Back to Journals » Journal of Inflammation Research » Volume 15
Fibrinogen-Like Protein 1 as a Novel Biomarker of Psoriasis Severity
Authors Sun X, Liu L, Chen S, Wang J, Cai X, Song J, Zhou M, Guo D, Kuai L, Ding X, Li B, Li X
Received 27 June 2022
Accepted for publication 9 August 2022
Published 15 August 2022 Volume 2022:15 Pages 4637—4647
DOI https://doi.org/10.2147/JIR.S378953
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Xiaoying Sun,1,2 Liu Liu,1,2 Siting Chen,1,2 Jiao Wang,1,2 Xiaoce Cai,1,2 Jiankun Song,3 Mi Zhou,1,2 Dongjie Guo,1,2 Le Kuai,1,2 Xiaojie Ding,1,2 Bin Li,2,4 Xin Li1,2
1Department of Dermatology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Institute of Dermatology, Shanghai Academy of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 3Dermatology of TCM, Shanghai Skin Diseases Hospital, Shanghai, People’s Republic of China; 4Department of Dermatology, Shanghai Skin Diseases Hospital, Shanghai, People’s Republic of China
Correspondence: Xin Li, Department of Dermatology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, 110 Ganhe Road, Hongkou District, Shanghai, 200437, People’s Republic of China, Tel +8613661956326, Email [email protected] Bin Li, Institute of Dermatology, Shanghai Academy of Traditional Chinese Medicine, 110 Ganhe Road, Hongkou District, Shanghai, 200437, People’s Republic of China, Tel +8618930568129, Email [email protected]
Background: Psoriasis is an immune-mediated chronic systemic inflammatory skin disease whose diagnosis and severity assessment pose challenges for clinicians worldwide. The use of serum biomarkers facilitates the early diagnosis and treatment of psoriasis.
Methods: This case–control study compared tumor necrosis factor α (TNF-α), interleukin (IL)-6, IL-17, IL-10, and fibrinogen-like protein 1 (FGL1) levels of 139 untreated psoriasis patients and 140 healthy controls. Serum samples were collected, and enzyme-linked immunosorbent assays were performed to quantify their levels. Subgroups were analyzed according to abnormal lipid metabolism status.
Results: Compared to controls, patients with psoriasis exhibited lower concentrations of serum TNF-α, IL-17, and FGL1 (P < 0.05). A correlation analysis showed that FGL1 was inversely correlated with high-density lipoprotein cholesterol and IL-17 in the psoriatic state. Stepwise multiple regression analysis revealed that FGL1 and total cholesterol were the independent determinants of Psoriasis Area and Severity Index (PASI) score in psoriasis patients. The area under the receiver operating characteristic curve of FGL1 assessing moderate-to-severe psoriasis and mild psoriasis was 0.70, while the area under the curve (AUC) assessing severe psoriasis and mild-to-moderate psoriasis was 0.67, better than that of IL-17. In addition, FGL1, but not IL-17, was able to identify psoriasis with abnormal lipid metabolism to a certain extent (AUC = 0.60).
Conclusion: In conclusion, serum FGL1 may be a promising biomarker for diagnosing and staging psoriasis. It may also be involved in its progression and comorbid abnormal lipid metabolism.
Keywords: psoriasis, disease severity, biomarker, fibrinogen-like protein 1, lipid metabolism
Background
Psoriasis is an immune-mediated systemic inflammatory skin disease that affects approximately 2–4% of the world’s population.1 Although its etiology is unclear, it presumably involves the interaction of environmental factors and a genetic predisposition.2 Its typical clinical signs are erythema, infiltration, scaling, and cribriform hemorrhage accompanied by pruritus or pain, seriously affecting patient quality of life.
