Back to Journals » Infection and Drug Resistance » Volume 13
Fecal Carriage Rate of Extended-Spectrum Beta-Lactamase-Producing Escherichia coli and Klebsiella pneumoniae Among Apparently Health Food Handlers in Dilla University Student Cafeteria
Authors Diriba K , Awulachew II E, Tekele L, Ashuro Z
Received 7 August 2020
Accepted for publication 7 October 2020
Published 23 October 2020 Volume 2020:13 Pages 3791—3800
DOI https://doi.org/10.2147/IDR.S269425
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Kuma Diriba,1 Ephrem Awulachew II,1 Lami Tekele,2 Zemachu Ashuro3
1Department of Medical Laboratory Sciences, Health Science and Medical College, Dilla University, Dilla, Ethiopia; 2Department of Biochemistry, Health Science and Medical College, Dilla University, Dilla, Ethiopia; 3School of Public Health, College of Health Science and Medicine, Dilla University, Dilla, Ethiopia
Correspondence: Kuma Diriba Email [email protected]
Background: The rapid spread of extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae among food handlers is a public health concern and has become a serious world threat. Klebsiella pneumoniae and Escherichia coli are the predominant ESBL-producing Enterobacteriaceae. This study aimed to assess the magnitude of ESBL producing K. pneumoniae and E. coli isolated from the stool of food handlers in the Dilla University Student Cafeteria.
Methods: A cross-sectional study was conducted from November to September 2018/2019. A total of 220 stool samples were cultured on MacConkey agar and a series of biochemical tests were performed for the identification of bacterial species. Double-disk synergy method was used for the detection of ESBL-producing strains.
Results: The rate of fecal carriage of E. coli and K. pneumoniae among the food handlers of the current study was 66.4%, of which 81.5% was E. coli, while 18.5% was K. pneumoniae. Of the total bacterial isolates, 25.3% were confirmed to be positive for ESBL production, of which 19.9% was E. coli, while 5.5% was K. pneumoniae. ESBL-producing fecal carriage showed high resistance to aztreonam, cefotaxime, ceftriaxone, cefuroxime, and ceftazidime, with resistance rates ranging from 88% to 100%, while the lowest resistance rate was observed against imipenem accounted < 26.3%. In this study, 91.9% of ESBL-producers were co-resistant to β-lactams antibiotics plus at least one of the non-β-lactam antibiotics. ESBL carriers were more common among food handlers who used antibiotics repeatedly, drunk unpasteurized milk, and ate raw meat.
Conclusion: The magnitude of ESBL-producing E. coli and K. pneumoniae fecal carriage is alarmingly high and a threat to human health. MDR to third-generation cephalosporins and non-β-lactam antibiotics is more common among ESBL producers. Detecting ESBL-producing organisms has a remarkable importance in clinical decision-making. Hence, a coordinated effort should be implemented for the prevention and control of the disease.
Keywords: extended-spectrum β-lactamase, Enterobacteriaceae, antibiotic resistance, Klebsiella pneumoniae, Escherichia coli, Ethiopia
Background
Enterobacteriaceae are a substantial, heterogeneous group of gram-negative rods whose natural habitat is the intestinal tract of human beings and animals.1 These microorganisms have emerged as one of the most important reasons for nosocomial and community-obtained infections.2–5 Enterobacteriaceae are typically associated with a range of infections,6 among which urinary tract infections, bloodstream infections, heath facility associated pneumonia, and some intra-abdominal infections are the most crucial.7,8 Studies conducted in different underdeveloped countries indicate a high case-fatality rate associated with bloodstream infection due to Enterobacteriaceae.9,10
Antibiotics play a vital role in decreasing the load of communicable diseases worldwide.11 Microbial resistance to antimicrobial agents is remarkably rising globally.12–14 This rapid spread of resistance among pathogenic microorganisms is a serious problem globally,2,4 because it limits the drug treatment alternative against infections.15 Antimicrobial resistance has presently been recognized as one of the most important problems facing human health by the World Health Organization (WHO).15,16 Frequent isolation of multidrug resistant (MDR) pathogens in both hospital and community acquired infections further intensified the problem of antimicrobial resistance.5
Nowadays, extended-spectrum β-lactamases (ESBLs) producing Klebsiella pneumoniae and Escherichia coli are a serious public health issue globally.1 They become resistant to beta-lactam antibiotics via the production of beta-lactamase enzymes that inactivate beta-lactam antibiotics and this continues to be the prominent cause of β-lactam antibiotic resistance among Enterobacteriaceae.17,18 It can rapidly develop resistance against a range of important broad-spectrum antimicrobials.19,20 Inappropriate and irrational use of antimicrobial drugs, poor sanitatation, and infection control practices in developing countries play a critical role in an increased prevalence of resistant bacteria in a community providing favorable conditions for resistant microorganisms to emerge and spread.21,22 This can lead to a proliferation of organisms with broad-spectrum β-lactamase activity that threatens the future of the β-lactam class in clinical care.21
The increasing rate of human infections caused by antimicrobial resistance strains of Enterobacteriaceae makes clinical management more difficult by prolonging the illness and compromising the treatment.5 This can have a potentially serious impact on human health. The situation is more common in developing countries where there is widespread and uncontrolled use of antibiotics.16 Data on extended-spectrum β-lactamases producing Enterobacteriaceae in Ethiopia are limited and there is no data associated with this. Therefore, this study aimed to assess the magnitude of ESBL producing Klebsiella pneumoniae and Escherichia coli isolated from the stool of apparently healthy food handlers in the Dilla University Student Cafeteria.
