β).14,15 It was found that synovial and intra-articular macrophages aggregated, and M1 synovial macrophages increased and released IL-1α/β, TNF-α, and IL-6, thereby accelerating cartilage degradation and synovitis in the KOA mice.16,17 The induction of M2 macrophages secreted IL-4, IL-10, and TGF-β, improved KOA tissue repair and regeneration in the microenvironment.16,18 In addition, the removal of all subsets of activated macrophages in synovial tissue aggravated the development of KOA, suggesting that M2 macrophages have a crucial protective effect on KOA.19 Therefore, we can consider treating knee osteoarthritis (KOA) by modifying macrophage phenotype and function, ie, the transformation of macrophage M1 phenotype to M2 phenotype.
Acupuncture, a common treatment in traditional Chinese medicine, has a prominent role in treating KOA, and has been widely reported both clinically and experimentally, in which “xiyan” (EX-LE5), “dubi” (ST35), “liangqiu” (ST34), and “xuehai” (SP10) are frequently used.20–23 Fire needling acupuncture (FNA), one of the acupuncture therapies, is done by puncturing a red-hot needle quickly into selected areas on the body to play the role of warming and activating effect and promoting harmonization. Clinically, it has been proved that fire needling acupuncture is effective and safe, and is widely used to treat pain, numbness, and skin diseases.24–30 Some studies have shown that the analgesic effect of fire needling acupuncture is superior to filiform acupuncture in treating KOA.31–33 In animal experiments, the efficacy of fire needling acupuncture has also been verified, such as acute gouty arthritis, moderate–severe acne, spinal cord injury and so on.34–36 Our previous investigation showed that fire needling acupuncture could improve motor skills and cartilage morphology, reduce inflammatory factors and increase anti-inflammatory factors in the serum of KOA rats.37 However, the mechanism of fire needling acupuncture treatment in KOA has yet to be determined.
This study aims to determine the mechanism of fire needling acupuncture in KOA treatment, especially its effect on macrophage polarization. Monosodium iodoacetate (MIA) was injected in mice to induce KOA. Here, KOA mice were used to investigate the effects of fire needling acupuncture on pain relief and improvements on the cartilage structure by promoting the polarization of M2 macrophages of the synovial membrane.
Materials and Methods
Animals
Six-week-old male C57BL/6 mice were obtained from the SPF Biotechnology Co., Ltd. (Beijing, China, Certificate number: SCXK 2019-0010). The mice were kept in a 12-hour light–dark cycle with ad libitum food and water. The environment’s temperature was maintained at 22–26°C. They were housed at least one week before the experiments. Each mouse was divided randomly into control group (CON), model group (MIA), and fire needling acupuncture group (FNA) (10 mice/group) by using the random envelope method. The number of animals used was based on previous experience. Every effort was made to reduce the mice number used in our study. Animal experiments were conducted according to the ethical guidelines formulated by the International Association for the Study of Pain.38 The study design, experiment conduct, statistical analysis, and results reporting were compliant with ARRIVE guidelines 2.0.39 All experiments were authorized by the Beijing Institute of Traditional Chinese Medicine’s Animal Experimentation Ethics Committee (Approval number: 20201113) and conducted following the Committee’s norms and regulations.
Reagents and Antibodies
Monosodium iodoacetate (Sigma, Saint Louis, Missouri, USA), isoflurane (RWD, Shenzhen, China), Ethylenediaminetetraacetic acid disodium salt, dihydrate (EDTA) (Solarbio, Beijing, China), citrate buffer (ZSbio, Beijing, China), HE (hematoxylin-eosin) staining kit (Solarbio, Beijing, China), modified Safranine O-Fast Green FCF cartilage stain kit (Solarbio, Beijing, China), toluidine blue O cartilage stain solution (Solarbio, Beijing, China), goat serum (Solarbio, Beijing, China), F4/80 antibody (Abcam, Cambridge, UK), CD86 antibody (Abcam, Cambridge, UK), CD206 antibody (Abcam, Cambridge, UK), second antibody goat anti-rabbit (Beyotime, shanghai, China), second antibody goat anti-mouse (Applygen, Beijing, China), DAPI (ZSbio, Beijing, China).
MIA-Induced KOA Model
KOA model was induced via intra-articular injection of MIA.40 Mice were anesthetized by isoflurane (2–3% induction, 1–1.5% maintenance) and their right knee joints were shaved. MIA (5μL, 5 mg/mL) mixed with sterile 0.9% normal saline was injected into the right knee joint through the subpatellar ligament using a 30-gauge needle inserted (Supplementary Figure 1). The knees were stretched and flexed gently to disperse the MIA throughout the articular cavity. The control group received 5μL 0.9% sterile normal saline at a similar injection site.