Dysregulation of the skin’s immune system is among the most important pathogenic mechanisms underlying psoriasis. Tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 are the earliest main pro-inflammatory factors in the inflammatory response that activates neutrophils and lymphocytes.3,4 Upon TNF-α and IL-6 production, dendritic cells transform into mature antigen-presenting cells and start producing cytokines such as IL-23 and IL-12, which further affect T cell differentiation.5 T helper (Th) 17 activation stimulates the overexpression of inflammatory cytokines, such as IL-17, in the skin and serum,6,7 acting as a key player in the development of psoriasis. In addition, studies have confirmed that TNF-α8,9 and IL-610,11 are involved in lipid metabolism, which may be related to metabolic diseases that complicate psoriasis symptoms. T-regulatory (Treg) cells, more specifically the Th17/Treg imbalance, play a key role in the pathogenesis of psoriasis.12 Treg cells can produce potent anti-inflammatory cytokines such as IL-10 that regulate the antigen presentation of dendritic cells, inhibit T cell activity, and stimulate B cell differentiation to play a regulatory role.13,14 IL-10 can also be secreted by macrophages, B cells, and natural killer cells.13,14
Fibrinogen-like protein 1 (FGL1) is a proliferation- and lipid metabolism–related protein secreted by the liver.15 Several studies have confirmed that FGL1 is an emerging checkpoint ligand for lymphocyte activation gene 3 (LAG3), a susceptibility gene for psoriasis.16 A study compared the skin lesions of patients with psoriasis and those with delayed skin hypersensitivity in remission and found lower LAG3 expression in the former.17 A Phase I clinical trial confirmed that targeting LAG3 could effectively treat psoriasis and reduce IL-17 secretion.18 FGL1 can bind to a specific domain of LAG3, trigger T-cell exhaustion, and inhibit T-cell activity.19 Studies have shown that FGL1 may be a new biomarker for immune-related diseases including Crohn’s disease and rheumatoid arthritis.20,21 Altogether, these findings suggest that FGL1 is associated with psoriasis pathogenesis and may be useful for diagnosing and staging its severity.
The diagnosis of psoriasis in the clinical setting currently relies primarily on the assessment of the patient’s history and clinical signs by experienced dermatologists. However, lesions in certain specific anatomical sites, such as the palms and soles, pudendal region, external auditory canal, and scalp present a challenge to both general practitioners and trained dermatologists. A definite diagnosis may be made using dermatoscopy, imaging technology, and other auxiliary examinations. However, only a few practitioners can interpret the associated images. Skin histopathology is the gold standard for the diagnosis of psoriasis; as a resource-intensive method, its full process includes an invasive skin biopsy, biopsy processing, and subsequent analysis. This method requires the collaboration of a specialized and trained dermatologist, laboratory technician, and dermatopathologist. Therefore, it may be difficult to apply in many healthcare settings, and it often takes weeks for a patient to receive a biopsy report, resulting in delayed or inappropriate care.
Treatment, conversely, is individualized according to disease severity. The area of the lesions in patients with psoriasis is an important indicator of severity; however, its measurement is highly subjective in patients with scattered lesions. The lack of an objective tool for assessing severity coupled with several clinical subtypes and multiple differential diagnoses is a major obstacle to early diagnosis and treatment.22,23 Therefore, a method to diagnose psoriasis and assess its severity is needed.
In this context, the detection of serum inflammatory factors and FGL1 in patients with psoriasis may be a useful approach to an uncomplicated diagnosis, post-treatment assessment, and exploration of its immune-related pathogenesis. Therefore, we hypothesized that psoriasis and its varying severity could alter the concentrations of inflammatory biomarkers and FGL1. Hence, this study aimed to compare the serum levels of TNF-α, IL-6, IL-17, IL-10, and FGL1 between patients with psoriasis and healthy controls as well as between patients with psoriasis of varying severities and explore their ability to identify psoriasis complicated by abnormal lipid metabolism.
Methods
The trial was designed in accordance with the STROBE Statement—Checklist of items that should be included in reports of case–control studies (Additional file 1).