Methods
Study Design and Period
An institutional-based cross-sectional study was carried out among food handlers to evaluate the magnitude and antimicrobial resistance pattern of ESBL-producing E. coli and K. pneumoniae from stools of apparently healthy food handlers in Dilla University Student Cafeteria, Southern Ethiopia from November 2018 to September 2019. Three sample selection sites were identified prior to data collection. To select representative participants, the final sample size was proportionally allocated to each stratum, and food handlers were selected using a systematic random sampling technique. Finally, a total of 220 were included in the study based on the single population proportion formula.
Data Collection and Analysis
Data related to sociodemographic characteristics and personal hygiene practices were collected via face-to-face interviewing of the patient or guardian of the patient using a well-structured questionnaire before laboratory sample collection. One environmental health and four medical laboratory professionals were recruited for data collection, supervision, and microbiological analysis. The data collectors were trained for 2 days by the principal investigator on observational data collection and specimen collection procedures.
Laboratory Data Collection and Analysis
After interviewing, all respondents were asked to give a fresh stool specimen in a sterile, clean, wide-mouthed plastic and leak proof container by a clean wooden applicator stick for microbiological analysis. The isolation and characterization of E. coli and K. pneumoniae were performed based on the standard procedure. Briefly, a mixture of a stool sample (1 mL) was transferred to the selective media, Hicrome ESBL agar base, to assess ESBL production in 2 hours (Oxoid, Ltd, UK). An inoculum from Hicrome ESBL agar base was cultured onto MacConkey agar (Oxoid, Ltd, UK). After overnight incubation at 37°C the growth of E. coli and K. pneumoniae were differentiated by their colony characteristics, pigment production (pink to colorless flat or mucoid colonies), motility, and Gram-staining techniques (Gram-negative rods, non-sporing, and non-capsulated).
A list of biochemical tests conducted for further identification of isolates were made by conformation of motility and other relevant biochemical tests. An isolate was considered as E. coli when it was indole positive (dark pink ring) and methyl-red positive, citrate negative (no change or remained green) and urea negative, gas and acid producer, and motile and was considered as K. pneumoniae when it is indole and methyl-red negative, citrate positive, urea slow producing, and non-motile. In case of delay, the isolated bacteria were kept at 2–8°C in the nutrient broth for no more than 24 hours until the antimicrobial sensitivity test was done.
Detection Method of ESBL-Producing E. coli and K. pneumoniae
The disk-diffusion method was used to screen ESBL producers using ceftazidime (30 μg), cefotaxime (30 μg), and ceftriaxone (30 μg) (Oxoid, UK) as per CLSI criteria. A suspension of pure colony was wrapped on Mueller–Hinton agar (MHA) (Oxoid, UK) with a sterile cotton swab, and antibiotic discs were placed at a distance of 20 mm from each other and incubated at 35±2°C for 16–18 hours. The isolates with diminished susceptibility with breakpoints of ≤22 mm for ceftazidime, ≤25 mm for ceftriaxone, and ≤27 mm for cefotaxime were suspected for ESBL production.15 Phenotypic confirmation of ESBL producers was performed using double disc synergy between the indicator cephalosporin and clavulanic acid, according to EUCAST guidelines.23 A disc of amoxicillin/clavulanic acid (20/10 μg) was placed in the center of the MHA plate, and then cefotaxime (30 μg), ceftazidime (30 μg), and aztreonam (30 μg) were placed at a distance of 20 mm edge to edge from the amoxicillin/clavulanic acid disk. After 24 hours incubation, the elevation of ≥5 mm zone inhibition between either of the cephalosporin disks and their respective amoxicillin/clavulanic acid disks was interpreted as an ESBL producer24 (Figure 1A and B).