Fire Needling Acupuncture
The mice of the FNA group received fire needling acupuncture treatment on the right “xiyan” (EX-LE5), “dubi” (ST35), “liangqiu” (ST34), and “xuehai” (SP10). These four acupoints were chosen based on traditional Chinese medicine, clinical practice, and literature analysis. They are found around the knee joint. They can dredge local qi and blood, invigorate channels, and activate collaterals, and are frequently used to treat knee problems.31,33 Acupoints used were referenced from the animal acupuncture point atlas and Experimental Acupuncture Science formulated by the Experimental Acupuncture Research Association of the Chinese Association of Acupuncture-Moxibustion (Supplementary Table 1).41,42 The electronic intelligent fire needling acupuncture (2mm length, 0.4mm diameter, “low temperature” 200°C, utility model, patent no. ZL201721678201.2) was applied in this study (Supplementary Figure 2) and supplied by Beijing Hanbotai Kanglai Technology Development Co., LTD. The needle was heated before being punctured and withdrawn from the acupoints (a needle ejection and withdrawal in 0.1s). The depth and temperature of the fire needle used during the fire needling acupuncture treatment were consistent. The mice were anesthetized (isoflurane, 2–3% induction, 1–1.5% maintenance) before commencing fire needling acupuncture. The mice had their right knee bent at 90 degrees and had them punctured once at each acupoint (Supplementary Figure 3). This was done from the second day after MIA injection once every other day, for a total of 2 weeks. The operation of the control group and the MIA group was the same as that of the FNA group, without fire needling acupuncture treatment.
Behavioral Testing
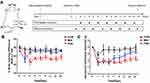
The mice were acclimated to the experimental environment in a quiet room for three consecutive days before performing the behavioral tests. The behavioral study researchers were blinded to avoid experimenter bias. Mice were also acclimated to the environment in which the experiment was conducted. The baseline tests were performed 3 days before MIA injection. Values determined as outliers were excluded from the calculation of the mean values. Behavioral analysis was conducted on days without fire needling acupuncture treatment (Figure 1A).
Weight-Bearing Test
An Incapacitance Meter (600MR, IITC Life Science, Woodland Hills, California, USA) was used to conduct the weight-bearing test. Each hind limb of mice rests on a separate sensor plate. The force applied by each hind limb was averaged over 5 seconds in grams with at least 10 seconds between each measurement. The weight on each hind limb was measured three times. The final value obtained was the mean of the values received. The percentage of weight-bearing difference onto the right hind limb was calculated: weight on the right limb/(weight on the right limb + weight on the left limb) ×100.43
Mechanical Withdrawal Threshold
The mechanical allodynia was assessed using a series of von Frey filaments (Stoelting, Kiel, WI, USA). The mice were placed in raised plexiglass chambers on a metal mesh floor and allowed to adapt for 30 minutes. A von Frey hair filament was applied perpendicular to the plantar surface of the right hind limb until the hair filament started to bend, which lasted 2 seconds. A positive withdrawal response indicated that the mice would sharply withdraw or lick their hind limb. The von Frey hair was applied from 1g force in ascending order until a withdrawal response was established. Once a positive withdrawal response was found, descending levels of von Frey hair pressure was used to test until no response occurred. The minimum force required to induce a response was recorded.44
Sample Collection
At post-injection of MIA after 15 days, perfusion was conducted before obtaining the right knee joints. The mice were anesthetized with pentobarbital sodium before being placed in the supine position. The chest cavity was exposed and 50ml normal saline was injected into the mice from hearts until the lung, liver, and limbs turned white. After 30mL 4% paraformaldehyde was injected, it could be seen that the tail and limbs shook. The right knee joints were harvested and fixed in 4% paraformaldehyde for 24h and decalcified in EDTA solution (10%, pH=7.4) for 20 days. After dehydration, the tissues were embedded in paraffin and cut into serially 4-μm thick sections.
Histological Analysis
The sections were stained with hematoxylin and eosin (HE), Safranin O-Fast Green (Saf-O), and toluidine blue. The procedure of staining followed standard protocols provided by the biotech company. HE staining slides were used to evaluate the synovitis score by three features of chronic synovitis (enlargement of the lining cell layer, density of resident cells, and inflammatory infiltrate) (Supplementary Table 2).45 The degree of cartilage damage of KOA was evaluated by Osteoarthritis Research Society International (OARSI) scoring criteria (Supplementary Table 3).46 KOA severity was the total score and/or the maximum score. The histology samples were examined under a light microscope. The investigators examined the sections independently. They were also blind to the nature of the sections.