Study Design
We recruited psoriasis vulgaris patients aged 18–80 years who had not received psoriasis-related treatment within the past 3 months and normal controls aged 18–80 years from the Department of Dermatology, Yueyang Integrated Traditional Chinese and Western Medicine Hospital affiliated with Shanghai University of Traditional Chinese Medicine between July 2020 and September 2021. The diagnostic criteria for psoriasis vulgaris were based on the Chinese Psoriasis Diagnosis and Treatment Guidelines24 compiled by the Psoriasis Professional Committee of the Dermatology and Venereology Branch of the Chinese Medical Association. Patients with psoriatic arthritis, familial hyperlipidemia, severe endocrine system diseases such as Cushing syndrome, severe mental illness, or cognitive impairment; who lacked personal behavioral ability; or who were judged unsuitable for participation were excluded. All procedures in this study were approved by the Medical Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine affiliated with the Shanghai University of Traditional Chinese Medicine (2020-071, Additional file 2) and followed the principles of the Declaration of Helsinki. All participants were informed in detail about the purpose and procedures before enrollment and signed an informed consent form (Additional file 3). Demographic data were collected; laboratory tests were performed to determine fasting blood lipids, including triglycerides (TG), total cholesterol (TC), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol; and peripheral blood was drawn for the determination of serum FGL1, IL-6, TNF-α, IL-10, and IL-17 concentrations. The participant could provide the test report without reevaluation if the lipid determination was conducted in the public hospital in our city in the past month. The Psoriasis Area and Severity Index (PASI) scores of the participants in the psoriasis group were assessed and recorded by the same investigator. PASI scores <3, 3 to <10, and ≥10 were defined as mild, moderate, and severe psoriasis, respectively.24 All blood samples were immediately centrifuged at 3000 rpm for 6 min, and the serum samples were transferred to clean Eppendorf tubes and stored at −80 °C until analysis.
Sample Size
The prevalence of psoriasis combined with metabolic syndrome is approximately 35%, while that of metabolic syndrome alone is approximately 20%.25 According to the sample size calculation formula of the case–control study with an unmatched design: 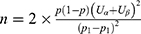 , a two-sided test was used with values of α = 0.05 and β = 0.20; therefore, a minimum sample size of 139 participants per group was required.
, a two-sided test was used with values of α = 0.05 and β = 0.20; therefore, a minimum sample size of 139 participants per group was required.
Diagnostic Criteria for Lipid Metabolism Disorders
The diagnosis of lipid metabolism disorder was made if any of the following diagnostic criteria were met26: body mass index (BMI) (weight/height2) ≥25 kg/m2; previous diagnosis of hyperlipidemia, hepatic adipose infiltration or fatty liver by abdominal ultrasonography; and TG ≥1.7 mmol/L and/or HDL cholesterol <0.9 mmol/L (male) or <1.00 mmol/L (female).
Enzyme-Linked Immunosorbent Assay Analysis of Peripheral Blood Cytokines in Patients with Psoriasis versus Controls
A human FGL1 enzyme-linked immunosorbent assay (ELISA) kit was purchased from Elabscience (Wuhan, China), while human high-sensitivity ELISA detection kits for IL-6, IL-10, IL-17A, and TNF-α were purchased from MultiSciences (Hangzhou, China). All serum protein levels were measured by the same investigator in psoriasis versus normal participants according to the manufacturer’s protocol.
Statistical Analysis
The demographics, serum cytokine levels and PASI scores of the two groups were analyzed, and a subgroup analysis was performed according to whether the participants had psoriasis complicated by metabolic disorders to explore the relationship between FGL1 and psoriasis, severity of psoriasis, and concomitant lipid metabolism disorders.
Qualitative indicators are described as frequency, percentage, or constituent ratio; quantitative indicators are described as mean, standard deviation (SD), or median (M) and interquartile range (IQR). Qualitative data were analyzed using the chi-squared test. Quantitative data were used to calculate statistically significant differences in serum lipid and cytokine levels between psoriasis patients and healthy subjects using the t-test for normally distributied variables and a non-parametric test (Mann–Whitney U-test) for non-normally distributed variables. A correlation analysis (Spearman correlation test) was used to analyze the correlation between blood lipid levels and cytokine concentrations of the two groups. Associations between variables and PASI scores in psoriasis were evaluated by univariate and stepwise multiple regression analyses. Receiver operating characteristic (ROC) analysis was used to diagnose the different psoriasis severities. There are no missing data in this study. The statistical analyses were performed using SPSS software version 20.0. A two-sided test was used uniformly for hypothesis testing, and statistical significance was set at P ≤ 0.05.