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility tests were performed for both Enterobacteriaceae by using the disc diffusion technique on Mueller–Hinton agar based on EUCAST guidelines23 for the listed antibiotics: Ampicillin (10 μg), aztreonam (30 μg), cefotaxime (30 μg), ceftriaxone (30 μg), ceftazidime (30 μg), cefoxitin (30 μg) cefuroxime (30 µg), ciprofloxacin (5 μg), nitrofurantoin (300 μg), trimethoprim-sulfamethoxazole (1.25/23.75 μg), tetracycline (30 μg) and imipenem (30 μg) (all from Oxoid, UK). The resistance and sensitivity results were interpreted according to the EUCAST guidelines.23 MDR was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories. The quality and performance of culture media, biochemical tests, and antimicrobial susceptibility discs were checked using a control strain (E. coli ATCC 25,922 and K. pneumoniae ATCC 700,603) obtained from the Ethiopian Public Health Institute, Addis Ababa.
Data Analysis
Descriptive statistical analysis was performed using SPSS version 24 software. The prevalence of ESBL producers and non-ESBL-producing isolates were categorized by personal hygiene related factors of food handlers and presented by calculating frequencies and percentages. The Chi-square test was used with appropriate correction for the observation. Multivariate logistic regression analysis was used to identify risk factors for colonization by ESBL. P-values<0.05 were considered statistically significant.
Ethical Consideration
Ethical clearance was obtained from the Ethical Review Committee of Dilla University Medical and Health Science College. Written informed consent was obtained from each study participant. Strict confidentiality was maintained during the interview process as well as anonymity was maintained during data processing and report writing. Food handlers who were found to be positive for enteric pathogens (parasite and bacterial) were referred to their respective staff medical center for appropriate anti-parasitic and antimicrobial treatments.
Results
Socio Demographic Characteristics of Study Participant
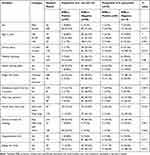
A total of 220 food handlers were included in this study. Most participants (>90%) were females which explains high prevalence of ESBLs in females. The majority of the study participants were between the age group of 20 and 40 years (84.5%) with a mean age of 31.2 (standard deviation=±8.8 years). Of the total respondents, 51.4% reported that they always perform periodic medical checkups, 81.8% always trimmed their finger weekly, and 63.2% always wear a clean apron. Similarly, 69.1% wear a hair garment regularly in their working area, and 79.5% reported that they always use soap and water for hand washing after visiting the toilet. The finding from observation also supported that personal hygiene was practiced by the food handlers. From the total, 97.3% use pipe water for drinking. About 86.8% of the study participants drunk unpasteurized milk, while 42.3% of the study participants ate raw meat (Table 1).
ESBL Production Fecal Carriage Rate of E. coli and K. pneumoniae from Stool Culture
In the present study, The rate of fecal carriage of E. coli and K. pneumoniae among the food handlers of the current study was found to be 146 (66.4%), of which 119 (81.5%) were E. coli, while 27 (18.5%) were K. pneumoniae. From the total bacterial isolates, 37 (25.3%) were confirmed to be positive for ESBL production. 29 (19.9%) of ESBL producing isolates were E. coli, while eight (5.5%) were K. pneumoniae (Figure 2).
 |
Figure 2 The distribution of ESBL-producing E. coli and K. pneumoniae isolated from the stool of apparently healthy food handlers. |
In this study, ESBL-producers and non ESBL producers isolates were predominantly reported in females with a prevalence rates of 35 (24.0%) and 97 (66.4%). E. coli and K. pneumoniae producing ESBL were predominantly reported among the age group of 20–40 years with a prevalence of 31 (21.2%). Most ESBL producers were reported in food handlers who did not attend a regular medical checkup with a prevalence of 25 (17.1%). The majority of E. coli and K. pneumoniae producing ESBL were reported among food handlers who did not wash their hands with soap, with the prevalence of 24 (16.4%). In the present study, the majority of the demographic characteristics study participants have no association with E. coli and K. pneumoniae producing ESBL. Only food handlers who used antibiotics in the last 3 months had a strong association with the ESBL carriage with P-values of 0.001 (Table 1).