Immunofluorescence
Section preparation was described above. After the sections were deparaffinized and rehydrated, they were incubated in a citrate buffer (pH 6.0) before a two-cycle thermostat water bath. Each cycle consists of five minutes of boiling and five minutes of stopping. After the water bath, 10% normal goat serum was used to block non-specific binding for 30 min at 37°C. The sections were later stained with the primary antibody (1:500) overnight at 4°C and were incubated for 1 h with species-matched Alexa 488 dye-labeled secondary antibodies (1:2000) at 37°C. These sections were subsequently covered in fluorescent mounting medium with DAPI. Fluorescent images were acquired using fluorescence microscopy (Axio ImagerA2, ZEN, Zeiss, Germany). Image J (Bethesda, USA) was used to detect the positive cell numbers by two researchers who were blinded from the grouping.
Statistical Analysis
All statistical analyses were presented as the mean ± SEM using the SPSS version 21.0 software. The differences between groups were analyzed using one-way analysis of variance (ANOVA) and Student’s t-test. The repeated-measure two-way ANOVA and Tukey’s multiple comparisons test were applied for the behavioral testing as indicated. P-values of <0.05 were considered significant.
Results
Fire Needling Acupuncture Effectively Relieves Pain Behavior in KOA Mice
Pain management is an essential aspect of the treatment of KOA. We measured weight-bearing difference and the mechanical allodynia reaction to assess pain in KOA mice, as described previously. Intra-articular injection of MIA induced the transfer of weight distribution in KOA mice to the uninjured limb, which was effectively different from the control mice on all days tested after injection. The FNA group showed weight-bearing difference relief when compared with the MIA group from the 3rd day post-injection (Figure 1B). Furthermore, there was a significant increase in mechanical withdrawal threshold from the CON group on the 3rd day post-injection of intra-articular saline. The MIA group exhibited a significant decrease in mechanical withdrawal threshold compared to the CON group. From the 7th day, the FNA group showed a great increase in mechanical withdrawal threshold than the MIA group until the end of observation (day 15 intra-articular injection of MIA) (Figure 1C). These results suggest that FNA effectively relieved pain behave in KOA mice.
Fire Needling Acupuncture Effectively Prevents KOA Development via Suppressing Inflammatory Response and Cartilage Damage in KOA Mice
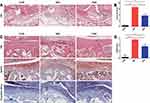
Histological analysis was performed further to investigate the therapeutic effect of fire needling acupuncture. We first examined synovial inflammation by HE staining. The whole joint of HE staining can be seen in Supplementary Figure 4. The mice that had saline injections showed intact synovium surface, one layer of the lining cell layer, normal synovial stroma cells, and no inflammatory infiltration. MIA injection resulted in the significant increase of synovium surface lining cells and synovial stroma cells, and infiltration of numerous lymphocytes or plasma cells. The FNA group showed 2–3 layers of lining cells, a few stroma cells, and a small number of lymphocytes or plasma cells infiltration (Figures 2A). The synovitis score was increased in MIA group mice 15 days after intra-articular injection compared to the CON group. It was comparable between the FNA and the MIA groups (Figure 2B). These indicated that the synovium by fire needling acupuncture treatment was closer to normal than MIA induced.
Next, cartilage damage in MIA-induced KOA was assessed by HE, Saf-O, and toluidine blue staining. Articular cartilage of saline injection showed intact and smooth surface with obvious tide line, neat cells, and evenly distributed proteoglycan, aggrecan and collagen. The injection of MIA resulted in severe cartilage damage, uneven coloring, abnormal distribution of chondrocytes, and loss of tide line and extracellular matrix components. After the fire needling acupuncture treatment, KOA mice exhibited cartilage surface fibrillation, partial dead chondrocytes, visible tide line, and expression of aggrecan, collagen, and proteoglycan (Figure 2C). The FNA group exhibited less cartilage damage than the MIA group, as confirmed by the OARSI score (Figure 2D). These results demonstrated that fire needling acupuncture therapy suppressed synovial inflammatory response and promoted structural improvement in the KOA mice.
