Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Feasibility Study for the Long-Term Management of Refractory Hyperkeratotic Eczema with Calcipotriol and Betamethasone Dipropionate (Daivobet®), Viaminate and Concomitant Conventional Therapies: A Retrospective Study
Authors Nabatanzi A , Mafuru M, Male M, Tian C, Zhang L, Wu T, Wu S, Huang C
Received 9 August 2020
Accepted for publication 26 September 2020
Published 29 October 2020 Volume 2020:13 Pages 789—794
DOI https://doi.org/10.2147/CCID.S276148
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Amelia Nabatanzi,1 Magesa Mafuru,2 Musa Male,3 Chunxia Tian,4 Lingyun Zhang,1 Ting Wu,1 Shidi Wu,1 Changzheng Huang1
1Department of Dermatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2Department of Pharmacology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 3Department of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 4Department of Integrated Traditional Chinese and Western Medicine, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
Correspondence: Changzheng Huang
Department of Dermatology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, People’s Republic of China
Tel +86-13971176116
Fax +86-27-85768188
Email [email protected]
Background: The available treatments for refractory hyperkeratotic eczema are inadequate with frustrating results. We, therefore, incorporated Calcipotriol and Betamethasone Dipropionate (Daivobet®), and Viaminate into the mainstay treatment to improve the clinical symptoms. The study aimed to evaluate the efficacy of Daivobet® and Viaminate as a potential treatment alternative for refractory hyperkeratotic eczema.
Patients and Methods: Between 2013 and 2015, 61 patients diagnosed with refractory hyperkeratotic eczema (RHE) who had shown inadequate response to conventional therapies were pooled from a single center. Besides, they were all treated with Daivobet®, Viaminate, and an occlusive dressing mixture containing 5% salicylic acid ointment and 25% zinc oxide paste following inadequate response to conventional therapies (corticosteroids plus 25% zinc oxide paste and 5% salicylic acid ointment). Investigators Global Assessment (IGA) and Patient-Oriented Eczema Measure (POEM) assessed baseline and outcome measures for the degree of hyperkeratinization (0-clear; 3-moderate; 4-severe).
Results: Of the 61 patients, 49 (80.3%) patients presented with moderate RHE and 12 (19.7%) with severe RHE. After 24 weeks of treatment, the period for loss of keratinization was significantly lower in patients with moderate RHE (3.9± 1.9 weeks) than those with severe RHE (10.8± 1.0 weeks) with a P-value < 0.01. Furthermore, they required a significantly shorter total treatment duration (10.6 ± 4.3 weeks) than those with severe RHE (20.3± 3.6 weeks) with a P-value of < 0.01. However, there were no significant differences in post hoc analysis at week 36 with P-values of 0.46 and 1.00 for IGA and POEM, respectively.
Conclusion: Our results showed that the incorporation of Viaminate and Daivobet® into mainstay treatment was effective and safe for the long-term management of RHE.
Keywords: refractory, hyperkeratosis, eczema, Viaminate, Daivobet®
Plain Language Summary
What is known?
- The available treatment options for patients with hyperkeratotic eczema are inadequate with dissatisfying results.
- Systemic use of corticosteroids is not recommended because of the serious adverse effects.
- Daivobet® is a two-compound product that has shown efficacy and safety in treating chronic inflammatory conditions with abnormal keratinization such as psoriasis and Olmsted syndrome.
What does this study add?
- Our results showed positive effects with the incorporation of Daivobet® and Viaminate to mainstay treatment without the use of rescue treatments such as topical calcineurin inhibitors.
- Both Daivobet® and Viaminate have an acceptable safety profile, and our designed treatment protocol improved the clinical severity and patient’s quality of life.
Introduction
Hyperkeratotic eczema is a monomorphic subtype of chronic eczema with a multifactorial etiology;1–4 clinically characterized by itching, fissures, and severe keratinization. The condition is highly stigmatizing and often requires long-term treatment. Data on the currently available treatments have shown no uniform efficacy for hyperkeratotic eczema despite treatment adherence and long-term use.5,6 Treatment guidelines do not recommend the long-term use of systemic corticosteroids, immunosuppressants, and photo-therapy because the risks outweigh the benefits.7 Therefore, there is a need for extensive research for safe and effective long-term treatments for refractory hyperkeratotic eczema (RHE).