Results
A total of 279 participants were included in this study: 139 with psoriasis and 140 healthy controls. Table 1 lists the demographic data, lipid metabolism, and serum cytokine levels of the two groups as well as the course, age at onset, and severity in the psoriasis group. According to PASI score, 25 (17.99%), 43 (30.94%), and 71 (51.08%) patients had mild, moderate, and severe psoriasis, respectively. There was no significant intergroup difference in age, and the psoriasis group included a higher proportion of men than the normal control group (P < 0.05). The median disease severity in the psoriasis group was 8.60, the median age at onset was 40.50 years, and the median disease duration was 12.50 years. A proportion of patients in the psoriasis group had hyperlipidemia or fatty liver than the normal controls, but no intergroup difference in BMI was noted. Among the four blood lipid-related indices, the serum HDL cholesterol and the abnormal proportion of this index differed between groups. ELISA revealed that FGL1, TNF-α, and IL-17 levels differed between the two groups (P < 0.05).
 |
Table 1 Basic and Clinical Characteristics of Psoriasis Patients versus Normal Controls |
We analyzed the relationship between serum cytokines and lipid metabolism levels in two groups (Table 2, Figure 1). First, interestingly, FGL1 was significantly negatively correlated with IL-17 and HDL cholesterol levels in the psoriatic state; in contrast, in normal controls, FGL1 was correlated with only HDL cholesterol. IL-17 was associated with only LDL cholesterol in normal controls and the proinflammatory factor IL-6 in addition to FGL1 in psoriasis. Second, IL-6, IL-10, and TNF-α were correlated in pairs in the normal controls, and all were related to TG metabolism. In psoriasis, IL-6 was not associated with TNF-α but was correlated with TC and LDL cholesterol. Finally, LDL cholesterol and HDL cholesterol were not correlated in normal controls, but the four lipid metabolism indexes were correlated in pairs in psoriasis patients.
 |
Table 2 Correlation Between the Serum Cytokine and Lipid Index Levels in Psoriasis Patients versus Normal Controls |
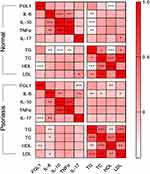 |
Figure 1 Heat map of correlation coefficients of serum cytokine levels and blood lipid indexes in psoriasis versus normal controls. *P < 0.05; **P < 0.01; ***P < 0.001. |
Afterward, we performed a subgroup analysis of serum cytokine levels according to lipid metabolism disorder status and found that the levels of all cytokines but IL-6 differed significantly between groups. For intragroup comparisons, we did not repeat the comparison between patients with psoriasis and healthy controls. As shown in Table 3, the serum FGL1 level of the psoriasis with lipid metabolism disorder group (M [IQR], 578.92 [296.37] ng/mL) was higher than that in the psoriasis without lipid metabolism disorder group (M [IQR], 501.05 [278.53] ng/mL), but no difference was seen in normal controls. Intragroup differences in IL-10 and TNF-α levels were observed in the two groups of normal controls but not in the psoriasis groups. However, there was no intragroup difference in IL-17 levels. We also compared PASI scores in psoriasis patients by lipid metabolism status and found no intergroup difference (P = 0.13).
 |
Table 3 Serum Levels of FGL1, IL-6, IL-10, TNF-α, and IL-17 of Psoriasis Patients versus Normal Controls by Lipid Metabolism |
Univariate associations of psoriasis severity are shown in Table 4. The PASI score was significantly correlated with serum concentrations of FGL1, TC, HDL cholesterol and LDL cholesterol. To avoid omitting important variables, those with P values <0.2 on univariate analysis were included. Stepwise multiple regression analysis revealed that FGL1 and TC were the independent determinants of PASI score in psoriasis patients (Table 5). Other variables tested in the model included age, psoriasis duration, and HDL and LDL cholesterol levels.
 |
Table 4 Univariate Associations with PASI Scores in Patients with Psoriasis |
 |
Table 5 Multivariable Predictors of PASI Scores in Psoriatic Patients |
Finally, we tested the diagnostic accuracy of FGL1 in different psoriasis disease states (Table 6). The ROC curves for FGL1 and IL-17 levels are shown in Figure 2. The area under the ROC curve (AUC) of FGL1 for differentiating normal controls from patients with psoriasis was 0.63, with a sensitivity of 72.9% and specificity of 49.7% (95% confidence interval [CI], 0.57–0.70). In this respect, IL-17 outperformed FGL1 with an AUC of 0.70 (95% CI, 0.64–0.76). The AUC, sensitivity, and specificity of FGL1 in patients with moderate-to-severe psoriasis vs those with mild psoriasis were 0.70, 44.4%, and 92.3%, respectively (95% CI, 0.61–0.80). For differentiating patients with severe psoriasis from those with mild-to-moderate psoriasis, FGL1 had an AUC, sensitivity, and specificity of 0.67, 45.9%, and 83.8% (95% CI, 0.59–0.75). However, IL-17 is useful for evaluating severe psoriasis and mild-to-moderate psoriasis with an AUC value of 0.61 (95% CI, 0.52–0.69), but it cannot differentiate between moderate-to-severe psoriasis and mild psoriasis. Meanwhile, for differentiating between psoriasis patients with versus without lipid metabolism disorders, FGL1 had an AUC sensitivity, and specificity of 0.60, 64.8%, and 51.0% (95% CI, 0.50–0.69).