Fire Needling Acupuncture Promotes the Polarization of Macrophages Towards M2 Phenotype Macrophages and Reduces Polarization of M1 Phenotype Macrophages in KOA Mice
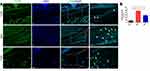
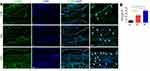
To determine whether fire needling acupuncture therapy can regulate macrophage polarization, we studied the expression of F4/80 (macrophage-specific marker), CD86 (M1 phenotypes macrophage-specific marker), and CD206 (M2 phenotypes macrophage-specific marker) by immunofluorescence. We first studied whether fire needling acupuncture might modulate the macrophages. The results showed that the expression of F4/80 positive cells was significantly suppressed after the fire needling acupuncture treatment (Figure 3), which indicated that the fire needling acupuncture treatment could reduce synovial inflammation in KOA mice. To explore whether the fire needling acupuncture treatment of KOA was related to the change in macrophage phenotypes, we further observed the expression of CD86 and CD206. The treatment of fire needling acupuncture reduced the expression of CD86 (Figure 4). However, the expression of CD206 was increased (Figure 5). We quantified the polarization of the macrophages among the groups and present their data in Figures 3–5. These findings suggested that fire needling acupuncture transformed macrophage polarization from the M1 to M2 phenotype and attenuated KOA.
Discussion
It has been well-documented that macrophage polarization in the KOA participates in the process of inflammation response and cartilage damage.47–49 In the present study, we established the mice MIA model to clarify the mechanism of fire needling acupuncture treatment on KOA by applying ST34, ST35, SP10, and EX-LE5 acupoints. Regarding behavioral tests, fire needling acupuncture reduced the weight-bearing difference and increased the mechanical withdrawal threshold in KOA. Fire needling acupuncture further decreased synovial inflammation and cartilage damage. More importantly, fire needling acupuncture modulated macrophage polarization by enhancing M2 phenotypes and reducing M1 in synovial KOA. This suggests that fire needling acupuncture alleviated the pain, inflammation response and cartilage damage in KOA mice by promoting macrophages from M1 to M2 phenotypes.
OA is a whole joint disease, and it is currently generally accepted that joint inflammation plays a crucial role in the pathogenesis and progression of OA.50 The level of synovial inflammation is associated with the degree of damage to articular cartilage in OA patients.51 Articular cartilage degeneration and extracellular matrix degradation metabolites can stimulate synovial inflammation, and pro-inflammatory factors released by synovial membranes, such as IL-1, IL-6, TNF-α, nitric oxide, neuropeptides, and prostaglandins can further aggravate the damage of articular cartilage.52 IFP and synovial membrane are intimately related and are considered a morpho-functional unit. IFP interacts with the synovial membrane by influencing synovial cell inflammation and promoting synovial cell fibrosis and cartilage damage.53–55 In addition, inflammation of IFP and synovial tissue may drive peripheral and central sensitization of KOA, in which cytokine production is involved in KOA pain and progression, including IL-1β, IL-6, and TNF-α.56–58 IFP, synovium, cartilage, and subchondral bone interact to intervene in the symptoms and development of KOA.53,57 Targeting synovitis might thus suppress KOA pain and progression.59 Although the etiology and pathogenesis of KOA are diverse, available evidence suggests that macrophages regulate joint inflammation through a variety of secretory mediators and thereby the severity of OA.16,60 A study found that the M1/M2 macrophages ratio in synovial fluid of KOA was higher than normal knees, and the ratio was correlated to the Kellgren–Lawrence grade.61 These results suggest that macrophage polarization plays a role in the progression of KOA. Macrophages can modify their phenotype in response to external signals, transform from M1 to M2 phenotype or M2 to M1 phenotype.40 Intra-articular injection of MIA, a standard model of KOA, causes the pain state, synovitis, and cartilage damage.62–64 Indeed, in our experiment, the MIA induced pain behaviors and histological changes in the synovium and cartilage. Tissue staining showed apparent synovitis, infiltration of inflammatory cells, severe destruction of cartilage, and osteophyte formation. Previous studies show that intervening with macrophages by modifying their phenotypic state can significantly reduce cartilage and abnormal reconstruction.16,49 In this study, immunofluorescence showed that M1 phenotype macrophages (CD86 positive cells) infiltration in the synovium was increased in the MIA group. Still, it was significantly decreased after fire needling acupuncture treatment. Moreover, fire needling acupuncture up-regulated M2 phenotype macrophages (CD206 positive cells) in the synovium by MIA-induction after 15 days. The tissue staining suggested that fire needling acupuncture therapy had a protective effect on articular cartilage in MIA-induced KOA mice. This was consistent with previous studies that modified the phenotypes of macrophages to improve cartilage damage.
