Back to Journals » OncoTargets and Therapy » Volume 12
FGFR2-BICC1: A Subtype Of FGFR2 Oncogenic Fusion Variant In Cholangiocarcinoma And The Response To Sorafenib
Authors Ying X, Tu J, Wang W , Li X, Xu C , Ji J
Received 9 June 2019
Accepted for publication 25 October 2019
Published 6 November 2019 Volume 2019:12 Pages 9303—9307
DOI https://doi.org/10.2147/OTT.S218796
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Xihui Ying,1 Jianfei Tu,1 Wenxian Wang,2 Xingliang Li,3 Chunwei Xu,4 Jiansong Ji1
1Department of Radiology, Lishui Central Hospital/Key Laboratory of Imaging Diagnosis and Minimally Invasive Interventional Research of Zhejiang Province, Lishui, Zhejiang 323000, People’s Republic of China; 2Department of Chemotherapy, Zhejiang Cancer Hospital, Hangzhou, Zhejiang 310022, People’s Republic of China; 3Department of Thoracic Disease Diagnosis and Treatment Center, Zhejiang Rongjun Hospital, Jiaxing, Zhejiang 314000, People’s Republic of China; 4Department of Pathology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou, Fujian 350014, People’s Republic of China
Correspondence: Jiansong Ji
Department of Radiology, Lishui Central Hospital/Key Laboratory of Imaging Diagnosis and Minimally Invasive Interventional Research of Zhejiang Province, Lishui, Zhejiang 323000, People’s Republic of China
Email [email protected]
Abstract: Fibroblast growth factor receptor (FGFR) family includes four highly conserved receptor tyrosine kinases. Particularly, FGFR2 has been identified as a potential target for tyrosine kinase inhibitor (TKI) treatment. Except for immunohistochemistry and fluorescence in situ hybridization, next-generation sequencing (NGS) technology represents a novel tool for FGFR2 detection that covers a wide range of fusion genes. In the present work, we present a case of cholangiocarcinoma who had FGFR2-BICC1 rearrangement detected by NGS. A 76-year-old female diagnosed with cholangiocarcinoma underwent four cycles of chemotherapy. The NGS assay showed that the tumor had a FGFR2-BICC1 rearrangement. The patient had a favorable tumor response to sorafenib. Herein, we report the first case with cholangiocarcinoma harboring FGFR2-BICC1 who is sensitive to sorafenib therapy.
Keywords: cholangiocarcinoma, NGS, FGFR2 rearrangement
Introduction
Cholangiocarcinoma is the second most diagnosed liver tumor next to hepatocellular carcinoma, which is characterized by its late diagnosis and poor prognosis. It is estimated that cholangiocarcinoma accounts about 3% of all gastrointestinal tumors and its overall 5-year survival rate was less than 10%.1,2 Current therapeutic options for cholangiocarcinoma include surgery and systematic chemotherapy. Standard chemotherapy treatment for cholangiocarcinoma is gemcitabine administered alone or in combination with cytotoxic agents such as cisplatin. However, patients underwent the above standard chemotherapy generally have a poor prognosis with a median survival of 6–12 months, highlighting the urgent need for more cholangiocarcinoma treatment modalities.3 Currently, some clinical trials utilizing target drugs in cholangiocarcinoma are under investigation. Most of the clinical trials focused on the efficacy of small molecule inhibitors targeting EGFR signaling pathway.4,5 The fibroblast growth factor receptor (FGFR) family includes four highly conserved receptor tyrosine kinases. FGFR2 have been reported as a novel target for tyrosine kinase inhibitor (TKI) treatment.6,7 Herein, we report a patient with cholangiocarcinoma who had a FGFR2-rearranged gene fusion and was sensitive to TKI-sorafenib, a multi-targeted small molecule.
Case Presentation
A written informed consent has been provided by the patient to have the case details and any accompanying images published and the study was approved by the Institutional Ethics Review Board of the hospital.