 |
Table 6 Parameters of Receiver Operating Characteristic Curve for Differentiating Psoriasis Disease Status |
Discussion
Based on the pathogenesis of psoriasis, this study explored the ability of serum cytokines, including IL-6, TNF-α, IL-17, IL-10, and serum FGL1, to differentiate between patients with psoriasis and normal controls and identify psoriasis severity. We found that serum FGL1, TNF-α, and IL-17 concentrations differed between psoriasis patients and normal controls. A correlation analysis of cytokines in psoriasis showed a significant correlation between FGL1 and IL-17. We also noted differences in FGL1, TNF-α, IL-17, and IL-10 in the subgroups of patients with psoriasis complicated with abnormal lipid metabolism. Univariate and multiple regression analyses revealed that FGL1 and TC were independent determinants of PASI score, although the regression-based mediation analysis indicated that the relationship between TC and PASI scores was not mediated by FGL1 (results not shown). However, in the correlation analysis, TC, IL-6, IL-17, and FGL1 were correlated, while TC, TG, HDL cholesterol, and LDL cholesterol were closely related; therefore, the correlation between TC and PASI score must be circumspectly recognized and determined by other in-depth studies.
Based on the pathogenesis of psoriasis, we took the above results and evaluated the diagnostic value of IL-17 and FGL1 in different psoriasis disease states. The ROC analysis results indicated that IL-17 was unable to distinguish mild from moderate-to-severe psoriasis, while the AUC value was lower than that of FGL1 for differentiating mild-to-moderate psoriasis from severe psoriasis. To the best of our knowledge, this is the first study to initially demonstrate that serum FGL1 levels in psoriasis patients are superior to serum IL-17 for assessing psoriasis severity, suggesting that FGL1 may be a promising future biomarker, and involved in the pathogenesis of psoriasis.
In the correlation analysis shown in Figure 1, some intergroup commonalities and differences are visible, which implies the effects of cytokines on blood lipid metabolism under physiological and pathological states of psoriasis. Considering the commonalities, FGL1 and IL-6 are involved in the metabolism of HDL cholesterol and TG, respectively. In disease states, IL-6 is more extensively involved in lipid metabolism, and it reportedly has activity as a driver of dyslipidemia in rheumatoid arthritis.27 Serum FGL1 concentrations in patients with rheumatoid arthritis are significantly higher than those in normal controls,20 which is in contrast to our finding that patients with psoriasis had lower FGL1 levels than normal controls, but the positive correlation trend between serum FGL1 level and its severity is consistent. Based on the function of FGL1,19 this may mean that it is unable to inhibit the activation and proliferation of effector T cells to participate in the pathogenesis of psoriasis, and with the aggravation of inflammation, FGL1 may be upregulated in a reactive manner. The negative correlation between FGL1 and IL-17 in the disease state implies the same. Of course, this requires verification. In a report on Crohn’s disease and FGL1, intestinal tissues, which involve local immunity, were examined.21 All of these findings suggest a fundamental role of FGL1 in regulating immune-mediated inflammation.