Of late, there is accumulating evidence showing the effective use of Calcipotriol and Betamethasone Dipropionate (Daivobet®) which is both tolerable and adherent for the treatment of other skin disorders with abnormal keratinization.8,9 Viaminate is a retinoic acid derivative attributed to the inhibition of keratin, differentiation of epithelial cells of the skin, and anti-inflammatory effects. Both Daivobet® and Viaminate have been used to treat psoriasis a chronic condition with abnormal keratinization and an ongoing inflammation.10,11 Because of their role in the pathophysiology, we designed a treatment protocol aimed at targeting: ① hyperkeratosis; ② inflammation; and ③ immune response. The approach used for all our patients included; Daivobet® ointment, Viaminate capsules, an occlusive dressing mixture of 25% zinc oxide paste, and 5% salicylic acid ointment.
The chronic course of RHE makes treatment very challenging with unsatisfactory results. This study aimed to evaluate the efficacy of a possible treatment alternative for RHE in patients who showed inadequate response with conventional therapies (corticosteroids plus 25% zinc oxide paste and 5% salicylic acid ointment). The treatment was used daily in order to mimic clinical practice, and the monthly therapeutic response was evaluated using photographic comparison.
Patients and Methods
Study Design and Participants
Between 2013 and 2015, 61 patients diagnosed with refractory hyperkeratotic eczema (RHE) with inadequate response to conventional therapies were pooled from the archive of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Inclusion Criteria
Patients aged 15 years old and above; hyperkeratotic eczema present for more than 1 year before enrollment; documentation of the inadequate response to conventional therapies within 6 months or more before enrollment; Investigators Global Assessment (IGA) score of 3 or higher on the scale of 0–4 (0= clear with no inflammatory lesions, 1= perceptible erythema, 2= mild erythema and papulation, 3= moderate erythema and papulation, 4= severe erythema and papulation); Patient-Oriented Eczema Measure (POEM) score of 8 or higher12 at baseline (0–2= clear/almost clear, 3–7= mild, 8–16= moderate, >17= severe).
Exclusion Criteria
Patients with mild RHE, history of psoriasis, and palmoplantar keratoderma, liver and kidney function disease, pregnant women, as well as breastfeeding mothers.
RHE was clinically defined as pruritus, fissures, and severe keratinization on the hands and feet.13 Severe keratinization and fissures were taken to be primary outcome of the disease and severe pruritus as secondary outcome.
Patients were stratified based on disease severity (IGA=3 and POEM=8-16 for moderate, and IGA=4 and POEM>17 for severe). The two co-primary endpoints were the percentage of patients with both IGA 0/1 (clear/almost clear; on a 0–4 scale) and >2-point reduction, as well as a 90% POEM (POEM-90) improvement efficacy from baseline and week 36. The secondary efficacy endpoint was defined as the percentage improvement or reduction of severe pruritus from baseline to week 24 and sustained to week 36.
Photographic documentations were done on day one of enrollment, and every time patients returned throughout the course of treatment and follow-up.
Treatment Procedure
Twenty-five percent Zinc oxide paste was mixed with 5% salicylic acid ointment. The formulation was applied on a clean gauze to cover the area every night before patients went to bed. During the day, patients used topical Daivobet twice a day every after 6 hours and took 25 mg Viaminate orally once a day (after a meal). The dosage of Viaminate capsules could be altered or terminated based on disease severity (terminated after clearance of keratinization or adjusted to a maximum of 50 mg for severe cases). No rescue treatments (such as topical calcineurin inhibitors or corticosteroids) were used during this study. After clearance of abnormal keratinization, patients were switched to daily use of vitamin E moisturizer and Daivobet® to manage erythema lesions (three times a week for a month and then twice a week for the following months). During follow-up, any patients reporting recurrence resumed Daivobet® treatment once a day for 1–2 weeks, and then the treatment terminated. Throughout the course of treatment, the demographics, clinical symptoms, and treatment efficacy of all patients were assessed. Patients were monitored for any adverse reactions such as toxicity, redness and dry skin, comorbid diseases including asthma, rhinitis and atopy, and drug interactions with concomitant medications. Study visits were done in person once a month for the first 24 weeks, followed by hotline inquiries for the remaining 12 weeks.