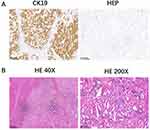
A 76-year-old female was admitted to our hospital in March 2011 because of a space-occupying lesion in the liver during routine physical examination. A magnetic resonance imaging (MRI) scan revealed intrahepatic multiple nodules in the right liver (Figure 1A). The patient underwent laparoscopy-guided liver biopsy for confirmation diagnosis. The patients were diagnosed with cholangiocarcinoma with immunohistochemical staining positive expression of CK19 and negative for HEP (Figure 2A). The patient was treated with floxuridine, oxaliplatin and pirarubicin in transcatheter hepatic arterial chemoembolization (TACE). After the TACE treatment, the patient also underwent the right hepatectomy under general anesthesia. Hematoxylin and eosin (H&E) staining confirmed a typical morphology of differentiated cholangiocarcinoma in the posterior portion of the right lobe, with invasion of the hepatic capsule and positive surgical margins (Figure 2B). Thus, the patient underwent floxuridine, oxaliplatin, pirarubicin and ultrafluid lipiodol, recombinant human interleukin in TACE in May 2011. Five years later, the disease progressed and patients subsequently underwent chemotherapy of gemcitabine cisplatin followed by hepatic support nutrition, i.e., intravenous injection with 80mg glycyrrhizin per day. On June 2018, the patient underwent lobaplatin and gemcitabine followed by hepatic support nutrition, i.e., intravenous injection with 80mg glycyrrhizin per day. However, the patient did not respond to the above treatment and disease progressed five months later (Figure 1B). Because of no response and disease progression, the patients underwent gene mutation examination. The NGS analysis of the peripheral blood revealed a FGFR2-BICC1 rearrangement, as shown in Figure 3 (3D Medicines, Shanghai China). In addition, immunohistochemistry analysis showed that FGFR2-BICC1 protein was highly expressed in cholangiocarcinoma (Figure 4). For effective mutation, the patient underwent oral sorafenib treatment in November 2018. After 1 month, the MRI scan showed a decrease in tumor size (Figure 1C). According to RECIST, the patient was considered to have a partial response (PR) to sorafenib (Figure 1D). During treatment with sorafenib, there were no adverse events such as rashes, gastrointestinal reactions, and cordis damage. Up to last follow-up, the disease is stable and the patient is being treated with sorafenib.
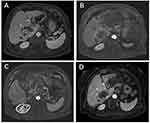 |
Figure 1 Magnetic resonance imaging for tumors. Magnetic resonance imaging (MRI) scans were performed at initial diagnosed (A), and before (B and C) and after (D) sorafenib therapy. |
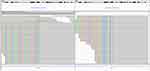 |
Figure 3 Integrative genomics viewer snapshot of FGFR2-BICC1. Alignment of NGS sequencing reads from the patient’s tumor reveals a FGFR2-BICC1 rearrangement. |
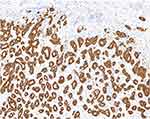 |
Figure 4 Detection of FGFR2-BICC1 protein in cholangiocarcinoma. Immunohistochemical staining of FGFR2-BICC1 was performed on the surgical biopsy of cholangiocarcinoma. |
Discussion
Sorafenib is a multi-targeted small molecule that suppresses tumor proliferation and angiogenesis through inhibiting the activity of fibroblast growth factor, vascular endothelial growth factor receptor (VEGFR) 2/3, RAF proto-oncogene, serine/threonine kinase and platelet-derived growth factor receptor (PDGFR).8 Numerous animal models and clinical studies indicated that sorafenib exhibits a significant safety and efficacy against a variety of cancers. Sorafenib also had been approved for the treatment of renal cell and hepatocellular carcinoma.9,10 However, limited clinical data can be available on the efficacy of sorafenib in patients with advanced cholangiocarcinoma. Several case reports demonstrated potential benefits of sorafenib on cholangiocarcinoma.11,12,13,14,15 Clinical trials from a non-controlled and single-arm study showed that sorafenib only had limited effects for patients with cholangiocarcinoma.16 However, other studies revealed that sorafenib monotherapy had rather low efficacy in cholangiocarcinoma.17 Therefore, the precise clinical efficacy of sorafenib on cholangiocarcinoma needs further investigation. Selection of cholangiocarcinoma patients who may benefit from TKI treatment based on the molecular characteristics of tumor specimens is necessary for clinical treatment.