Located on human chromosome 8 (8p22-21.3), FGL1 is a 68-kD protein belonging to the fibrinogen family.28,29 It contains beta and gamma subunits at the carboxy terminus and is highly homologous to fibrinogen but lacks the platelet-binding sites, cross-linking regions, or thrombin-sensitive sites necessary for fibrin clot formation.18 Under physiological conditions, FGL1 is mainly secreted by hepatocytes and involved in hepatocyte mitosis and hepatic energy utilization (including lipid metabolism and blood sugar regulation),19,30–32 and its expression can be considered a defense mechanism against exogenous injury or as a stimulator of self-protection mechanisms. Partial hepatectomy and IL-6 induce FGL1-promoter activity.33 However, high FGL1 levels can act on myoblasts via the phosphorylated Jun N-terminal kinase pathway or on HepG2 cells via the hepatocyte-dependent extracellular regulated protein kinase (ERK) 1/2 pathway, resulting in insulin resistance.14,31 In addition, FGL1 blockade as a therapeutic target reportedly combats obesity via a mechanism may be related to the canonical ERK1/2-CCAAT/enhancer-binding protein β–peroxisome proliferator–activated receptor γ pathway.34
Since the discovery of FGL1 as a major inhibitory ligand of LAG-3,19 FGL1 has become a promising biomarker with predictive value for resistance to programmed cell death protein 1/programmed cell death 1 ligand 1 therapy and an effective target for cancer immunotherapy.19 Recent studies reported that LAG3 deficiency is significantly associated with elevated HDL cholesterol levels and the risk of myocardial infarction.35 There are conflicting reports on the association between elevated HDL cholesterol and a reduced risk of myocardial infarction (MI), and gene association study revealed that LAG3 may be the gene significantly associated with high HDL cholesterol and increased risk of MI.36 This indicates that LAG3/FGL1 may be a new intervention target for psoriasis prevention and treatment as well as comorbid psoriasis with metabolic syndrome and cardiovascular disease.
Our study has some limitations. First, we cannot rule out that glucose metabolism and other comorbidities in patients with psoriasis may cause serum FGL1 concentration fluctuations. Second, only serum samples were collected from patients with psoriasis; thus, cytokine expressions in skin lesions was not explored. Likewise, we did not observe the treatment effect on the aforementioned cytokines. Third, by design, observational studies do not elucidate causal relationships; thus, further studies are needed to determine the role of FGL1 in the pathogenesis of psoriasis and its usefulness as a biomarker for diagnosis and the assessment of treatment effects.
Conclusion
To summarize, the study initially demonstrated that serum FGL1 concentrations were superior to serum IL-17 for determining psoriasis severity. Considering the diagnostic accuracy of FGL1 in this study, we believe that serum FGL1 could be a valuable biomarker. We also found that FGL1 level was negatively correlated with HDL cholesterol level, suggesting that FGL1 may also be involved in the progression of psoriasis complicated by abnormal lipid metabolism. However, given the exploratory design of our study, the exact role of FGL1 in the pathogenesis of psoriasis requires further in-depth studies and validation.
Abbreviations
AUC, area under the curve; BMI, body mass index; CI, confidence interval; ERK, extracellular regulated protein kinase; ELISA, enzyme-linked immunosorbent assay; FGL1, fibrinogen-like protein 1; HDL, high-density lipoprotein; IL, interleukin; LAG3, lymphocyte activation gene 3; LDL, low-density lipoprotein; M, median; MI, myocardial infarction; PASI, Psoriasis Area and Severity Index; QR, interquartile range; ROC, receiver operating characteristic; SD, standard deviation; SE, standard error; TC, total cholesterol; TG, triglycerides; Th, T helper; TNF-α, tumor necrosis factor-α; Treg, T-regulatory.
Reporting Checklist
The authors have completed the STROBE checklist.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Ethics Committee of Shanghai Yueyang Integrated Medicine Hospital (#2020-071) (Additional file 2). Informed consent was obtained from all participants before study inclusion (Additional file 3). The authors are accountable for all aspects of the work and certify that questions related to the accuracy or integrity of any part of this work will be appropriately investigated and resolved.
Acknowledgments
The authors acknowledge all study participants.
Author Contributions
XYS, XL, and BL contributed to the study concept and design; LL, XCC, and JW provided administrative support; XYS, STC, JKS, MZ, DJG, LK, and XJD enrolled the patients. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the National Key Research and Development Program of China (No. 2018YFC1705301), the National Natural Science Foundation of China (Nos. 82074427 to XL and 81973860 to BL), the Natural Science Foundation of Shanghai (No. 19ZR1458700), the Shanghai Sailing Program (No. 20YF1450400), and the Hospital-level Fund of Yueyang Integrated Traditional Chinese and Western Medicine Hospital affiliated to Shanghai University of Traditional Chinese Medicine (No. 2019YYQ31).