The ethics committee of Union Hospital of Tongji Medical College of Huazhong University of Science and Technology approved the study. All patients and parents of patients under 18 years signed informed consent. The study was conducted in accordance with the Declaration of Helsinki.
Statistical Analysis
Continuous variables were expressed as mean ± SD and median, while categorical variables as the percentage of distribution of each category. Comparisons between the two groups were performed using Student’s t-test for continuous variables and Chi-square (χ2) or Fisher’s exact test for categorical data. Data were analyzed using IBM Statistical Package for Social Sciences (SPSS) program, version 20. A two-tailed P-value of ˂0.05 level was considered statistically significant.
Results
Patients’ Characteristics
The baseline demographics and clinical characteristics of patients pooled are shown in Table 1. Of the 61 patients diagnosed and treated for RHE that were pooled for the study, 40 were male and 21 were female. The proportion of males was higher than that of females in a ratio of 2:1. The mean age and standard deviation of the patients were 47.28 ±11.05 years.
 |
Table 1 Baseline Demographics and Clinical Characteristics |
Of the 61 patients, 49 (80.3%) patients presented with moderate RHE and 12 (19.7%) patients with severe RHE. Moreover, a total of 88.5% complained of itching. Twenty-nine percent had involvement of palms, 27% involved plantaris, 24.6% involved lower legs, and 18.0% involved feet and ankles. Generally, the duration of the disease ranged from 1 to 8 years with the median disease duration of 3 years.
Assessment of Treatment Period
After 24 weeks of treatment, the period for loss of keratinization was significantly lower in patients with moderate RHE (3.9±1.9 weeks) than those with severe RHE (10.8±1.0 weeks) with a P-value<0.01 as shown in Table 2 and Figure 1. Furthermore, they required a significantly shorter total treatment duration (10.6±4.3 weeks) than those with severe RHE (20.3±3.6 weeks) with a P-value of <0.01.
 |
Table 2 Assessment of Treatment Period for Moderate and Severe Disease |
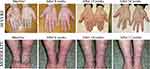 |
Figure 1 Showing linear changes of loss of keratinization from baseline to after treatment. |
Secondary Outcomes
Generally, both treatment groups reported similar secondary outcomes. After 24 weeks of treatment, severe pruritus in both groups improved from 88.5% to 11.5% (P<0.01) (Table 3).
 |
Table 3 Efficacy Outcomes |
Post Hoc Analysis
Post hoc endpoints of >2-point improvement or reduction in IGA, as well as POEM-90 improvement were achieved between 24 and 36 weeks. At week 36, the co-primary endpoints (IGA 0/1 and POEM-90) were 77.6% and 85.7%, respectively, in patients with moderate RHE, and 66.7% and 83.3%, respectively, in patients with severe RHE (p-values of 0.46 and 1.00 for IGA and POEM, respectively) (Table 4).
 |
Table 4 Post Hoc Analysis from Week 24 to Week 36 |
Discussion
The long-term prognosis of refractory hyperkeratotic eczema (RHE) highly depends on a well-designed treatment protocol than the prevention of causative factors; this is because the chronic course of the disease may change its morphology.3 On account of this rationale, our pilot study attempted to assess the off-label incorporation of Daivobet® and Viaminate into mainstay treatment to evaluate the clinical basis of an alternative treatment for RHE.
Currently, the available data have shown no uniform efficacy for hyperkeratotic eczema.5,6 A case study by Egawa K reported 80% improvement of hyperkeratotic palmoplantar eczema with Daivobet® monotherapy after 2–8 weeks of treatment9 while Yang M and Chang JM in their case study reported that 1 out of the 3 patients exhibited a 90% resolution of hyperkeratotic lesions in 5 weeks and the remaining 2 patients showed clearance after 24 weeks.6 However, these two studies did not report on the extent of severity, we, therefore, attempted to analyze the use of Viaminate and Daivobet® in patients with moderate and severe RHE.
In this study, we observed an optimal loss of keratinization and a shorter total treatment period in all the patients. Besides, the patients with moderate RHE exhibited an earlier response to the treatment (3.9±1.9 weeks) than those with severe RHE (10.8±1.0 weeks) (Table 2). The earlier loss of keratinization could be because of the combination of Daivobet® with Viaminate compared to Daivobet® monotherapy. Furthermore, patients with moderate RHE required 10.6±4.3 weeks of total treatment while those with severe RHE required 20.3±3.6 weeks. However, it is hard to compare with any of the previous studies since the patients were not stratified according to severity of the disease.
