Back to Journals » Infection and Drug Resistance » Volume 15
Femoral Head Necrosis Due to Brucella Infection in China: Case Series and Literature Review
Authors Wang J , Zhang Y, Zhao C, Zhang Q
Received 24 July 2022
Accepted for publication 28 September 2022
Published 10 October 2022 Volume 2022:15 Pages 5857—5865
DOI https://doi.org/10.2147/IDR.S383584
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Jie Wang, Yao Zhang, Changsong Zhao, Qiang Zhang
Department of Orthopedics, Beijing Ditan Hospital, Capital Medical University, Beijing, 100015, People’s Republic of China
Correspondence: Qiang Zhang, Email [email protected]
Introduction: The causes of femoral head necrosis mainly include trauma, corticosteroid, and alcohol, among which Brucella is highly unusual.
Patients and Methods: This paper reported three rare cases of femoral head necrosis due to Brucella, summarizing and analyzing the epidemiological history, clinical manifestations, laboratory examinations, imaging findings, individualized therapy, and biopsy results of patients with this disease. A literature review related to brucellosis and femoral head necrosis was conducted.
Results: All three patients had an epidemic history of brucellosis. They suffered from hip pain and limitation of movement for months, and femoral head necrosis was confirmed by magnetic resonance imaging (MRI). Pathological Giemsa staining or real-time polymerase chain reaction (real-time PCR) confirmed Brucella melitensis (B. melitensis) as the cause of osteonecrosis. Each patient received individualized therapy according to the degree of osteonecrosis, which was based on anti-brucellosis medications and hip puncture and irrigation decompression. They were followed up for more than two years and rehabilitated well.
Conclusion: Brucella infection should always be considered the cause of femoral head necrosis for patients with hip pain, especially for those with an epidemic history; early diagnosis and individual stepwise therapy can prevent the progression of osteonecrosis.
Keywords: Brucella, femoral head necrosis, core decompression, total hip arthroplasty, case series
Introduction
Trauma, corticosteroid, alcohol, hip dysplasia, decompression sickness, homeopathy, Gaucher’s disease, and human immunodeficiency virus (HIV) are some pathogens responsible for femoral head necrosis, among which trauma, corticosteroid, and alcohol are the most common.1–5 Brucella primarily infects humans’ bone and joint system, resulting in spondylitis, sacroiliitis, and so on; pyogenic hip arthritis is relatively rare, and osteonecrosis of the femoral head is even more unusual.6 There have been only three cases of osteonecrosis of the femoral head caused by Brucella, mainly speculated due to the absence of a conclusive basis of bacterial culture or pathological diagnosis.7–9 This paper describes three unusual cases of femoral head necrosis due to Brucella; we also discuss possible pathogenesis, the novelty of our cases, individualized stepwise therapy, and prognosis.
Patients and Methods
This study collected data on the epidemiological history, clinical manifestations, laboratory examinations, imaging findings, therapy, and pathological biopsy results of three patients with femoral head necrosis due to Brucella infection. These three patients were followed up for more than two years. We discussed the possible etiology, previous case reports, diagnosis methods, and treatment options of this type of bacterial osteonecrosis of the femoral head.
Case Presentation
Case 1
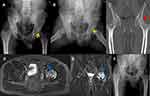
The patient is a 37-year-old Chinese male. In April 2014, he was diagnosed with brucellosis due to fever and fatigue, and his symptoms disappeared after anti-brucellosis medications for three months. In October 2014, the patient developed left hip pain, worsened by weight-bearing, and was admitted to the orthopedics department of our hospital in December 2014 due to the deterioration of symptoms. He is a sheep herdsman without any history of trauma, alcoholism, or hormone use. Physical examination revealed that the active and passive motion of the left hip was somewhat restricted due to pain. X-ray demonstrated that the left femoral head exhibited a strip-like low-density area but no evidence of stenosis (Figure 1A and B). CT scan revealed cavity necrosis in the left femoral head (Figure 1C). MRI revealed necrosis of the left femoral head, and effusion in the left joint cavity (Figure 1D and E). Blood test showed WBC 6.85 × 109/L with neutrophils accounted for 61.8%, CRP 3.5 mg/L, ESR 2.00 mm/h. SAT was positive at a titer of 1:320, and all tests for tuberculosis and rheumatoid arthritis were negative. Having been diagnosed with brucellar hip arthritis complicated by osteonecrosis of the left femoral head, the patient underwent puncture and irrigation of the left hip joint and core decompression and bone grafting. B. melitensis was detected in hip joint effusion and osteonecrosis of femoral head by real-time PCR. HE staining of a core biopsy of the left femoral head revealed amorphous necrotic material (Figure 2A); Giemsa staining can demonstrate scattered positive Brucella (Figure 2B). Triple anti-infective therapy was administered after the operation: rifampicin (0.6g po qd; Guangdong Hengjian Pharmaceuticals), doxycycline (0.1g po bid; Jiangsu Lianhuan Pharmaceuticals), and levofloxacin (0.2g ivgtt bid; Yangtze River Pharmaceuticals). Upon discharge from the hospital two weeks after the operation, his left hip pain was substantially relieved, and the antibiotic regimen was adjusted to rifampicin (0.6g po qd; Guangdong Hengjian Pharmaceuticals) and doxycycline (0.1g po bid; Jiangsu Lianhuan Pharmaceuticals) for six months. In follow-up, the symptoms of left hip pain and movement limitation disappeared almost half a year later, and there was no obvious abnormality in the blood test or hip X-ray (Figure 1F). In the telephone follow-up in April 2022, the patient’s recovery proceeded well.