Disclosure
The authors declare no competing interests or financial or personal relationships that could bias the work described in this manuscript.
References
1. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi:10.1001/jama.2020.4006
2. Langley RG, Krueger GG, Griffiths CE. Psoriasis: epidemiology, clinical features, and quality of life. Ann Rheum Dis. 2005;64(suppl_2):ii18–ii23. doi:10.1136/ard.2004.033217
3. Chiricozzi A, Romanelli P, Volpe E, et al. Scanning the immunopathogenesis of psoriasis. Int J Mol Sci. 2018;19(1):179. doi:10.3390/ijms19010179
4. Morizane S, Yamasaki K, Mühleisen B, et al. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J Investig Dermatol. 2012;132(1):135–143. doi:10.1038/jid.2011.259
5. Surcel M, Huica R, Constantin C, et al. Biomarkers insights in psoriasis-regulatory cytokines. Curr Biomark. 2018;7(1):3–11. doi:10.2174/2468422807666180320125713
6. Fotiadou C, Lazaridou E, Sotiriou E, et al. Targeting IL-23 in psoriasis: current perspectives. Psoriasis. 2018;8:1–5. doi:10.2147/PTT.S98893
7. Rendon A, Schakel K. Psoriasis pathogenesis and treatment. Int J Mol Sci. 2019;20(6):1475. doi:10.3390/ijms20061475
8. Xiuping C, Keli X, Lidian C, et al. TNF-alpha, a potent lipid metabolism regulator. Cell Biochem Funct. 2009;27(7):407–416. doi:10.1002/cbf.1596
9. Stachowiak M, Flisikowski K. Analysis of allele-specific expression of seven candidate genes involved in lipid metabolism in pig skeletal muscle and fat tissues reveals allelic imbalance of ACACA, LEP, SCD, and TNF. J Appl Genet. 2019;60(1):97–101. doi:10.1007/s13353-019-00485-z
10. Hua Q, Reina D, Kavita I-W, et al. Origin and function of stress-induced IL-6 in murine models. Cell. 2020;182(6):1660. doi:10.1016/j.cell.2020.08.044
11. Patin F, Baranek T, Vourc’h P, et al. Combined metabolomics and transcriptomics approaches to assess the IL-6 blockade as a therapeutic of ALS: deleterious alteration of lipid metabolism. Neurotherapeutics. 2016;13(4):905–917. doi:10.1007/s13311-016-0461-3
12. Owczarczyk-Saczonek A, Czerwi´nska J, Placek W. The role of regulatory T cells and anti-inflammatory cytokines in psoriasis. Acta Dermatovenerol Alp Panonica Adriat. 2018;27:17–23.
13. Saxena A, Khosraviani S, Noel S, et al. Interleukin-10 paradox: a potent immunoregulatory cytokine that has been difficult to harness for immunotherapy. Cytokine. 2015;74(1):27–34. doi:10.1016/j.cyto.2014.10.031
14. Trifunovi CJ, Miller L, Debeljak Ž, et al. Pathologic patterns of interleukin 10 expression: a review. Biochem Med. 2015;25:36–48. doi:10.11613/BM.2015.004
15. Wu HT, Ou HY, Hung HC, et al. A novel hepatokine, HFREP1, plays a crucial role in the development of insulin resistance and type 2 diabetes. Diabetologia. 2016;59(8):1732–1742. doi:10.1007/s00125-016-3991-7
16. Sheng Y, Jin X, Xu J, et al. Sequencing-based approach identified three new susceptibility loci for psoriasis. Nat Commun. 2014;5(1):4331. doi:10.1038/ncomms5331
17. Gulati N, Suárez-Fariñas M, Correa da Rosa J, et al. Psoriasis is characterized by deficient negative immune regulation compared to transient delayed-type hypersensitivity reactions. F1000research. 2015;4:149. doi:10.12688/f1000research.6581.1
18. Ellis J, Marks Daniel JB, Srinivasan N, et al. Depletion of LAG3 T cells translated to pharmacology and improvement in psoriasis disease activity: a phase I randomized study of mAb GSK2831781. Clin Pharmacol Ther. 2020;109(5):1293–1303. doi:10.1002/cpt.2091
19. Wang J, Sanmamed MF, Datar I, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG3. Cell. 2019;176(1–2):334–347. doi:10.1016/j.cell.2018.11.010
20. Liu S, Guo Y, Lu L, et al. Fibrinogen-like protein 1 is a novel biomarker for predicting disease activity and prognosis of rheumatoid arthritis. Front Immunol. 2020;11:579228. doi:10.3389/fimmu.2020.579228
21. Sun X-L, Qiao L-C, Gong J, et al. Proteomics identifies a novel role of fibrinogen-like protein 1 in Crohn’s disease. World J Gastroenterol. 2021;27(35):5946–5957. doi:10.3748/wjg.v27.i35.5946