Treatment improved pruritus and provided extended benefits for pruritus, such as improved quality of life.14 After treatment, our patients demonstrated reduced improvements of pruritus from 88.5% to 11.5%. This is because the calcipotriol in Daivobet® complex regulates the release of cytokines and suppresses the generation of inflammatory cytokines.15
During post hoc analysis, a 77.6% reduction of IGA>2 was observed in patients with moderate RHE and 66.7% reduction of IGA>2 in patients with severe RHE. Moreover, 85.7% POEM-90 was achieved in patients with moderate RHE and 83.3% in patients with severe RHE. The findings from this pilot study demonstrated that the incorporation of Daivobet® and Viaminate into the mainstay treatment greatly improved clinical symptoms at stringent measures of >2 IGA reduction and POEM-90. Moreover, the symptom improvement exhibited in this study, greatly exceeded the 50% improvement from baseline which is regarded as a meaningful clinical response.16
The low flare rates and mild recurrence observed may be attributed to an earlier termination of Daivobet® and Viaminate consequently resulting in the transient anti-inflammatory effects, and yet the inflammatory process is ongoing. The incorporation of Viaminate was on account that it has anti-inflammatory effects, regulates the immune function and differentiation of skin epithelial cells.10 In addition to the anti-inflammatory properties of Daivobet®, it also suppresses keratinocyte proliferation and differentiation, and reduces the mitotic rate of the epidermis.17–19 We maintained the use of the occlusive dressing mixture daily during the night to increase absorption of the Daivobet® ointment. This could explain the rapid loss of keratinization in this study.
The chronic course of RHE requires long-term management, and currently, the treatment guidelines do not recommend the long-term use of systemic corticosteroids, immunosuppressants, and photo-therapy because the risks such as skin irritation, atrophy, and folliculitis outweigh the benefits.7 In the present study, the safety profile was consistent with other studies,6 and there were no new safety signals reported. The use of Daivobet® a formulation of the fixed-dose combination of two synthetics caused a steroid-sparing effect and conversely, the steroid may suppress skin irritation caused by calcipotriol.15 Besides, there were no signals for hepatotoxicity observed with Viaminate as previously reported.20 The difference in our findings as regards to adverse reactions could be because of the early terminating of Viaminate and the intermittent use of Daivobet® once keratinization had been cleared.
The current guidelines of managing hyperkeratotic eczema recommend conventional therapies as the mainstay treatment21,22 because of their treatment adherence in chronic diseases. However, the incorporation of Daivobet® and Viaminate in our study, greatly improved the clinical symptoms of RHE with better prognosis and acceptable safety while mimicking treatment protocols in daily clinical practice. These findings may guide the relevant use of an adherent, anti-inflammatory, and anti-immune responsive drugs in patients with chronic and/or RHE. That being said, our findings should be interpreted with caution because of the retrospective nature of the study, lack of a control group along with its small sample size. Hence, the present study is preliminary and a step towards a hypothesis-based study.
In conclusion, our results showed that the incorporation of Viaminate and Daivobet® into mainstay treatment was effective and safe for the long-term management of RHE. Therefore, findings from this pilot study may serve as a reference for designing a randomized control trial of RHE patients to expound more on the long-term efficacy of this treatment in hypersensitive individuals.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Jungersted JM, Hogh JK, Hellgren LI, Wilkinson S, Jemec GB, Agner T. Hand eczema and stratum corneum ceramides. Clin Exp Dermatol. 2015;40(3):243–246. doi:10.1111/ced.12502
2. Molin S, Diepgen TL, Ruzicka T, Prinz JC. Diagnosing chronic hand eczema by an algorithm: a tool for classification in clinical practice. Clin Exp Dermatol. 2011;36(6):595–601. doi:10.1111/j.1365-2230.2010.04001.x
3. Agner T, Aalto-Korte K, Andersen KE, et al. Classification of hand eczema. J Eur Acad Dermatol Venereol. 2015;29(12):2417–2422. doi:10.1111/jdv.13308
4. Johansen JD, Hald M, Andersen BL, et al. Classification of hand eczema: clinical and aetiological types. Based on the guideline of the Danish Contact Dermatitis Group. Contact Dermatitis. 2011;65(1):13–21.