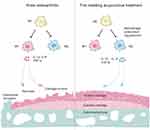
Fire needling acupuncture therapy is considered a distinctive treatment of traditional Chinese medicine therapy. Fire needling acupuncture has the mechanical effect of acupuncture alongside the thermal effect, which can invigorate the meridian and better regulate the microenvironment. Fire needling acupuncture treatment of KOA has a long history and has been widely used in clinics.65–67 The previous study suggested that fire needling acupuncture significantly advances the quality of life of KOA patients by improving pain symptoms and dysfunction, and its short-term and long-term clinical effects are superior to filiform needling.31,33 However, there are few animal studies on the fire needling acupuncture therapy of KOA and the mechanism of fire needling acupuncture treatment in KOA remains unclear. Our previous experimental studies showed that fire needling acupuncture ameliorated joint and systemic inflammation in KOA rats by MIA-induced, inhibited M1 macrophage pro-inflammatory cytokines (IL-1α, TNF-α), and regulated M2 macrophage anti-inflammatory cytokines (IL-10, TGF-β).37 In this experiment, FNA reduced proinflammatory cytokines (IL-1β, TNF-α) and increased anti-inflammatory cytokine IL-10 (Supplementary Figure 5), which is consistent with previous research. Therefore, we further verified that fire needling acupuncture interferes with macrophage polarization. This experiment is the first in the field to explore the intervention of the fire needling acupuncture in KOA macrophage polarization (Figure 6). The existing fire needle’s properties are simulated in the self-developed electronic intelligent fire needle. This electronic fire needle replicated the desired characteristics of the fire needle’s heat, accuracy, and speed for quantitative and qualitative analysis. We believe that the effect of fire needling acupuncture therapy is realized through the dynamic adjustment of the body. Through the warming, activating, and a little damage effect of fire needling, the human body can accelerate regional and systemic self-regulation, thus promoting harmonization of the local environment and achieving overall rehabilitation of the body.68 We found that fire needling acupuncture effectively alleviated pain in the MIA-induced KOA mice model, and suppressed inflammatory responses and cartilage damage. This provides some theoretical support for fire needling acupuncture therapy of KOA in clinical practice.
There are some limitations to this study. Firstly, this study did not establish a fire needle group with non-acupoint areas, and further research is needed to determine the acupoints’ specific effect on fire needling acupuncture in KOA. Secondly, only one model was used in this study, and whether other models (eg, destabilization of the medial meniscus (DMM), anterior cruciate ligament transection (ACLT) and so on) can produce the same effect would need further exploration. Thirdly, we only detected the surface markers of macrophages at one time point without longitudinal observation. Thus the change of the surface marker expression of macrophages in the treatment process remains unclear. As for macrophage polarization, we only focused on the expression of surface markers. Further studies of critical cells, critical cytokines, and different subtypes would need to be conducted to obtain a greater clarity for macrophage polarization.
Conclusion
In summary, for the first time, our study revealed that fire needling acupuncture is an effective non-pharmacological therapy for inhibiting pain behavior and inflammatory response and promoting cartilage repair by modulating macrophage polarization from M1 to M2 phenotypes. This study improves our understanding of the mechanisms of fire needling acupuncture therapy in the treatment of KOA.
Data Sharing Statement
Study data can be shared on individual requests to the corresponding author at [email protected].
Funding
This work was supported by the following funding sources: the China National Natural Science Foundation (82074547, 82074179, 82004435), the National Key Research and Development Plan (2019YFC1709703), the National Administration of Traditional Chinese Medicine: 2019 Project of Building Evidence-Practice Capacity for TCM (No. 2019XZZX-ZJ002), the China Association for Science and Technology Young Talent Lifting Project (2019-2021ZGZJXH-QNRC001), the Capital Health Development Scientific Research Project Excellent Young Talents (Capital Development 2020-4-2236), and the Beijing Municipal Education Commission Science and Technology Plan General Project (KM202110025005).
Disclosure
The authors declare no potential conflicts of interest concerning this article’s research, authorship, and/or publication.