 |
Figure 2 (A) Core biopsy of the right femoral head revealed necrotic material stained with HE. (B) Giemsa staining demonstrated scattered positive Brucella (red circle). |
Case 2
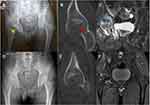
The patient is a 33-year-old Chinese male. He was diagnosed with brucellosis in a local hospital in October 2017 due to right hip pain and wavy fever. Following the administration of anti-brucellosis drugs, the fever subsided, but the pain in the right hip did not. In January 2018, the patient visited the orthopedic department of our hospital due to aggravation of right hip pain and decreased hip joint mobility. History indicates that the patient’s family raised sheep for a long time, and there was no history of trauma, alcohol consumption, or hormone use. The physical examination revealed severe tenderness in the right hip region, longitudinal percussion pain in the right lower limb, and limited passive and active movement of the right hip. There was no abnormality in the chest X-ray or CT. X-ray of the hip joint showed low signal on the right femoral head, joint narrowing and even invagination of the joint space (Figure 3A). CT showed cavity necrosis and low-density patches of shadows in the right femoral head (Figure 3B). MRI of the hip joint indicates effusion of the right hip joint complicated by necrosis of the right femoral head, and the inflammatory fluid diffused with extra-articular (Figure 3C). Blood tests indicated WBC 4.60×109/L with neutrophils accounting for 61.1%, CRP59.3mg/L, ESR29.0mm/hr, and procalcitonin (PCT) 0.05ng/mL. T-SPOT.TB assay (T-SPOT), as well as serum anti-tuberculosis antibody tests, were negative. RBPT was positive, and SAT was positive at a titer of 1:640. The above examination eliminated tuberculosis, and a preliminary diagnosis of brucellar hip arthritis and osteonecrosis of the femoral head was made. Despite taking anti-brucellosis medications for two weeks, which included rifampicin (0.6g po qd; Guangdong Hengjian Pharmaceuticals), doxycycline (0.1g po bid; Jiangsu Lianhuan Pharmaceuticals), and levofloxacin (0.2g ivgtt bid; Yangtze River Pharmaceuticals), the right hip’s symptoms did not improve. This patient underwent puncture and irrigation of the right hip joint and core decompression with bone grafting of the femoral head. Using real-time PCR, B. melitensis was detected in joint effusions and osteonecrosis of the femoral head. HE staining in osteonecrosis of the femoral head revealed partial dead bone and inflammatory cellulose exudate (Figure 4A); Giemsa staining showed scattered positive Brucella (Figure 4B). When discharged from the hospital three weeks after surgery, the patient’s right hip pain was significantly reduced; blood tests showed a CRP level of 24.7mg/L and an ESR level of 18.00mm/hr. The antibiotic regimen was adjusted to rifampicin (0.6g po qd; Guangdong Hengjian Pharmaceuticals) and doxycycline (0.1g po bid; Jiangsu Lianhuan Pharmaceuticals) after discharge, with a total therapy duration of six months. Following a follow-up four years later, the pain in the right hip had completely disappeared, and the hip joint was no longer limited in movement; neither the blood tests nor the imaging examinations (Figure 3D–F) revealed any abnormalities.