22. Feldman SR, Goffe B, Rice G, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9(9):504–513.
23. Puzenat E, Bronsard V, Prey S, et al. What are the best outcome measures for assessing plaque psoriasis severity? A systematic review of the literature. J Eur Acad Dermatol Venereol. 2010;24:10–16. doi:10.1111/j.1468-3083.2009.03562.x
24. Psoriasis Professional Committee of Dermatology and venereology Branch of Chinese Medical Association. Guidelines for the diagnosis and treatment of psoriasis in China (2018 simplified version). Chin J Dermatol. 2019;52(4):223–230.
25. Yanyan F, Junling L, Xiongming P. Prevalence of psoriasis with metabolic syndrome in Xinjiang. Chin J Leprosy Skin Dis. 2016;32(03):153–155.
26. Metabolic Syndrome Research Collaborative Group of Diabetes Branch of Chinese Medical Association. Suggestions of diabetes branch of Chinese medical association on metabolic syndrome. Chin J Diabetes. 2004;12(5):156–161.
27. Robertson J, Porter D, Sattar N, et al. Interleukin-6 blockade raises LDL via reduced catabolism rather than via increased synthesis: a cytokine-specific mechanism for cholesterol changes in rheumatoid arthritis. Ann Rheum Dis. 2017;76(11):1949–1952. doi:10.1136/annrheumdis-2017-211708
28. Chan KY, Lai PB, Squire JA, et al. Positional expression profiling indicates candidate genes in deletion hotspots of hepatocellular carcinoma. Mod Pathol. 2006;19(12):1546–1554. doi:10.1038/modpathol.3800674
29. Nayeb-Hashemi H, Desai A, Demchev V, et al. Targeted disruption of fibrinogen like protein-1 accelerates hepatocellular carcinoma development. Biochem Biophys Res Commun. 2015;465(2):167–173. doi:10.1016/j.bbrc.2015.07.078
30. Demchev V, Malana G, Vangala D, et al. Targeted deletion of fibrinogen like protein 1 reveals a novel role in energy substrate utilization. PLoS One. 2013;8(3):e58084. doi:10.1371/journal.pone.0058084
31. Jung TW, Chung YH, Kim HC, et al. Hyperlipidemia- induced hepassocin in the liver contributes to insulin resistance in skeletal muscle. Mol Cell Endocrinol. 2018;470:26–33. doi:10.1016/j.mce.2017.10.014
32. Ou HY, Wu HT, Lin CH, et al. The hepatic protection effects of hepassocin in hyperglycemic crisis. J Clin Endocrinol Metab. 2017;102(7):2407–2415. doi:10.1210/jc.2016-3287
33. Yu HT, Yu M, Li CY, et al. Specific expression and regulation of hepassocin in the liver and down-regulation of the correlation of hnf1alpha with decreased levels of hepassocin in human hepatocellular carcinoma. J Biol Chem. 2009;284(20):13335–13347. doi:10.1074/jbc.M806393200
34. Wu HT, Chen SC, Fan KC, et al. Targeting fibrinogen-like protein 1 is a novel therapeutic strategy to combat obesity. FASEB J. 2020;34(2):2958–2967. doi:10.1096/fj.201901925R
35. Ani M, Honghuang L, Chansuk K, et al. Lymphocyte activation gene-3-associated protein networks are associated with HDL-cholesterol and mortality in the trans-omics for precision medicine program. Commun Biol. 2022;5(1):362. doi:10.1038/s42003-022-03304-0
36. Rodriguez A. High HDL-cholesterol paradox: SCARB1-LAG3-HDL axis. Curr Atheroscler Rep. 2021;23(1):5. doi:10.1007/s11883-020-00902-3
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