Antimicrobial Resistance Pattern of ESBL-Producing and Non-ESBL-Producing E. coli and K. pneumonia
In the current study, ESBL-producing isolates showed significantly higher resistance not only towards the third generation cephalosporins but also towards other antimicrobial agents tested (P>0.001). Both E. coli and K. pneumoniae ESBL producers were highly resistant to Aztreonam, cefotaxime, ceftriaxone, cefuroxime, and ceftazidime, with resistance rates ranging from 87–100%. Almost all ESBL-producing and non-ESBL-producing isolates were highly resistant to ampicillin and tetracycline, with a resistance rate of 89.5%. Higher resistance rates of ESBL producing isolate were also reported among ciprofloxacin, trimethoprim–sulfamethoxazole, and nitrofurantoin with resistance rates ranged from 67–75%. In the current study, ESBL producing isolates were the least resistant to imipenem with a resistance rate of 13.8% (Table 2).
 |
Table 2 Antimicrobial Resistance Patterns of ESBLs-Positive and ESBLs-Negative E. coli and K. pneumonia Isolated from Food Handlers in Dilla University Student Cafeteria, Southern Ethiopia (n=220) |
Antibiogram Pattern of Multidrug-Resistant E. coli and K. pneumoniae
In this study, around 34 (91.9%) ESBL-producing isolates were resistant to both β-lactam groups and tetracycline, while 26 (70.3%) ESBL-producing isolates were Co-resistant to β-lactams groups and ciprofloxacin and 20 (54.1%) ESBL producers were resistant to both β-lactams groups and trimethoprim sulfamethoxazole. Other ESBL-producing isolates were co-resistant to β-lactams groups, and three, four, and five types of non-β lactam antibiotics with resistance rates of 17 (45.9%), 12 (32.4%), and 8 (21.6%), respectively (Table 3). .
 |
Table 3 Multidrug Resistance Pattern of E. coli and K. pneumonia Fecal Carriage Isolated from Food Handlers in Dilla University Student Cafeteria, Southern Ethiopia (n=220) |
Discussion
The emergence and rapid spread of multidrug resistance strains of ESBL producing Enterobacteriaceae is a serious public health issue worldwide. Rapid expansion of ESBLs highly affects the activity of broad-spectrum antibiotics, creating major therapeutic difficulties with a significant impact on the outcomes for patients.25 Few data are available on the prevalence of fecal carriage of ESBL producing Enterobacteriaceae, and little attention was given to it in Africa. In this study, we examined the fecal carriage rate of ESBL producers E. coli or K. pneumoniae in food handlers working at the Dilla University student cafeteria.
The phenotypic information obtained in the current study indicates a significant prevalence of ESBL producers. The overall magnitude of fecal carriage of ESBL producing E. coli or K. pneumoniae among apparently health food handlers was 25.3%. This indicates a remarkably high number of ESBL productions among food handlers working in food preparation. Our finding is consistent with studies conducted in India,26 Italy,27 Egypt,28 and in Turkey,29 with prevalence ranging from 15–33%. However, it is lower than the study conducted in Central Africa,30 Tanzania,31 Nigeria,32 Burkina Faso,33 and Ghana,34 with prevalence ranging from 40–59%. The variation might be explained by methodological differences, differences in study area, quality of media used, or type of specimen collected. The use of low quality, inappropriate use of antibiotics, and weak infection prevention measures may additionally make a contribution to the high magnitude of ESBL production.