 |
Figure 4 (A) HE staining showed partial dead bone and inflammatory cellulose exudate in osteonecrosis of the femoral head. (B) Giemsa staining revealed scattered positive Brucella (red circle). |
Case 3
The patient is a 50-year-old Chinese male. He was admitted to the local hospital in November 2019 due to progressive right hip pain with left hip pain for five months. X-ray revealed that the right femoral head collapsed significantly, the joint space became narrow, and there were multiple areas of low density in the right femoral head; the left femoral head shape was regular, joint space was normal, and low-density area was visible in the left femoral head (Figure 5A and B). CT showed severe bilateral hip dysplasia, uneven bone density in the left femoral head, and a huge cavity in the right femoral head (Figure 5C). MRI of the hip joint showed bilateral effusion and bilateral osteonecrosis of the femoral head, particularly on the right side (Figure 5D). Blood tests revealed a WBC level of 4.70×109/L with neutrophils comprising 58.30%, CRP36.0mg/L, ESR52.00mm/hr. SAT was positive at a titer of 1:800. The patient was diagnosed with bilateral brucellar hip arthritis and bilateral osteonecrosis of the femoral head in the local hospital. Despite receiving anti-brucellosis medications for one month, his symptoms did not improve, and he was admitted to our orthopedics department for surgical treatment in December 2019. The patient had previously worked in a dairy factory for more than five years, and many of his colleagues suffered from brucellosis; he had no history of trauma, drinking, or hormone use. Physical examination revealed that both hips were tender, axial percussion was positive, active and passive hip movement was limited, especially on the right side, and a shortening deformity of the right lower limb is evident. In addition to hip joint puncture and irrigation, triple anti-infective therapy was administered after admission: rifampicin (0.6g po qd; Guangdong Hengjian Pharmaceuticals), doxycycline (0.1g po bid; Jiangsu Lianhuan Pharmaceuticals), and levofloxacin (0.2g ivgtt bid; Yangtze River Pharmaceuticals). Real-time PCR analysis of an effusion in the right hip joint revealed the presence of B. melitensis. We can confirm that Brucella is responsible for the necrosis of the right femoral head. Following three weeks of anti-brucellosis medications, inflammatory indexes returned to normal, and right total hip arthroplasty was performed (Figure 5E); the left femoral head was not treated since it has a regular shape. After being discharged from the hospital four weeks after the surgery, both hips were significantly less painful. The antibiotic regimen was adjusted to rifampicin (0.6g po qd; Guangdong Hengjian Pharmaceuticals) and doxycycline (0.1g po bid; Jiangsu Lianhuan Pharmaceuticals) after discharge; the total duration of the anti-brucellosis treatment is six months. This patient was hospitalized again in September 2020 due to progressive pain in the left hip, and all right hip symptoms had disappeared. Both the infection index and the SAT of the patients were standard; an X-ray of the hip joint indicated that the left femoral head collapsed and the joint space narrowed (Figure 5F and G). A left total hip arthroplasty was performed on the patient successfully. The patient’s symptoms disappeared entirely at the follow-up in January 2022; there were also no abnormalities in blood tests and X-ray of the hip (Figure 5H). The clinical characteristics of three patients are summarized in Table 1.
 |
Table 1 The Clinical Characteristics of Three Patients |
Discussion
The unique anatomical structure of the femoral head makes it more prone to ischemic necrosis than other bone tissues.10 Aseptic and bacterial necroses are the two forms of osteonecrosis of the femoral head. Bacterial osteonecrosis of the femoral head is rare, requiring bacterial invasion and vascular obstruction.1,11 Staphylococcus aureus, Streptococcus, and Pseudomonas aeruginosa are the primary organisms responsible for orthopedic infection; bacteria like Brucella are relatively uncommon.12–14. Suppurative osteomyelitis occurs when the bone becomes infected, and pus enters the Haversian canal and the bone’s perforating canal, obstructing the bone’s small nutritive arteries and disrupting the blood supply to the femoral head.15,16 There have only been three reports of osteonecrosis of the femoral head due to Brucella infection to date (Table 2): In the first two cases of osteonecrosis of the femoral head, Brucella was presumed as the likely cause due to a lack of diagnostic bases such as pathology and bacterial culture;7,8 in the third case, the diagnosis and treatment methods were outdated and the follow-up period was too short.9 Our study confirmed that Brucella could cause osteonecrosis of the femoral head: Brucella was detected by Giemsa staining, and B. melitensis was identified by real-time PCR testing. This article contains more comprehensive information; the basis of bacterial osteonecrosis is more precise; we adopted individualized stepwise therapy according to the Ficat stages, the follow-up period is more extended, and the prognosis is well.