FGFR genes are involved in multiple biological processes, including cell transformation, angiogenesis, apoptosis, embryonic development, and tissue repair. Mutations and amplification of FGFR gene members have been shown to serve as therapeutic targets in a wide range of human malignancies, such as gastric, lung, and bladder cancers.18,19 Previous studies have demonstrated that overexpression of FGFR fusion proteins promotes tumor cell proliferation and migration.20 Bladder cancer cells harboring FGFR3 fusion proteins were shown to be more susceptible to pharmacologic inhibition both in vitro and in vivo.21 Transcriptome sequencing in patients with metastatic cholangiocarcinoma reveals novel genetic rearrangement, including FGFR2-BICC1, which was further confirmed by real-time PCR.22
Bicc1 gene encodes a RNA-binding protein and has been identified in many species, ranging from C. elegans to humans. Loss of Bicc1 in Drosophila disrupts the anterior follicle cell migration and affects anterior-posterior patterning, leading to embryos development disorder.23 Knockdown of the zebrafish homologue of Bicc1 induces cystic kidneys in vivo.24 Recently, the association between Bicc1 level and various mood disorders are demonstrated in animals and humans.25,26 It remains to be determined whether patients with FGFR2-BICC1 fusion exhibit unique clinicopathologic manifestations.
In conclusion, the present case provides a first report of a cholangiocarcinoma patient harboring FGFR2-BICC1 fusion who are responded to sorafenib therapy, suggesting the critical role for the diagnostic application of NGS in precision medicine.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Najran P, Lamarca A, Mullan D, et al. Update on treatment options for advanced bile duct tumours: radioembolisation for advanced cholangiocarcinoma. Curr Oncol Rep. 2017;19(8):50–58. doi:10.1007/s11912-017-0603-8
2. Cai Y, Cheng N, Ye H, Li F, Song P, Tang W. The current management of cholangiocarcinoma: a comparison of current guidelines. Biosci Trends. 2016;10(7):92–102. doi:10.5582/bst.2016.01048
3. Rahnemai-Azar AA, Weisbrod A, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: molecular markers for diagnosis and prognosis. Surg Oncol. 2017;26(16):125–137. doi:10.1016/j.suronc.2016.12.009
4. Andersen JB, Spee B, Blechacz BR, et al. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142(12):1021–1031. doi:10.1053/j.gastro.2011.12.005
5. Rizvi S, Gores GJ. Emerging molecular therapeutic targets for cholangiocarcinoma. J Hepatol. 2017;67(16):632–644. doi:10.1016/j.jhep.2017.03.026
6. Sia D, Losic B, Moeini A, et al. Massive parallel sequencing uncovers actionable FGFR2-PPHLN1 fusion and ARAF mutations in intrahepatic cholangiocarcinoma. Nat Commun. 2015;6(5):6087–6091. doi:10.1038/ncomms7087
7. Graham RP, Barr FEG, Pestova E, et al. Fibroblast growth factor receptor 2 translocations in intrahepatic cholangiocarcinoma. Hum Pathol. 2014;45(18):1630–1638. doi:10.1016/j.humpath.2014.03.014
8. Chen J, Jin R, Zhao J, et al. Potential molecular, cellular and microenvironmental mechanism of sorafenib resistance in hepatocellular carcinoma. Cancer Lett. 2015;367(1):1–11. doi:10.1016/j.canlet.2015.06.019
9. Jindal A, Thadi A, Shailubhai K. Hepatocellular carcinoma: etiology and current and future drugs. J Clin Exp Hepatol. 2019;9(8):221–232. doi:10.1016/j.jceh.2019.01.004
10. Randrup HC, Grimm D, Bauer J, Wehland M, Magnusson NE. Effects and side effects of using sorafenib and sunitinib in the treatment of metastatic renal cell carcinoma. Int J Mol Sci. 2017;18(6):534–539.