In the present study, E. coli was the leading fecal carriage of ESBL producing Enterobacteriaceae with a prevalence of 19.9%, followed by K. pneumoniae (5.5%). Our finding agrees with studies conducted in India,26,27 Central Africa,30 Central India,35 and Burkina Faso,33 where E. coli was the predominant ESBL producer than K. pneumoniae. However, studies conducted in Uganda,36 Gambia,37 and elsewhere38 reported a higher prevalence of K. pneumoniae than E. coli. Those bacteria can result in the transfer of the resistance genes to other strains of E. coli and K. pneumoniae within the gastrointestinal tract that can result in fatal consequences. In addition, when those carriers are admitted to hospitals they can easily transmit the infection to other hospitalized patients. Quick adaptation of those strains to the harsh environment, upregulation of intrinsic resistance mechanisms, and rapid acquisition and transferring of drug resistance genes through mobile genetic elements could be a possible explanation for an elevated overall drug resistance prevalence rate against different categories of drugs.
In the current study, the fecal carriage of ESBLs among food-handlers working in the cafeteria was significantly associated with food handlers who used antibiotics repeatedly in the last 3 months and those food handlers who did not attend regular medical check studies.39,40 In most parts of developing countries including Ethiopia, antibiotics were used without prescription, which can lead to overuse or misuse of antibiotics. This can contribute to the emergence and spread of antimicrobial-resistant strains. Moreover, in developing countries, poor personal hygiene practices may increase the prevalence of bacterial gastrointestinal infections which may play a role in rising antimicrobial resistance. This increases the risk of treatment failure with potentially serious consequences. This can be overcome by performing bacterial culture and performing an antimicrobial susceptibility test to select the most effective antibiotics for suspected cases of gastrointestinal infected patients.
In the present study, ESBL-producing E. coli and K. pneumoniae were highly resistant to ampicillin, cefotaxime, aztreonam, ceftazidime, and ceftriaxone, with resistance rates ranging from 88–100%, while the isolates were highly susceptible to imipenem rating 86.2%. Our finding agrees with studies conducted in Gambia,41 Burkina Faso,33 Ghana,34 Saudi Arabia,42 Israel,43 Poland,44 Sierra Leone,45 and Lebanon.46 This indicates ESBL-producing Enterobacteriaceae were rapidly emerging in developing and developed countries. In this study, ESBL-producing E. coli and K. pneumoniae were not only resistant to third-generation cephalosporins but also to other non-β lactams groups of antibiotics. The finding of this study showed that imipenem had a better performance against ESBL-producing Enterobacteriaceae than other antibiotics including the cephalosporin group. This is inconsistent with a study conducted by Moges et al,47,48 who reported that imipenem had higher performance for the treatment of ESBL-producing Enterobacteriaceae.
Our findings showed that greater than 90% of the ESBL producing isolates were MDR. The MDR nature of ESBL-producing E. coli and K. pneumoniae may be explained by the fact that they are plasmid-mediated enzymes which are carrying multi-resistant genes by plasmid, transposon, and integron and they are readily transferred to other bacteria through conjugation, transduction, or transformation. The carrier of those bacteria can easily disseminate to the community and hospitalized patients, which can result in remarkable consequences. As a result, nowadays bacteria with multiple resistance to antibiotics are widely distributed in hospitals and communities and became a serious problem throughout the world.49 This is the most challenging and alarming condition in the management of infectious diseases associated with ESBL-producing Enterobacteriaceae.
Limitations of the Study
The limitations of this study were the lack of a molecular characterization using PCR. Most of the participants were unable to provide the previous prescribed antibiotics to show association with the fecal carriage of ESBLs (recall bias). Most of the study participants were female, which tends to show the gender bias.
Conclusion
In this study, the magnitude of fecal carriage of ESBL-producing E. coli and K. pneumoniae is alarmingly high and a threat to human health. ESBL-producing fecal carriage showed high resistance to aztreonam, cefotaxime, ceftriaxone, cefuroxime, and ceftazidime. The better option for the treatment of ESBL-producing E. coli and K. pneumonia in this study is imipenem. The occurrence of multidrug resistance to third-generation cephalosporins and non-β lactam antibiotics is more common among ESBL producers. In the present study, ESBL-producing isolates were co-resistant to β-lactams antibiotics plus at least one of the non β-lactam antibiotics. Only food handlers who used antibiotics repeatedly in the last 3 months were significantly associated with the ESBL fecal carriage. The rise of ESBL-producing Enterobacteriaceae requires strict infection prevention and control strategies and strengthening of the diagnostic capacity of laboratory professionals for the detection and surveillance of antibiotic resistance that have a significant role in clinical decision-making. Hence a coordinated effort should be implemented. Molecular confirmation and characterization using PCR of the ESBL producers to reveal the mobile genetic elements, resistance, and virulence of the detected isolates is also recommended as a future direction in the study area.
