Back to Journals » Cancer Management and Research » Volume 12
Feasibility Study of Adjuvant Chemotherapy with Carboplatin and Nab-Paclitaxel for Completely Resected NSCLC
Authors Katsurada N , Tachihara M , Hatakeyama Y, Koyama K, Yumura M, Kiriu T , Dokuni R , Hazama D, Tokunaga S , Tamura D, Nakata K , Yamamoto M , Kamiryo H , Kobayashi K, Tanaka Y, Maniwa Y, Nishimura Y
Received 27 November 2019
Accepted for publication 22 January 2020
Published 3 February 2020 Volume 2020:12 Pages 777—782
DOI https://doi.org/10.2147/CMAR.S239647
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Naoko Katsurada,1 Motoko Tachihara,1 Yukihisa Hatakeyama,1 Kiyoko Koyama,1 Masako Yumura,1 Tatsunori Kiriu,1 Ryota Dokuni,1 Daisuke Hazama,1 Shuntaro Tokunaga,1 Daisuke Tamura,1 Kyosuke Nakata,1 Masatsugu Yamamoto,1 Hiroshi Kamiryo,1 Kazuyuki Kobayashi,1 Yugo Tanaka,2 Yoshimasa Maniwa,2 Yoshihiro Nishimura1
1Division of Respiratory Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan; 2Division of General Theocratic Surgery, Kobe University Graduate School of Medicine, Kobe, Japan
Correspondence: Motoko Tachihara
Division of Respiratory Medicine, Department of Internal Medicine, Kobe University Graduate School of Medicine, 7-5-1 Kusunoki-cho, Chuo-ku, Kobe 650-0017, Japan
Tel +81-78-382-5660
Fax +81-78-382-5661
Email [email protected]
Purpose: Adjuvant chemotherapy with cisplatin (CDDP) plus vinorelbine is the standard regimen for the treatment of non-small cell lung cancer (NSCLC). However, CDDP elicits severe toxic effects, including emesis, neurotoxicity, and renal damage; carboplatin (CBDCA) may be a feasible alternative for CDDP-unfit patients. CBDCA plus paclitaxel (PTX) adjuvant chemotherapy showed a survival benefit for patients with stage IB tumors > 4 cm in size, while CBDCA plus nanoparticle albumin-bound (nab)-PTX showed greater efficacy and lower neurotoxicity than CBDCA plus PTX in advanced NSCLC. Here, we investigated the feasibility of using CBDCA plus nab-PTX as adjuvant chemotherapy for NSCLC.
Patients and Methods: Patients with completely resected stage II or III NSCLC, with an Eastern Cooperative Oncology Group performance status of 0– 1 and adequate kidney function, received four cycles of postoperative adjuvant chemotherapy with CBDCA (AUC=5 mg/mL/min, on day 1) and nab-PTX (100 mg/m2, on days 1, 8, and 15) administered every 4 weeks within 8 weeks after surgery. The study was designed as a prospective, single-center, Phase II study. The primary endpoint was the completion rate of chemotherapy; secondary endpoints were two-year relapse-free survival (RFS) and safety. The expected completion rate was 80%, with a 50% lower limit.
Results: Of 21 enrolled patients, 18 (85.7%) were CDDP-unfit owing to age (≥ 75 years old [n=11, 52.4%]) or mild renal impairment (n=7, 33.3%). Nineteen of the 21 enrolled patients were assigned to the intervention. The most common grade 3 or 4 adverse events were neutropenia (n=15, 78.9%) and anemia (n=3, 15.8%). The completion rate for the four cycles was 63.2% (95% CI, 38.4– 83.7). Two-year RFS was 56.8% (95% CI, 29.7– 76.9).
Conclusion: The completion rate for CBDCA plus nab-PTX as adjuvant chemotherapy for CDDP-unfit NSCLC patients did not reach treatment feasibility. Further dose modifications may be required in future studies.