11. Chakunta HR, Sunderkrishnan R, Kaplan MA, Mostofi R. Cholangiocarcinoma: treatment with sorafenib extended life expectancy to greater than four years. J Gastrointest Oncol. 2013;4(6):30–32. doi:10.3978/j.issn.2078-6891.2012.046
12. Pinter M, Sieghart W, Reisegger M, Wrba F, Peck-Radosavljevic M. Sorafenib in unresectable intrahepatic cholangiocellular carcinoma: a case report. Wien Klin Wochenschr. 2011;123:61–64. doi:10.1007/s00508-010-1522-y
13. LaRocca RV, Hicks MD, Mull L, Foreman B. Effective palliation of advanced cholangiocarcinoma with sorafenib: a two-patient case report. J Gastrointest Cancer. 2007;38(15):154–156. doi:10.1007/s12029-008-9028-9
14. Luo X, Jia W, Huang Z, et al. Effectiveness and safety of sorafenib in the treatment of unresectable and advanced intrahepatic cholangiocarcinoma: a pilot study. Oncotarget. 2017;8(15):17246–17257. doi:10.18632/oncotarget.12825
15. Poddubskaya EV, Baranova MP, Allina DO, et al. Personalized prescription of tyrosine kinase inhibitors in unresectable metastatic cholangiocarcinoma. Exp Hematol Oncol. 2018;7(11):21. doi:10.1186/s40164-018-0113-x
16. Pan TT, Wang W, Jia WD, Xu GL. A single-center experience of sorafenib monotherapy in patients with advanced intrahepatic cholangiocarcinoma. Oncol Lett. 2017;13(9):2957–2964. doi:10.3892/ol.2017.5847
17. El-Khoueiry AB, Rankin CJ, Ben-Josef E, et al. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs. 2012;30(7):1646–1651. doi:10.1007/s10637-011-9719-0
18. Kang X, Lin Z, Xu M, Pan J, Wang ZW. Deciphering role of FGFR signalling pathway in pancreatic cancer. Cell Prolif. 2019;52(5):e12605. doi:10.1111/cpr.12605
19. Porta R, Borea R, Coelho A, et al. FGFR a promising druggable target in cancer: molecular biology and new drugs. Crit Rev Oncol Hematol. 2017;113(12):256–267. doi:10.1016/j.critrevonc.2017.02.018
20. Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17(4):318–332. doi:10.1038/nrc.2017.8
21. Takamura T, Horinaka M, Yasuda S, et al. FGFR inhibitor BGJ398 and HDAC inhibitor OBP-801 synergistically inhibit cell growth and induce apoptosis in bladder cancer cells. Oncol Rep. 2018;39(8):627–632. doi:10.3892/or.2017.6127
22. Arai Y, Totoki Y, Hosoda F, et al. Fibroblast growth factor receptor 2 tyrosine kinase fusions define a unique molecular subtype of cholangiocarcinoma. Hepatology. 2014;59(8):1427–1434. doi:10.1002/hep.26890
23. Fu Y, Kim I, Lian P, et al. Loss of Bicc1 impairs tubulomorphogenesis of cultured IMCD cells by disrupting E-cadherin-based cell-cell adhesion. Eur J Cell Biol. 2010;89(7):428–436. doi:10.1016/j.ejcb.2010.01.002
24. Bouvrette DJ, Sittaramane V, Heidel JR, Chandrasekhar A, Bryda EC. Knockdown of bicaudal C in zebrafish (Danio rerio) causes cystic kidneys: a nonmammalian model of polycystic kidney disease. Comp Med. 2010;60(5):96–106.
25. Ota KT, Andres W, Lewis DA, Stockmeier CA, Duman RS. BICC1 expression is elevated in depressed subjects and contributes to depressive behavior in rodents. Neuropsychopharmacology. 2015;40(3):711–718. doi:10.1038/npp.2014.227
26. Chen S, Jiang H, Xu Z, et al. Serum BICC1 levels are significantly different in various mood disorders. Neuropsychiatr Dis Treat. 2019;15(15):259–265. doi:10.2147/NDT
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.