Data Sharing Statement
The data set used for analysis during the current study are available from the corresponding author on reasonable request.
Ethics Statement
A protocol for patient recruitment and participation in the study followed the principles of the Declaration of Helsinki and was approved by the Dilla University Health Research Ethics Review Committee under protocol unique number 005/2019-01, date 10-01-2019. During data collection, each child’s parent/guardian was informed about the aim of the study. Written consent was obtained from the parent/legal guardian for each patient before the start of data collection. The clinical samples were specifically collected for this research. Samples with positive culture results were communicated to physicians in order for patients to get treatment according to the drug susceptibility results of the isolates.
Additional Information
All data relevant to the study are included in the article.
Acknowledgments
The authors would like to acknowledge Dilla University for opening this opportunity for researchers to come with problem-solving project ideas and scientific questions, and Dilla University food and nutrition research center for funding this study. I acknowledge, Laboratory Department staff and all study participants for their cooperation during sample collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Dilla University Food and Nutrition Research Center.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Chiu C-W, Li M-C, Ko W-C, et al. Clinical impact of gram-negative non fermenters on adults with community-onset bacteremia in the emergency department. J Microbiol Immunol Infect. 2015;48(1):92–100. doi:10.1016/j.jmii.2013.08.004
2. Ventola CL. The antibiotic resistance crisis: part 1: causes and threats. Pharm Ther. 2015;40(4):277.
3. Gelband H, Molly Miller P, Pant S, et al. The state of the world’s antibiotics 2015. Wound Heal S Afr. 2015;8(2):30–34.
4. de La Blanchardière A, Dargère S, Guérin F, et al. Non-carbapenem therapy of urinary tract infections caused by extended-spectrum β-lactamase-producing Enterobacteriaceae. Med Mal Infect. 2015;45(5):169–172. doi:10.1016/j.medmal.2015.03.003
5. Health U, Services H. Antibiotic resistance threats in the United States, 2013. Center Dis Control Prev. 2013;1–113.
6. Kakati B, Agarwal S, Gupta S. Emerging issues regarding management of MDR non-fermenting gram negative ventilator associated pneumonia in a rural catering tertiary care hospital. J Med Sci Clin Res. 2015;4(10):13232–13238. doi:10.18535/jmscr/v4i10.69
7. Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791. doi:10.3201/eid1710.110655
8. Paterson DL. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Infect Control. 2006;34(5):S20–S8. doi:10.1016/j.ajic.2006.05.238
9. Dogan A, Lasch P, Neuschl C, et al. ATR-FTIR spectroscopy reveals genomic loci regulating the tissue response in high fat diet fed BXD recombinant inbred mouse strains. BMC Genom. 2013;14(1):386.
10. Aiken AM, Mturi N, Njuguna P, et al. Risk and causes of paediatric hospital-acquired bacteraemia in Kilifi District Hospital, Kenya: a prospective cohort study. Lancet. 2011;378(9808):2021–2027. doi:10.1016/S0140-6736(11)61622-X
11. Abdallah H, Wintermans B, Reuland E, et al. Extended-spectrum β-lactamase-and carbapenemase-producing Enterobacteriaceae isolated from Egyptian patients with suspected blood stream infection. PLoS One. 2015;10(5).
12. McDonald M, Blondeau JM. Emerging antibiotic resistance in ocular infections and the role of fluoroquinolones. J Cataract Refract Surg. 2010;36(9):1588–1598. doi:10.1016/j.jcrs.2010.06.028
13. Organization WH. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; 2014.
14. Duthey B. Priority Medicines for Europe and the World” a Public Health Approach to Innovation. WHO Background paper; 2013:6.
15. Fournier PE, Richet H, Weinstein RA. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis. 2006;42(5):692–699. doi:10.1086/500202
16. Bassetti M, Ginocchio F, Mikulska M. New treatment options against gram-negative organisms. In: Annual Update in Intensive Care and Emergency Medicine 2011. Springer; 2011:501–515.