Keywords: nab-paclitaxel, carboplatin, adjuvant chemotherapy, non-small cell cancer
Introduction
Lung cancer is the leading cause of cancer death worldwide.1 Even if completely resected in early stages, lung cancer recurrence is not uncommon. According to the Japanese nationwide registry of cases of resected lung cancer, the 5-year survival rates by pathological stage in the seventh edition of the TNM classification were 64.1% for stage IIA, 56.1% for stage IIB, and 47.9% for stage IIIA.2 Adjuvant chemotherapy has the potential to improve survival rates. A Lung Adjuvant Cisplatin Evaluation (LACE) study showed a 5-year survival benefit of 5.4% with cisplatin (CDDP)-based adjuvant chemotherapy, and 5-year survival showed an 8.9% improvement with CDDP plus vinorelbine as an adjuvant chemotherapy.3 CDDP-based adjuvant chemotherapy is the standard treatment regimen for patients with completely resected stage II or III non-small cell lung cancer (NSCLC). However, according to a LACE study, the completion rate for CDDP plus vinorelbine adjuvant chemotherapy was only 67%.4 Additionally, CDDP can elicit severe toxic effects, including renal damage, frequent emetic toxicity, and neurotoxicity, and requires large fluid loads to protect the kidney. Consequently, CDDP is unsuitable for patients with comorbidities or elderly patients.5
Carboplatin (CBDCA) has been proposed as a feasible alternative to CDDP for these patients. CBDCA has significantly less toxicity, including reduced nausea and vomiting, and can be administered to patients with mild renal impairment.6 In a meta-analysis of patients with advanced NSCLC, CBDCA and CDDP showed a similar survival advantage; however, chemotherapy with CDDP plus a third-generation drug was associated with longer survival compared with CBDCA plus a third-generation agent in a subgroup analysis, and the CDDP-based regimen had a higher objective response rate when compared with that of CBDCA.6 The Cancer and Leukemia Group B (CALGB) 963 trial investigated adjuvant chemotherapy for stage IB NSCLC, and showed that CBDCA plus paclitaxel (PTX) produced a survival benefit in patients presenting with tumors larger than 4 cm in diameter, although no overall survival benefit was indicated.7 CALGB 963 is the only large study to show the efficacy of adjuvant chemotherapy with CBDCA plus a third-generation drug, and no large clinical trial to date has shown a survival benefit for patients with completely resected stage II and III NSCLC using a CBDCA-based chemotherapy regimen.
Nanoparticle albumin-bound (nab)-PTX is a 130-nm albumin-bound formulation of paclitaxel that can reach the tumor microenvironment more efficiently than solvent-based (sb)-PTX.8 A large multicenter international randomized Phase III trial in patients with advanced NSCLC (CA 031) showed that CBDCA (area under the concentration-time curve [AUC]=6 mg/mL/min, on day 1) plus nab-PTX (100 mg/m2, on days 1, 8, 15) administered every 3 weeks produced a significantly higher overall response rate, as well as a 10% improvement in overall survival, than sb-PTX.9 Moreover, nab-PTX also showed significantly less neuropathy, neutropenia, arthralgia, and myalgia than sb-PTX. Based on these findings, CBDCA plus nab-PTX would be expected to have similar efficacy and more safety as adjuvant chemotherapy for NSCLC patients than CDDP-based adjuvant therapies.
To investigate the feasibility of utilizing CBDCA plus nab-PTX as adjuvant chemotherapy for patients with completely resected stage II or III NSCLC, we conducted a phase II study of CBDCA (AUC=5 mg/mL/min, on day 1) plus nab-PTX (100 mg/m2, on days 1, 8, 15) administered every 4 weeks. In the CA 031 trial, CBDCA (AUC=6 mg/mL/min) was administered on day 1 and nab-PTX (100 mg/m2) on days 1, 8, and 15 every 3 weeks, and dose reductions and delays in nab-PTX administration were frequently observed because of toxicities.9 In the present study, therefore, we reduced the CBDCA dose to AUC=5 mg/mL/min, and extended the interval between every cycle to 4 weeks.