 |
Table 2 Literature Review of Femoral Head Necrosis Due to Brucella Infection |
The diagnosis of femoral head necrosis was precise in these three patients based on the past epidemiological history, current medical history, physical examination, auxiliary imaging examination, and pathological results, but they were also complicated by bacterial hip arthritis. As the severity of brucellar hip arthritis in case 1 is less than that in cases 2 and 3, the joint space in case 1 is average, while the joint space in cases 2 and 3 is significantly reduced. We infer that the pathogenesis of this rare bacterial femoral head necrosis may include bacterial invasion, vascular blockage and destruction of synovial tissue (Figure 6), the decrease of synovial fluid secretion and the destruction of inflammatory factors make articular cartilage lose nutrition, further soften, necrosis and exfoliate.
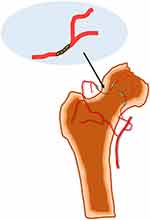 |
Figure 6 Rare bacterial avascular necrosis of the femoral head caused by Brucella infection, its pathogenesis may include bacterial invasion, vascular blockage and destruction of synovial tissue. |
Brucellosis is a zoonotic disease worldwide; it is most prevalent in herders, slaughterhouse workers, and dairy farmers.17–19 Internationally, there are six species of Brucella, with B. melitensis being the most common strain in China, followed by Brucella abortus (B. abortus) and Brucella suis (B. suis); B. melitensis is virulent, and the clinical symptoms of the patients are severe; the virulence of B. abortus is relatively weaker, and the clinical symptoms of the patients are mild.20 Spondylitis and sacroiliitis are the most common manifestations of brucellosis when it invades the bone and joint, of which spondylitis is the most common manifestation.6
There are three main types of laboratory detection of brucellosis: bacterial culture, serological detection, and molecular biological detection. Bacterial cultures are the gold standard for diagnosing brucellosis; however, the false-negative rate is high; bone marrow cultures are more sensitive than blood cultures.21 Several serological tests are widely used, including RBPT, SAT, and enzyme-linked immunosorbent assay (ELISA). RBPT is mainly used for primary screening.22 It is recommended by the World Health Organization (WHO) that the diagnostic criteria for SAT titer be set at 1:160, but the threshold should be set at 1:320 or higher in endemic regions; the titer of SAT is not physically related to infection, and it can remain high after the disease was cured.23,24 ELISA provides high sensitivity and specificity; it can monitor infection and curative effects.25 Molecular biological detection entails using real-time PCR to identify strains.26 It is recommended to use various detection methods to reduce the rate of missed diagnoses in patients with an epidemiological history.
For uncomplicated brucellosis, the antimicrobial regimen consisted of rifampicin and doxycycline for six weeks; treatment for brucellosis affecting the bone and joint system generally involves a triple regimen at least three months: rifampicin, doxycycline, and levofloxacin/ceftriaxone.27,28 These three patients were treated with antibiotics for half a year; they were followed for an extended period without recurrences or adverse effects.
Osteonecrosis of the femoral head may cause secondary osteoarthritis in the late stages due to the collapse of the femoral head and narrowing of the joint space, which inevitably leads to joint pain and loss of function.29 Conservative treatment is appropriate for patients in Ficat stages I or IIa; the effect of core decompression is evident in patients with femoral head cysts and dead bone in Ficat stage IIb.30,31 Patients with Ficat stages III and IV are contraindicated for conservative treatment and require total hip arthroplasty in the majority of cases.32 Following conservative treatment and core decompression, patients with osteonecrosis of the femoral head should undergo routine X-ray and computed tomography examinations; if the condition worsens, a total hip replacement may be necessary. In this paper, case 1 had Ficat stage IIb of the left femoral head; case 2 had Ficat stage IIb of the right femoral head; case 3 had Ficat stage IV of the right femoral head at the first admission and Ficat stage III of the left femoral head at the second admission. Brucellar hip arthritis with osteonecrosis of the femoral head is rare and does not have a standard treatment. Osteonecrosis may be exacerbated by tension within the hip joint caused by infection;7–9 based on anti-infection combined with hip puncture and irrigation decompression, we provided individualized stepwise therapy for osteonecrosis of the femoral head according to the Ficat stage, and the prognosis of all three patients was well. Clinicians should be aware of the possibility of bacterial osteonecrosis of the femoral head in patients with an epidemiological history of brucellosis; osteonecrosis can result in severe complications if it is not diagnosed and treated at an early stage.