5. Menne T, Johansen JD, Sommerlund M, Veien NK. Danish Contact Dermatitis G. Hand eczema guidelines based on the Danish guidelines for the diagnosis and treatment of hand eczema. Contact Dermatitis. 2011;65(1):3–12. doi:10.1111/j.1600-0536.2011.01915.x
6. Yang M, Chang JM. Successful treatment of refractory chronic hand eczema with calcipotriol/betamethasone ointment: a report of three cases. Exp Ther Med. 2015;10(5):1943–1946. doi:10.3892/etm.2015.2729
7. Diepgen TL, Andersen KE, Chosidow O, et al. Guidelines for diagnosis, prevention and treatment of hand eczema. J Dtsch Dermatol Ges. 2015;13(1):e1–22. doi:10.1111/ddg.12510_1
8. Takeichi T, Tsukamoto K, Okuno Y, et al. A combination of low-dose systemic etretinate and topical calcipotriol/betamethasone dipropionate treatment for hyperkeratosis and itching in Olmsted syndrome associated with a TRPV3 mutation. J Dermatol Sci. 2017;88(1):144–146. doi:10.1016/j.jdermsci.2017.05.012
9. Egawa K. Topical vitamin D3 derivatives in treating hyperkeratotic palmoplantar eczema: a report of five patients. J Dermatol. 2005;32(5):381–386. doi:10.1111/j.1346-8138.2005.tb00911.x
10. JG Q. Compound glycyrrhizic acid glucoside tablets combined with Viaminate Capsules in treatment of vulgaris psoriasis. Mod J Integrated Trad Chin Western Med. 2012;21(17):
11. Wang XD. Viaminate Capsules combined with Tripterygium wilfordii multi glycoside and compound liquorice acid glycoside in the treatment of plaque psoriasis in 80 cases of observation. J Binzhou Med Univ. 2014;21(17):2.
12. Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch Dermatol. 2004;140(12):1513–1519. doi:10.1001/archderm.140.12.1513
13. Yang YS, Byun YS, Kim JH, Park CW, Kim HO. Hyperkeratotic hand eczema due to use of rubber gloves while driving. Ann Dermatol. 2014;26(6):769–771. doi:10.5021/ad.2014.26.6.769
14. Erturk IE, Arican O, Omurlu IK, Sut N. Effect of the pruritus on the quality of life: a preliminary study. Ann Dermatol. 2012;24(4):406–412. doi:10.5021/ad.2012.24.4.406
15. Kragballe K, Austad J, Barnes L, et al. A 52-week randomized safety study of a calcipotriol/betamethasone dipropionate two-compound product (Dovobet/Daivobet/Taclonex) in the treatment of psoriasis vulgaris. Br J Dermatol. 2006;154(6):1155–1160. doi:10.1111/j.1365-2133.2006.07236.x
16. Blauvelt A, de Bruin-weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, Phase 3 trial. Lancet. 2017;389(10086):2287–2303. doi:10.1016/S0140-6736(17)31191-1
17. Li L, Liu P, Li J, et al. [Psychosocial factors of chronic hand eczema]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2017;42(2):179–183. Chinese.
18. Kouris A, Armyra K, Christodoulou C, et al. Quality of life, anxiety, depression and obsessive-compulsive tendencies in patients with chronic hand eczema. Contact Dermatitis. 2015;72(6):367–370. doi:10.1111/cod.12366
19. Cazzaniga S, Ballmer-Weber BK, Grani N, et al. Chronic hand eczema: a prospective analysis of the Swiss CARPE registry focusing on factors associated with clinical and quality of life improvement. Contact Dermatitis. 2018;79(3):136–148. doi:10.1111/cod.13041
20. Zhang H, Zhang J, Dong H. Fatal hepatotoxicity due to viaminate. Am J Med Sci. 2018;356(1):84–86. doi:10.1016/j.amjms.2018.01.001
21. Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682.
22. Katoh N, Ohya Y, Ikeda M, et al. Clinical practice guidelines for the management of atopic dermatitis 2018. J Dermatol. 2019;46(12):1053–1101. doi:10.1111/1346-8138.15090
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