Materials and Methods
Patients and Treatment
Patients over 20 years of age with completely resected stage II to IIIA NSCLC were enrolled. Additional inclusion criteria were an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1, patients who were judged unsuitable to receive the CDDP-based regimen or did not want to receive the CDDP-based regimen as adjuvant chemotherapy, and adequate kidney (level of serum creatinine ≤1.5 mg/dL), liver, and bone marrow function. We set no upper age limit on enrolment. Surgery have completed 4–8 weeks before enrollment. Patients were excluded who had infectious diseases, fever, other malignancy, peripheral neuropathy and severe comorbidities such as cardiac diseases, uncontrolled diabetes mellitus. Pregnant and lactating women were also excluded. patients were administered four cycles of CBDCA (AUC=5 mg/mL/min, on day 1) and nab-PTX (100 mg/m2, on days 1, 8, and 15) as postoperative adjuvant chemotherapy every 4 weeks within 8 weeks after surgery. Chemotherapy was stopped if tumor recurrence was detected or the following discontinuation criteria were met: 1) the next cycle was delayed for more than 28 days; 2) occurrence of drug-induced pneumonitis grade 2 or worse; 3) detection of grade 4 or worse nonhematologic toxicities; and 4) deterioration of the patient’s condition due to severe toxicity or worsening comorbidities.
This study was approved by the ethics committee of Kobe University (250029) and was conducted in accordance with the Helsinki declaration. All patients provided written informed consent before enrollment. This study is registered in the University Medical Hospital Information Network in Japan (UMIN000011910). https://upload.umin.ac.jp/cgi-ope bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000013609&language=J
Assessment of Patients
Pathological stages were determined based on the seventh edition of the Union for International Cancer Control Manual of Clinical Oncology.10 Toxicities were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 (http://ctep.cancer. gov/reporting/ctc.html).
After completion or discontinuation of the study regimen, all the patients were followed up until death or the cut-off date (July 2019). All the patients underwent a physical examination, laboratory tests, and chest x-ray every 3 months, and chest and abdominal computed tomography (CT) was performed every 6 months. Other tests were performed if necessary. Chest and abdominal CT, brain magnetic resonance imaging or brain CT, and 18F-FDG-PET were performed 2 years after registration.
Statistical Analyses
The study was designed as a prospective, single-center, phase II trial. The primary endpoint was to evaluate the completion rate of four cycles of CBDCA plus nab-PTX as adjuvant chemotherapy for CDDP-unfit NSCLC patients, while the secondary endpoints were to evaluate two-year relapse-free survival (RFS) and safety. The sample size was calculated assuming an 80% completion rate with a lower limit of 50%, with the alpha level set at 0.05 (both-sided) and detection power of 80%. The minimum sample size was calculated as 19, and was set at 21 assuming a 10% exclusion or dropout rate. Relapse-free survival was analyzed using the Kaplan–Meier method to estimate the median points with 95% confidential intervals (CIs). All statistical analyses were performed using EZR version 1.38 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (version 3.3.2; R Foundation for Statistical Computing, Vienna, Austria).11
Results
A total of 21 patients were enrolled in this study from January 2014 to January 2019. Two patients were excluded before intervention because one experienced a recurrence and the other presented with new ground glass opacities before starting therapy. Therefore, 19 patients were assigned to the intervention (Figure 1). The baseline characteristics of the intention-to-treat patients are shown in Table 1. The median age was 73 years (range, 53–83). Five patients (23.8%) were at stage IIA, 3 (14.3%) at stage IIB, and 13 (61.9%) at stage IIIA. The histopathological subtypes included 14 (66.7%) adenocarcinomas and 6 (28.6%) squamous cell carcinomas.