Conclusion
Brucella infection is a rare cause of femoral head necrosis. MRI and other tests should be performed in time to exclude femoral head necrosis when hip pain occurs in patients with an epidemic history of brucellosis. A timely diagnosis and individual stepwise treatment based on the Ficat stage can prevent osteonecrosis from progressing.
Patient Consent and Ethics Statement
The three patients provided informed consent for publication of this study and accompanying images. The Ethics Committee of the Beijing Ditan Hospital of Capital Medical University approved the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work received no funding.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Cohen-Rosenblum A, Cui Q. Osteonecrosis of the femoral head. Orthop Clin North Am. 2019;50(2):139–149. doi:10.1016/j.ocl.2018.10.001
2. Schaeffer EK, Ponton E, Sankar WN, et al.; International Hip Dysplasia (IHD) Study Group. Interobserver and intraobserver reliability in the salter classification of avascular necrosis of the femoral head in developmental dysplasia of the Hip. J Pediatr Orthop. 2022;42(1):e59–e64. doi:10.1097/BPO.0000000000001979.
3. Briceño-Souza E, Méndez-Domínguez N, Cárdenas-Dajdaj R, Chin W, Huchim-Lara O. Dysbaric osteonecrosis associated with decompression sickness in a fishing diver. Undersea Hyperb Med. 2019;46:217–220.
4. Katz K, Horev G, Grunebaum M, Yosipovitch Z. The natural history of osteonecrosis of the femoral head in children and adolescents who have Gaucher disease. J Bone Joint Surg Am. 1996;78(1):14–19. doi:10.2106/00004623-199601000-00003
5. Munhoz lima ALL, Oliveira PR, Carvalho VC, et al. Osteonecrosis of the femoral head in people living with HIV: anatomopathological description and p24 antigen test. HIV AIDS. 2018;10:83–90. doi:10.2147/HIV.S162305
6. Unuvar GK, Kilic AU, Doganay M. Current therapeutic strategy in osteoarticular brucellosis. North Clin Istanb. 2019;6(4):415–420. doi:10.14744/nci.2019.05658
7. Wang P, Sun W, Shi L, Li T. Osteonecrosis of the femoral head due to brucellosis: a case report. BMC Infect Dis. 2020;20(1):202. doi:10.1186/s12879-020-4945-8
8. Salarvand S, Nazer M, Shokri S, Bazhvan S, Pournia Y. Brucellosis-induced avascular necrosis of the hip in a middle-aged person. Iran J Public Health. 2012;41(12):86–88.
9. Gedalia A, Howard C, Einhorn M. Brucellosis induced avascular necrosis of the femoral head in a 7 year old child. Ann Rheum Dis. 1992;51(3):404–406. doi:10.1136/ard.51.3.404
10. Johnson EO, Soultanis K, Soucacos PN. Vascular anatomy and microcirculation of skeletal zones vulnerable to osteonecrosis: vascularization of the femoral head. Orthop Clin North Am. 2004;35(3):285–291. doi:10.1016/j.ocl.2004.03.002
11. Wideman RF, Prisby RD. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Front Endocrinol (Lausanne). 2013;3:183. doi:10.3389/fendo.2012.00183
12. Urish KL, Cassat JE. Staphylococcus aureus osteomyelitis: bone, bugs, and surgery. Infect Immun. 2020;88(7):e00932–19. doi:10.1128/IAI.00932-19
13. Griffin AT, Timbrook T, Harting J, Christensen D. Streptococcus anginosus group and osteomyelitis: a single centre clinical experience. Postgrad Med J. 2013;89(1051):262–265. doi:10.1136/postgradmedj-2012-131663
14. Pliska NN. Pseudomonas aeruginosa as the main causative agent of osteomyelitis and its susceptibility to antibiotics. Drug Res. 2020;70(6):280–285. doi:10.1055/a-1150-2372
15. Marx RE, Tursun R. Suppurative osteomyelitis, bisphosphonate induced osteonecrosis, osteoradionecrosis: a blinded histopathologic comparison and its implications for the mechanism of each disease. Int J Oral Maxillofac Surg. 2012;41(3):283–289. doi:10.1016/j.ijom.2011.12.016