References
1. Sharma L. Osteoarthritis of the knee. N Engl J Med. 2021;384(1):51–59. doi:10.1056/NEJMcp1903768
2. Glyn-Jones S, Palmer AJ, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi:10.1016/S0140-6736(14)60802-3
3. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393(10182):1745–1759. doi:10.1016/S0140-6736(19)30417-9
4. Goldring MB, Berenbaum F. Emerging targets in osteoarthritis therapy. Curr Opin Pharmacol. 2015;22:51–63. doi:10.1016/j.coph.2015.03.004
5. Mandl LA. Osteoarthritis year in review 2018. Clin Osteoarthr Cartil. 2019;7(3):59–364.
6. Liang X, Ding Y, Zhang Y, et al. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;3(9):045–1059.
7. Loeser RF, Goldring SR, Scanzello CR, et al. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–1707. doi:10.1002/art.34453
8. Robinson WH, Lepus CM, Wang Q, et al. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12(10):580–592. doi:10.1038/nrrheum.2016.136
9. Raghu H, Lepus CM, Wang Q, et al. CCL2/CCR2, but not CCL5/ CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017;76(5):914–922. doi:10.1136/annrheumdis-2016-210426
10. Atukorala I, Kwoh CK, Guermazi A, et al. Synovitis in knee osteoarthritis: a precursor of disease? Ann Rheum Dis. 2016;75(2):390–395. doi:10.1136/annrheumdis-2014-205894
11. Bondeson J, Wainwright SD, Lauder S, et al. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8(6):R187. doi:10.1186/ar2099
12. Zhu X, Lee CW, Xu H, et al. Phenotypic alteration of macrophages during osteoarthritis: a systematic review. Arthritis Res Ther. 2021;23(1):110. doi:10.1186/s13075-021-02457-3
13. Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. doi:10.1146/annurev-physiol-022516-034339
14. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
15. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122(3):787–795. doi:10.1172/JCI59643
16. Zhang H, Lin C, Zeng C, et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann Rheum Dis. 2018;77(10):1524–1534. doi:10.1136/annrheumdis-2018-213450
17. Lopa S, Leijs MJ, Moretti M, Lubberts E, van Osch GJ, Bastiaansen-Jenniskens YM. Arthritic and non-arthritic synovial fluids modulate IL10 and IL1RA gene expression in differentially activated primary human monocytes. Osteoarthritis Cartilage. 2015;23(11):1853–1857. doi:10.1016/j.joca.2015.06.003
18. Schulert GS, Fall N, Harley JB, et al. Monocyte microRNA expression in active systemic juvenile idiopathic arthritis implicates microRNA-125a-5p in polarized monocyte phenotypes. Arthritis Rheumatol. 2016;68(9):2300–2313. doi:10.1002/art.39694
19. Wu CL, McNeill J, Goon K, et al. Conditional macrophage depletion increases inflammation and does not inhibit the development of osteoarthritis in obese macrophage fas-induced apoptosis-transgenic mice. Arthritis Rheumatol. 2017;69(9):1772–1783. doi:10.1002/art.40161
20. Liu W, Fan Y, Wu Y, et al. Efficacy of acupuncture-related therapy in the treatment of knee osteoarthritis: a network meta-analysis of randomized controlled trials. J Pain Res. 2021;14:2209–2228. doi:10.2147/JPR.S315956
21. Shi GX, Tu JF, Wang TQ, et al. Effect of electro-acupuncture (EA) and manual acupuncture (MA) on markers of inflammation in knee osteoarthritis. J Pain Res. 2020;13:2171–2179. doi:10.2147/JPR.S256950
22. Shi X, Yu W, Wang T, et al. Electroacupuncture alleviates cartilage degradation: improvement in cartilage biomechanics via pain relief and potentiation of muscle function in a rabbit model of knee osteoarthritis. Biomed Pharmacother. 2020;123:109724. doi:10.1016/j.biopha.2019.109724
23. Deng KF, Sheng FF, Wei XC, et al. Electroacupuncture combined with thunder-fire moxibustion for knee osteoarthritis of cold-damp type: a randomized controlled trial. World J Acupunct Moxib. 2020;30(01):33–38. doi:10.1016/j.wjam.2020.02.009
24. Luo Y, Kuai L, Song N, et al. Efficacy and safety of fire needle therapy for nodular prurigo: a quantitative study. Evid Based Complement Alternat Med. 2019;2019:8797056. doi:10.1155/2019/8797056