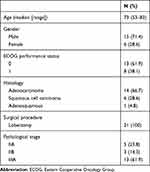 |
Table 1 Patient Characteristics |
 |
Figure 1 CONSORT flow chart of the study. |
Twelve patients completed the four cycles of adjuvant chemotherapy, representing a completion rate of 63.2% (95% CI, 38.4–83.7). First-step dose reduction was required in 6 patients (31.6%) owing to the occurrence of grade 3 neutropenia (n=5) and the physicians’ discretion (n=1). Nab-PTX administration on days 8 and 15 was skipped in 1 of 57 cycles and 41 of 57 cycles (71.9%), respectively. The most frequent causes for skipping administration were grade 3 or 4 neutropenia. For nab-PTX, the median cumulative dose was 685 mg/m2, and the median dose intensity was 46 mg/m2/week. For CBDCA, the median cumulative dose was 941 mg/m2, and the median dose intensity was 59 mg/m2/week. The relative dose intensity was 61.0% (range 8.3–100.0) for nab-PTX and 89.1% (range 25.0–114.3) for CBDCA (Table 2). The reasons for early discontinuation of chemotherapy before completion of four cycles were febrile neutropenia (n=2), patient requiring a two-step dose reduction due to neutropenia (n=1), empyema (n=1), drug-induced pneumonitis (n=1), renal failure (n=1), diarrhea (n=1), and patient refusal due to fatigue (n=1). The treatment adverse events are summarized in Table 3. The most common grade 3 or 4 adverse event was neutropenia (n=15, 78.9%), followed by anemia (n=3, 15.8%). Febrile neutropenia, grade 3 pneumonitis, and grade 1 peripheral sensory neuropathy were observed in 10.5%, 5.3%, and 31.6% of the 19 patients, respectively.
 |
Table 2 Dose Delivery |
 |
Table 3 Common Treatment-Related Adverse Events |
All 19 patients who had intervention were followed up, and the two-year RFS was 56.8% (95% CI, 29.7–76.9). The median follow-up time was 33.4 months (range, 5.4–66.1 months). The RFS curve is shown in Figure 2.
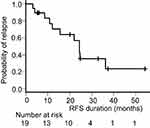 |
Figure 2 Kaplan–Meier curve of recurrence-free survival (RFS) of patients receiving assigned intervention. |
Discussion
In the present study, the completion rate for CBDCA plus nab-PTX as adjuvant chemotherapy for CDDP-unfit NSCLC patients did not reach the assumed lower limit for treatment feasibility. The completion rate for the present study was similar to that previously reported for CDDP-based adjuvant chemotherapy (63.2% vs 57%).4 Only one other study has evaluated the feasibility of utilizing CBDCA plus nab-PTX as adjuvant chemotherapy for completely resected NSCLC.12 Here, the authors reported a completion rate of 75.9% for adjuvant chemotherapy with 3-week cycles of nab-PTX (100 mg/m2) on days 1 and 8, followed by CBDCA (AUC=6 mg/mL/min) on day 1,12 with a median relative dose intensity of 82% for nab-PTX and 78% for CBDCA. Several reasons could help explain the lower completion rate of the present study. First, the patients in our study were older, with a median age of 73 years, compared to 69 years for the previous study. Second, patients who were judged unsuitable to receive the CDDP-based regimen were assigned to the current study. Most of these patients (n=18, 85.7%) were unfit for CDDP owing to age (over 75 years old [n=11, 52.4%]) or mild renal impairment (n=7, 33.3%). Moreover, there were more PS 1 patients in our study than in the previous study (38.1% vs 10.3%). Although age in itself is not a contraindication to a CDDP-based regimen, frailer patients, or those with an increased number and/or severity of comorbidities, may have been included in our study than in the previous trial. To date, no prospective randomized studies of adjuvant chemotherapy for elderly patients have been carried out. A few retrospective or post hoc analyses of prospective studies have indicated that adjuvant chemotherapy for elderly patients may be feasible, and may also produce a significant survival benefit, although older patients received a lower dose intensity than younger ones.13,14 However, the proportion of very elderly patients in these studies was very small, and these eligible patients may have had fewer comorbidities than that normally observed in regular clinical practice, so caution is needed to judge adaptation of adjuvant chemotherapy to elderly patients. Pooled analysis of the LACE study showed that patients with better PS gained more benefit from adjuvant chemotherapy, and administration of a CDDP-based adjuvant chemotherapy regimen was harmful to PS 2 patients.13