16. Sodnom-Ish B, Eo MY, Seo MH, Lee JH, Kim SM. Decompressive effects of draining tube on suppurative and sclerosing osteomyelitis in the jaw. BMC Musculoskelet Disord. 2021;22(1):469. doi:10.1186/s12891-021-04340-3
17. Oyetola WD, Diallo K, Kreppel K, et al. Factors influencing the transborder transmission of brucellosis in cattle between Côte d’Ivoire and Mali: evidence from literature and current key stakeholders. Front Vet Sci. 2021;8:630580. doi:10.3389/fvets.2021.630580
18. Acharya D, Hwang SD, Park JH. Seroreactivity and risk factors associated with human brucellosis among cattle slaughterhouse workers in South Korea. Int J Environ Res Public Health. 2018;15(11):2396. doi:10.3390/ijerph15112396
19. Tulu D. Bovine brucellosis: epidemiology, public health implications, and status of brucellosis in Ethiopia. Vet Med. 2022;13:21–30. doi:10.2147/VMRR.S347337
20. Deng Y, Liu X, Duan K, Peng Q. Research progress on brucellosis. Curr Med Chem. 2019;26(30):5598–5608. doi:10.2174/0929867325666180510125009
21. Basu S, Shetty A. Laboratory diagnosis of tropical infections. Indian J Crit Care Med. 2021;25(Suppl 2):S122–S126. doi:10.5005/jp-journals-10071-23813
22. Tadesse G. Brucellosis seropositivity in animals and humans in Ethiopia: a meta-analysis. PLoS Negl Trop Dis. 2016;10(10):e0005006. doi:10.1371/journal.pntd.0005006
23. Hasanjani Roushan MR, Moulana Z, Mohseni Afshar Z, Ebrahimpour S. Risk factors for relapse of human Brucellosis. Glob J Health Sci. 2015;8(7):77–82. doi:10.5539/gjhs.v8n7p77
24. Roushan MR, Amiri MJ, Laly A, Mostafazadeh A, Bijani A. Follow-up standard agglutination and 2-mercaptoethanol tests in 175 clinically cured cases of human brucellosis. Int J Infect Dis. 2010;14(3):e250–3. doi:10.1016/j.ijid.2009.05.008
25. Xu N, Wang W, Chen F, Li W, Wang G. ELISA is superior to bacterial culture and agglutination test in the diagnosis of brucellosis in an endemic area in China. BMC Infect Dis. 2020;20(1):11. doi:10.1186/s12879-019-4729-1
26. Ali Ahmadi M, Saadati D, Najimi M, Ganjali H, Shah Karami F. Comparison of PCR and conventional serological methods for detection of Brucella spp. in ovine and caprine blood serum. Arch Razi Inst. 2021;76(3):445–452. doi:10.22092/ari.2020.128449.1415
27. Vollmar P, Zange S, Zöller L, Erkel J, Robert Thoma B. Brucellose [Brucellosis, an overview and current aspects]. Dtsch Med Wochenschr. 2016;141(14):1014–1018. German. doi:10.1055/s-0042-101723
28. Shakir R. Brucellosis. J Neurol Sci. 2021;420:117280. doi:10.1016/j.jns.2020.117280
29. Larson E, Jones LC, Goodman SB, Koo KH, Cui Q. Early-stage osteonecrosis of the femoral head: where are we and where are we going in year 2018? Int Orthop. 2018;42(7):1723–1728. doi:10.1007/s00264-018-3917-8
30. Martinot P, Dartus J, Justo A, et al.; French Society of Orthopaedic Surgery and Traumatology (SoFCOT). Does augmented core decompression decrease the rate of collapse and improve survival of femoral head avascular necrosis? Case-control study comparing 184 augmented core decompressions to 79 standard core decompressions with a minimum 2 years’ follow-up. Orthop Traumatol Surg Res. 2020;106(8):1561–1568. doi:10.1016/j.otsr.2020.03.040
31. Grassi M, Salari P, Massetti D, Papalia GF, Gigante A. Treatment of avascular osteonecrosis of femoral head by core decompression and platelet-rich plasma: a prospective not controlled study. Int Orthop. 2020;44(7):1287–1294. doi:10.1007/s00264-020-04628-4
32. Erivan R, Caputo T, Riouach H, et al. Primary total hip replacement in Ficat-Arlet stage 3 and 4 osteonecrosis: a retrospective study at a minimum 12-year follow-up. Eur J Orthop Surg Traumatol. 2020;30(5):845–850. doi:10.1007/s00590-020-02644-z
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.