17. Paterson DL, Bonomo RA. Extended-spectrum β-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657–686. doi:10.1128/CMR.18.4.657-686.2005
18. Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi:10.1016/S1473-3099(08)70041-0
19. Alvarez-Uria G, Gandra S, Mandal S, Laxminarayan R. Global forecast of antimicrobial resistance in invasive isolates of Escherichia coli and Klebsiella pneumoniae. Int J Infect Dis. 2018;68:50–53. doi:10.1016/j.ijid.2018.01.011
20. Murray TS, Peaper DR. The contribution of extended-spectrum β-lactamases to multidrug-resistant infections in children. Curr Opin Pediatr. 2015;27(1):124–131. doi:10.1097/MOP.0000000000000182
21. Bevan ER, Jones AM, Hawkey PM. Global epidemiology of CTX-M β-lactamases: temporal and geographical shifts in genotype. J Antimicrob Chemother. 2017;72(8):2145–2155. doi:10.1093/jac/dkx146
22. Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54(3):969–976. doi:10.1128/AAC.01009-09
23. Testing E. Data from the EUCAST MIC Distribution Website. European Committee on Antimicrobial Susceptibility Testing Data from the EUCAST MIC Distribution Website. 2016.
24. Drieux L, Brossier F, Sougakoff W, Jarlier V. Phenotypic detection of extended‐spectrum β‐lactamase production in Enterobacteriaceae: review and bench guide. Clin Microbiol Infect. 2008;14:90–103. doi:10.1111/j.1469-0691.2007.01846.x
25. Adler A, Katz DE, Marchaim D. The continuing plague of extended-spectrum β-lactamase–producing Enterobacteriaceae infections. Infect Dis Clin. 2016;30(2):347–375.
26. Indernath S, Priyadharsini RI, Manikkanan M, Babu SV. Prevalence and Phenotypic Characterization of ESBL Producing E. Coli and Klebsiella Among the Fecal Isolates of Normal Population.
27. Rao SP, Rama PS, Gurushanthappa V, Manipura R, Srinivasan K. Extended-spectrum beta-lactamases producing Escherichia coli and Klebsiella pneumoniae: a multi-centric study across Karnataka. J Lab Physicians. 2014;6(1):7. doi:10.4103/0974-2727.129083
28. Fam N, Leflon-Guibout V, Fouad S, et al. CTX-M-15-producing Escherichia coli clinical isolates in Cairo (Egypt), including isolates of clonal complex ST10 and clones ST131, ST73, and ST405 in both community and hospital settings. Microb Drug Resist. 2011;17(1):67–73. doi:10.1089/mdr.2010.0063
29. Nijssen S, Florijn A, Bonten M, Schmitz F, Verhoef J, Fluit A. Beta-lactam susceptibilities and prevalence of ESBL-producing isolates among more than 5000 European Enterobacteriaceae isolates. Int J Antimicrob Agents. 2004;24(6):585–591. doi:10.1016/j.ijantimicag.2004.08.008
30. Girlich D, Bouihat N, Poirel L, Benouda A, Nordmann P. High rate of faecal carriage of extended-spectrum β-lactamase and OXA-48 carbapenemase-producing Enterobacteriaceae at a university hospital in Morocco. Clin Microbiol Infect. 2014;20(4):350–354. doi:10.1111/1469-0691.12325
31. Moyo SJ, Aboud S, Kasubi M, Lyamuya EF, Maselle SY. Antimicrobial resistance among producers and non-producers of extended spectrum beta-lactamases in urinary isolates at a tertiary hospital in Tanzania. BMC Res Notes. 2010;3(1):348. doi:10.1186/1756-0500-3-348
32. Ogefere HO, Aigbiremwen PA, Omoregie R. Extended-spectrum beta-lactamase (ESBL)–producing gram-negative isolates from urine and wound specimens in a tertiary health facility in southern Nigeria. Trop J Pharm Res. 2015;14(6):1089–1094. doi:10.4314/tjpr.v14i6.22
33. Ouedraogo A-S, Sanou M, Kissou A, et al. High prevalence of extended-spectrum ß-lactamase producing Enterobacteriaceae among clinical isolates in Burkina Faso. BMC Infect Dis. 2016;16(1):326.
34. Obeng-Nkrumah N, Twum-Danso K, Krogfelt KA, Newman MJ. High levels of extended-spectrum beta-lactamases in a major teaching hospital in Ghana: the need for regular monitoring and evaluation of antibiotic resistance. Am J Trop Med Hyg. 2013;89(5):960–964.