In this study, the treatment schedule and dose were modified compared with those of the CA 031 regimen. In the CA 031 study, the dose intensity of nab-PTX was 82 mg/m2/week, and that of CBDCA 166 mg/m2/week.9 In the present study, dose delivery was significantly lower than that of CA 031. In a study for elderly patients with advanced NSCLC, CBDCA (AUC=6 mg/mL/min) was administered on day 1, and nab-PTX (75 mg/m2, lower than in our study) was administered on days 1, 8, 15 every 4 weeks.15 The nab-PTX dose intensity was similar to that of the present study (45 mg/m2/week vs 46 mg/m2/week); however, the completion rate was not provided for that study. Further treatment schedule and dose modifications may be needed for adjuvant chemotherapy settings.
In the present study, hematologic adverse events were frequent, although grade 3 or 4 sensory neuropathy was not observed. Although the toxicity profile was similar to that found in the CA 031 study, the frequency of grade 3 or 4 neutropenia was higher in the present study (78.9% vs 47%).The reason may be that the median age of patients in the present study were older than that of CA 031 study (73 years vs 60 years)9 and 33% of patients in this study had mild renal impairment. And surgery may influence patient’s conditions. This findings indicates that dose modification for adjuvant chemotherapy is needed for elderly patients because of the severe hematologic toxicity elicited with our regimen.
The two-year RFS in this study was similar to that recorded in a recent study of CDDP-based adjuvant chemotherapy (56.8% vs 62%).16 The regimen used in the present study might be effective; however, the dose intensity was lower than for the standard CBDCA plus nab-PTX regimen currently used for advanced NSCLC, and 5-year survival data are required to properly evaluate its efficacy.
The present study had some limitations. First, the sample size was relatively small. Nonetheless, it was large enough to evaluate the feasibility of adjuvant chemotherapy as a phase II study. Second, selection bias may have occurred because another CDDP-based adjuvant chemotherapy study was being conducted in our institution in the same period. Therefore, most of our patients were unfit for a CDDP-based regimen. However, such patient status is similar to that seen in regular clinical practice, where CDDP-fit patients are usually administered CDDP plus vinorelbine, the standard adjuvant chemotherapy regimen. Therefore, the present study reflects adjuvant chemotherapy settings in regular clinical practice. Although the anticipated tolerability was not achieved in our study, the feasibility of using adjuvant chemotherapy with CBDCA plus nab-PTX has previously been reported.12 Consequently, a phase III clinical trial comparing the efficacy of CBDCA plus nab-PTX with that of a CDDP-based regimen as adjuvant chemotherapy is warranted if the regimen can be rendered safer and less toxic.
Conclusions
This regimen of CBDCA plus nab-PTX as adjuvant chemotherapy for CDDP-unfit NSCLC patients was not tolerable. Dose and schedule modification may be required in future studies.
Data Sharing Statement
The overview of this trial has been registered under the UMIN Clinical Trials Registry Identifier UMIN000011910. Individual patient data are confidential and thus they cannot be made publicly available.
Acknowledgments
The authors would like to thank all patients and investigators who participated in this study. The abstract of this paper was presented at the world conference on lung cancer 2019 as a poster presentation with interim findings. The poster’s abstract was published in Journal of Thoracic Oncology. https://www.jto.org/article/S1556-0864(19)32599-7/abstract.