25. Zhang Y, Jiang JS, Kuai L, et al. Efficacy and safety of fire needle therapy for flat warts: evidence from 29 randomized controlled trials. Evid Based Complement Alternat Med. 2021;2021:9513762. doi:10.1155/2021/9513762
26. Pan HD, Qi XL, Wang L, et al. Whether Fire-needle therapy benefits plaque psoriasis: a multicenter, randomized, and controlled trial. Chin J Integr Med. 2019;25(4):259–263. doi:10.1007/s11655-018-2940-8
27. Gao Y, Liu MY, Wang S, et al. Combination of fire needle and filiform needle for frozen shoulder in different clinical stages. World J Acupunct Moxib. 2019;29(2):119–122. doi:10.1016/j.wjam.2019.04.007
28. Wang B, Hu J, Zhang N, et al. Clinical study of fire acupuncture with centro-square needles for knee osteoarthritis. Zhongguo Zhen Jiu. 2017;37(5):463–466. doi:10.13703/j.0255-2930.2017.05.003
29. Chen Z, Zhou D, Wang Y, et al. Fire needle acupuncture or moxibustion for chronic plaque psoriasis: study protocol for a randomized controlled trial. Trials. 2019;20(1):674. doi:10.1186/s13063-019-3736-2
30. Han WB, Wang X, Liang ZF, et al. cases of popliteal cyst treated by fire needling and cupping therapy. World J Acupunct Moxib. 2020;30(01):45–48. doi:10.1016/j.wjam.2020.02.002
31. Zhang HX, Chen YY, Ouyang BS. Clinical observation of epiphora as sequela of peripheral facial paralysis treated with pricking technique of fire needling therapy at Chengqi(ST1). World J Acupunct Moxib. 2020;30(03):198–201. doi:10.1016/j.wjam.2020.05.004
32. Wu H, Shi P, Wang J. Effects of fire needle therapy on knee osteoarthritis: meta analysis. Chin J Inf TCM. 2014;21(5):10–14.
33. Fu YB, Chen JW, Li B, et al. Effect of fire needling on mild to moderate knee osteoarthritis and related serum inflammatory cytokines. Zhongguo Zhen Jiu. 2021;41(5):493–497. doi:10.13703/j.0255-2930.20200708-k0003
34. Chen H, Zhou GX, He XJ, Guo F, Chen JM, Qian X. Efficacy of fire needle on acute gouty arthritis induced by monosodium urate in rat. J Tradit Chin Med. 2021;41(4):564–570. doi:10.19852/j.cnki.jtcm.2021.03.008
35. Xing M, Yan X, Sun X, et al. Fire needle therapy for moderate-severe acne: a PRISMA systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2019;44:253–260. doi:10.1016/j.ctim.2019.04.009
36. Xu J, Cheng S, Jiao Z, et al. Fire needle acupuncture regulates wnt/erk multiple pathways to promote neural stem cells to differentiate into neurons in rats with spinal cord injury. CNS Neurol Disord Drug Targets. 2019;18(3):245–255. doi:10.2174/1871527318666190204111701
37. Li ZJ, Wang X, Sun JQ, et al. Effect of fire-needle intervention on joint function, cartilage impairment and inflammatory response in knee osteoarthritis rats. Zhen Ci Yan Jiu. 2020;45(3):220–226. doi:10.13702/j.1000-0607.190963
38. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi:10.1016/0304-3959(83)90201-4
39. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. BMJ Open Sci. 2020;4:e100115. doi:10.1136/bmjos-2020-100115
40. Zhang RX, Ren K, Dubner R. Osteoarthritis pain mechanisms: basic studies in animal models. Osteoarthrit Cartil. 2013;21(9):1308–1315. doi:10.1016/j.joca.2013.06.013
41. China Association of Acupuncture and Moxibustion. Designation and location of common acupoints in experimental animals – part 3: mice. Acupunct Res. 2022;46(05):445–446.
42. China Association of Acupuncture and Moxibustion. Designation and location of common acupoints in experimental animals – part 2: rats. Acupunct Res. 2022;46(04):351–352.
43. Deshmukh V, O’Green AL, Bossard C, et al. Modulation of the wnt pathway through inhibition of CLK2 and DYRK1A by lorecivivint as a novel, potentially disease-modifying approach for knee osteoarthritis treatment. Osteoarthrit Cartil. 2019;27(9):1347–1360. doi:10.1016/j.joca.2019.05.006
44. Fernihough J, Gentry C, Malcangio M, et al. Pain related behaviour in two models of osteoarthritis in the rat knee. Pain. 2004;112(1–2):83–93. doi:10.1016/j.pain.2004.08.004