35. Alfola MMHR, Kamel Z, Nada MGED, Rashed LA, El-Awady BA. Phenotypic and genotypic characterization of ESBL-producing Escherichia coli and Klebsiella pneumonia isolates from patient’s urine specimens. Int Arab J Antimicrob Agents. 2017;6(4).
36. Kateregga JN, Kantume R, Atuhaire C, Lubowa MN, Ndukui JG. Phenotypic expression and prevalence of ESBL-producing Enterobacteriaceae in samples collected from patients in various wards of Mulago Hospital, Uganda. BMC Pharmacol Toxicol. 2015;16(1):14. doi:10.1186/s40360-015-0013-1
37. Sanneh B, Kebbeh A, Jallow HS, et al. Prevalence and risk factors for faecal carriage of extended spectrum β-lactamase producing Enterobacteriaceae among food handlers in lower basic schools in West Coast Region of The Gambia. PLoS One. 2018;13(8):e0200894. doi:10.1371/journal.pone.0200894
38. Abera B, Kibret M, Mulu W, Kirk M. Extended-spectrum beta (β)-lactamases and antibiogram in Enterobacteriaceae from clinical and drinking water sources from Bahir Dar City, Ethiopia. PLoS One. 2016;11(11):e0166519. doi:10.1371/journal.pone.0166519
39. Calbo E, Romaní V, Xercavins M, et al. Risk factors for community-onset urinary tract infections due to Escherichia coli harbouring extended-spectrum β-lactamases. J Antimicrob Chemother. 2006;57(4):780–783. doi:10.1093/jac/dkl035
40. Briongos‐Figuero L, Gómez‐Traveso T, Bachiller‐Luque P, et al. Epidemiology, risk factors and comorbidity for urinary tract infections caused by extended‐spectrum beta‐lactamase (ESBL)‐producing enterobacteria. Int J Clin Pract. 2012;66(9):891–896. doi:10.1111/j.1742-1241.2012.02991.x
41. Okomo UA. Neonatal Infections; a Hospital-Based Study in the Gambia Examining Aetiology and Associated Maternal Colonisation. London School of Hygiene & Tropical Medicine; 2018.
42. Alyamani EJ, Khiyami AM, Booq RY, Majrashi MA, Bahwerth FS, Rechkina E. The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia. Ann Clin Microbiol Antimicrob. 2017;16(1):1. doi:10.1186/s12941-016-0177-6
43. Schwaber MJ, Navon-Venezia S, Schwartz D, Carmeli Y. High levels of antimicrobial coresistance among extended-spectrum-β-lactamase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2005;49(5):2137–2139. doi:10.1128/AAC.49.5.2137-2139.2005
44. Esiobu N, Armenta L, Ike J. Antibiotic resistance in soil and water environments. Int J Environ Health Res. 2002;12(2):133–144.
45. Leski TA, Taitt CR, Bangura U, et al. High prevalence of multidrug resistant Enterobacteriaceae isolated from outpatient urine samples but not the hospital environment in Bo, Sierra Leone. BMC Infect Dis. 2016;16(1):167. doi:10.1186/s12879-016-1495-1
46. Dagher C, Salloum T, Alousi S, Arabaghian H, Araj GF, Tokajian S. Molecular characterization of carbapenem resistant Escherichia coli recovered from a tertiary hospital in Lebanon. PLoS One. 2018;13(9):e0203323. doi:10.1371/journal.pone.0203323
47. Moges F, Setegn Eshetie WA, Mekonnen F, et al. High prevalence of extended-spectrum beta-lactamase-producing gram-negative pathogens from patients attending Felege Hiwot Comprehensive Specialized Hospital, Bahir Dar, Amhara region. PLoS One. 2019;14(4):e0215177. doi:10.1371/journal.pone.0215177
48. Arabaghian H, Salloum T, Alousi S, Panossian B, Araj GF, Tokajian S. Molecular characterization of carbapenem resistant Klebsiella pneumoniae and Klebsiella quasipneumoniae isolated from Lebanon. Sci Rep. 2019;9(1):1–12. doi:10.1038/s41598-018-36554-2
49. Hamdani SS, Bhat BA, Tariq L, et al. Antibiotic resistance: the future disaster. Int J Res Appl Sci Biotechnol. 2020;7(4):133–145. doi:10.31033/ijrasb.7.4.16
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.