Disclosure
Dr Yoshihiro Nishimura reports grants from Taiho Pharmaceutical Co., Ltd, during the conduct of the study. The authors report no other conflicts of interest in this study.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.v68.6
2. Okami J, Shintani Y, Okumura M, et al.; For the Japanese Joint Committee of Lung Cancer Registry. Demographics, safety and quality, and prognostic information in both the seventh and eighth editions of the TNM classification in 18,973 surgical cases of the Japanese joint committee of lung cancer registry database in 2010. J Thorac Oncol. 2019;14(2):212–222. doi:10.1016/j.jtho.2018.10.002
3. Pignon JP, Tribodet H, Scagliotti GV, et al.; For the LACE Collaborative Group. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26(21):3552–3559. doi:10.1200/JCO.2007.13.9030
4. Douillard JY, Tribodet H, Aubert D, et al. Adjuvant cisplatin and vinorelbine for completely resected non-small cell lung cancer: subgroup analysis of the lung adjuvant cisplatin evaluation. J Thorac Oncol. 2010;5(2):220–228. doi:10.1097/JTO.0b013e3181c814e7
5. Cheong KA, Chrystal K, Harper PG. Management of the elderly patient with advanced non-small cell lung cancer. Int J Clin Pract. 2006;60(3):340–343. doi:10.1111/ijcp.2006.60.issue-3
6. Hotta K, Matsuo K, Ueoka H, Kiura K, Tabata M, Tanimoto M. Meta-analysis of randomized clinical trials comparing cisplatin to carboplatin in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2004;22(19):3852–3859. doi:10.1200/JCO.2004.02.109
7. Strauss GM, Herndon JE
8. Desai N, Trieu V, Yao Z, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–1324. doi:10.1158/1078-0432.CCR-05-1634
9. Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–2062. doi:10.1200/JCO.2011.39.5848
10. Sobin LH, Gospodarowicz MK, Wittekind C. International Union Against Cancer, Ebrary Inc, TNM Classification of Malignant Tumours. Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell; 2009.
11. Kanda Y. Investigation of the freely available easy-to-use software EZR” for medical statistics. Bone Marrow Transpl. 2012;48(3):452–458. doi:10.1038/bmt.2012.244
12. Saji H, Marushima H, Miyazawa T, et al. Feasibility study of adjuvant chemotherapy with modified weekly nab-paclitaxel and carboplatin for completely resected non-small-cell lung cancer: FAST-nab. Anticancer Drugs. 2017;28(7):795–800. doi:10.1097/CAD.0000000000000512
13. Früh M, Rolland E, Pignon JP, et al. Pooled analysis of the effect of age on adjuvant cisplatin-based chemotherapy for completely resected non-small-cell lung cancer. J Clin Oncol. 2008;26(21):3573–3581. doi:10.1200/JCO.2008.16.2727
14. Pepe C, Hasan B, Winton TL, et al. National Cancer Institute of Canada and Intergroup Study JBR.10. Adjuvant vinorelbine and cisplatin in elderly patients: national Cancer Institute of Canada and Intergroup Study JBR.10. J Clin Oncol. 2007;25(12):1553–1561. doi:10.1200/JCO.2006.09.5570
15. Miyauchi E, Inoue A, Usui K, et al. Phase II study of modified carboplatin plus weekly nab-paclitaxel in elderly patients with non-small cell lung cancer: North Japan Lung Cancer Study Group Trial 1301. Oncologist. 2017;22:640–e59. doi:10.1634/theoncologist.2017-0059
16. Kenmotsu H, Ohde Y, Wakuda K, et al. Survival data for postoperative adjuvant chemotherapy comprising cisplatin plus vinorelbine after complete resection of non-small cell lung cancer. Cancer Chemother Pharmacol. 2017;80(3):609–614. doi:10.1007/s00280-017-3400-z
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