45. Krenn V, Morawietz L, Burmester GR, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology.2006. 49(4):358–364.
46. Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. 2006;14(1):13–29. doi:10.1016/j.joca.2005.07.014
47. Zhou F, Mei J, Han X, et al. Kinsenoside attenuates osteoarthritis by repolarizing macrophages through inactivating NF-κB/MAPK signaling and protecting chondrocytes. Acta Pharm Sin B. 2019;9(5):973–985. doi:10.1016/j.apsb.2019.01.015
48. Zhou F, Mei J, Yang S, et al. Modified ZIF-8 nanoparticles attenuate osteoarthritis by reprogramming the metabolic pathway of synovial macrophages. ACS Appl Mater Interfaces. 2020;12(2):2009–2022. doi:10.1021/acsami.9b16327
49. Hu Y, Gui Z, Zhou Y, et al. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic Biol Med. 2019;145:146–160. doi:10.1016/j.freeradbiomed.2019.09.024
50. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartil. 2013;21:16–21. doi:10.1016/j.joca.2012.11.012
51. Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. doi:10.1016/j.bone.2012.02.012
52. Madry H, Luyten FP, Facchini A. Biological aspects of early osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2012;20(3):407–422. doi:10.1007/s00167-011-1705-8
53. Macchi V, Stocco E, Stecco C, et al. The infrapatellar fat pad and the synovial membrane: an anatomo-functional unit. J Anat. 2018;233(2):146–154. doi:10.1111/joa.12820
54. Gross JB, Guillaume C, Gegout-Pottie P, et al. The infrapatellar fat pad induces inflammatory and degradative effects in articular cells but not through leptin or adiponectin. Clin Exp Rheumatol. 2017;35(1):53–60.
55. Klein-Wieringa IR, de Lange-brokaar BJ, Yusuf E, et al. Inflammatory cells in patients with endstage knee osteoarthritis: a comparison between the synovium and the infrapatellar fat pad. J Rheumatol. 2016;43(4):771–778. doi:10.3899/jrheum.151068
56. Neogi T, Guermazi A, Roemer F, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: the multicenter osteoarthritis study. Arthritis Rheumatol. 2016;68(3):654–661. doi:10.1002/art.39488
57. Belluzzi E, Stocco E, Pozzuoli A, et al. Contribution of infrapatellar fat pad and synovial membrane to knee osteoarthritis pain. Biomed Res Int. 2019;2019:6390182. doi:10.1155/2019/6390182
58. Schaible HG, von Banchet GS, Boettger MK, et al. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60–69. doi:10.1111/j.1749-6632.2009.05301.x
59. Belluzzi E, Olivotto E, Toso G, et al. Conditioned media from human osteoarthritic synovium induces inflammation in a synoviocyte cell line. Connect Tissue Res. 2019;60(2):136–145. doi:10.1080/03008207.2018.1470167
60. Wu CL, Harasymowicz NS, Klimak MA, Collins KH, Guilak F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthrit Cartil. 2020;28(5):544–554. doi:10.1016/j.joca.2019.12.007
61. Liu B, Zhang M, Zhao J, et al. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp Ther Med. 2018;16(6):5009–5014. doi:10.3892/etm.2018.6852
62. Li A, Zhang Y, Lao L, et al. Serotonin receptor 2A/C is involved in electroacupuncture inhibition of pain in an osteoarthritis rat model. Evid Based Complement Alternat Med. 2011;2011:619650. doi:10.1093/ecam/neq016
63. van Osch GJ, van der Kraan PM, Van Den Berg WB. van den Berg WB. Site-specific cartilage changes in murine degenerative knee joint disease induced by iodoacetate and collagenase. J Orthop Res. 1994;12(2):168–175. doi:10.1002/jor.1100120204
64. van der Kraan PM, Vitters EL, van de Putte LB, et al. Development of osteoarthritic lesions in mice by “metabolic” and “mechanical” alterations in the knee joints. Am J Pathol. 1989;135(6):1001–1014.
65. Wang Y, Xie X, Zhu X, et al. Fire-needle moxibustion for the treatment of knee osteoarthritis: a meta-analysis. Evid Based Complement Alternat Med. 2016;2016:1392627. doi:10.1155/2016/1392627
66. Wu J, Tian C. Observation on therapeutic effect of fire needle on osteoarthritis of type of accumulation of pathogenic cold due to yang deficiency. Chin Med Mod Distance Educ China. 2014;32:2969–2978.
67. Wang Z. Treatment of 39 cases of knee osteoarthritis with therapy of fire-needle and Chinese medicine. TCM Res. 2015;28:45–47.
68. Luopeng Z, Liu L, Yizhan W, et al. Preliminary study on the “breaking” and “building” effects of fire acupuncture therapy and its clinical application. J Tradit Chin Med. 2019;60(14):1255–1257.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.