Back to Journals » Journal of Pain Research » Volume 13
Feasibility of a Randomized Controlled Trial of Paediatric Interdisciplinary Pain Management Using Home-Based Telehealth
Authors Hilyard A , Kingsley J , Sommerfield D, Taylor S , Bear N, Gibson N
Received 24 May 2019
Accepted for publication 18 December 2019
Published 1 May 2020 Volume 2020:13 Pages 897—908
DOI https://doi.org/10.2147/JPR.S217022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Michael A Ueberall
Anna Hilyard,1,2 Julia Kingsley,1 David Sommerfield,1,3,4 Susan Taylor,1,5 Natasha Bear,6 Noula Gibson7,8
1Complex Pain Service, Perth Children’s Hospital, Perth, Western Australia, Australia; 2Discipline of Psychology, College of Science, Health, Engineering and Education, Murdoch University, Perth, Western Australia, Australia; 3Department of Anaesthesia and Pain Management, Perth Children’s Hospital, Perth, Australia; 4Medical School, Faculty of Health and Medical Sciences, The University of Western Australia, Perth, Australia; 5School of Occupational Therapy, Social Work and Speech Pathology, Faculty of Health Sciences, Curtin University, Perth, Western Australia, Australia; 6Department of Child Research, Child Adolescent Health Service, Perth, Western Australia, Australia; 7Physiotherapy Department, Perth Children’s Hospital, Perth, Western Australia, Australia; 8School of Physiotherapy and Exercise Science, Faculty of Health Sciences, Curtin University, Perth, Western Australia, Australia
Correspondence: Anna Hilyard
Complex Pain Service, Perth Children’s Hospital, Locked Bag 2010, Nedlands, Western Australia 6909, Australia
Tel +6186456 4341
Email [email protected]
Purpose: Chronic pain is common in adolescents. Evidence-based guidelines recommend interdisciplinary treatment, but access is limited by geography. The development of hybrid programs utilizing both face-to-face and videoconference treatment may help overcome this. We developed a 7-week hybrid pediatric interdisciplinary pain program (Hybrid-PIPP) and wished to compare it to individual face-to-face sessions (Standard Care). Our objective was to test the feasibility of a protocol that used a matched pair un-blinded randomized controlled design to investigate the efficacy and cost-effectiveness of the Hybrid-PIPP compared to Standard Care.
Patients and Methods: Parent–adolescent dyads were recruited from tertiary pediatric clinics and matched by disability before randomization to minimize allocation bias. The adolescents (aged 11– 17) had experienced primary pain for > 3 months. Hybrid-PIPP involved 11 hrs of group therapy and 4 individual videoconference sessions. Standard care was provided by the same clinical team, using the same treatment model and similar intensity as the Hybrid-PIPP. The intention was to recruit participants for 3 Hybrid-PIPP groups with a comparison stream. Recruitment was ceased after 2 groups due to the high participant disability requiring more intensive intervention.
Results: Eighteen dyads were screened and 13 randomized (7 Hybrid-PIPP, 6 Standard Care, 2 unsuitable, 3 unallocated when the study was stopped). The study met a priori feasibility criteria for staff availability; recruitment rate; treatment completion; and data collection. Global satisfaction ratings were similar in both streams (SC median 7, range 5– 9 and Hybrid-PIPP median 8.5, range 5– 10). Challenges were identified in both streams. A future modified Hybrid-PIPP was considered acceptable if the intensity is increased to manage the high level of disability. Standard care was considered inefficient. No adverse events were reported.
Conclusion: The study determined that the protocol met a priori feasibility criteria, but to be practicable in a real world, health environment requires significant modifications.
Registration: ANZTR(ACTRN2614000489695).
Keywords: videoconferencing, telemedicine, interdisciplinary pain management, hybrid treatment, allied health, pediatric pain management
Introduction
Chronic pain is a significant issue for children and adolescents with a prevalence rate of between 11% and 38%.1 Common presentations include widespread pain, and pain localized to the abdomen, back, head, and musculoskeletal system.1 Left untreated, chronic pain can have a significant impact on a young person’s life trajectory, affecting their physical and mental health into adult life.2–4
Evidence-based guidelines recommend an interdisciplinary approach to treatment using programs that incorporate cognitive behavioral therapy into functional restoration programs.5 This interdisciplinary model involves practitioners working together to provide a comprehensive and integrated treatment package, using a common treatment philosophy, and constant communication between team members that operate from the same physical location.6 The treatment usually involves the parents or caregivers who are provided with strategies to encourage more adaptive responses to their child’s pain.5 Access to this type of treatment is limited by the scarcity of clinical expertise7 and geographical barriers.8
The use of electronic information and communication technology to deliver health services (known as eHealth)9 has the potential to improve access to a wide range of treatment including pain management programs. This term describes a broad range of service delivery models including phone, videoconferencing, web, or app-based treatment delivery platforms. Services may be delivered solely via digital technology, or combined with traditional face-to-face treatments10 in programs known as “hybrid”.11
This feasibility study focuses specifically on videoconferencing. Recent technological advances mean that encrypted video links can be easily shared between clinicians and patients using a variety of devices such as computers, smartphones or iPads without the requirement for the patient to have special software, accounts or dial-in details.12 Examples include Health Direct Australia VideoCall (https://about.healthdirect.gov.au/video-call). This technology extends the reach of healthcare to the patient’s community environment such as their home, school or work.13
Before the large-scale adoption of this type of technology, it is essential that the benefits are rigorously tested in real-world environments.13 Changing the delivery mechanism of a treatment may arguably change its nature, so it is important to be aware of this when analyzing efficacy; for example, interacting with a clinician during a videoconference is mechanistically different from interacting with an app on a mobile phone. Research into videoconference pain treatment interventions is emerging but study numbers are low. Despite some promising studies, three systematic reviews evaluating the delivery of adult pain interventions via various eHealth modalities have been unable to unequivocally demonstrate the benefit of videoconferencing due to the low number of studies using this technology.9,14,15 Pediatric literature related to videoconferencing is increasing. A systematic review of technology-based family interventions for a variety of pediatric chronic illnesses identified 23 relevant studies of which the overwhelming majority (87%) featured a hybrid design. Although three studies investigated technology-based interventions for chronic pain or headache, none of these used videoconferencing. The mixed results and varying intervention targets for different chronic diseases made it hard for the authors to draw summative conclusions and they recommended further research evaluating both clinical and economic outcomes, and the identification of the “active ingredients” of the interventions.11
We wished to evaluate whether videoconferencing could be used to deliver components of a pediatric interdisciplinary pain program (PIPP). We developed a program that included a combination of group face-to-face sessions and individual videoconference into the home that we called Hybrid-PIPP. We reasoned that this would provide better outcomes than treatment delivered via individual face-to-face sessions (Standard Care) because the group therapy environment would provide positive peer modelling opportunities, reinforcement, and social support. The objective of this study was to assess the feasibility of conducting a randomized controlled trial to test the efficacy and cost-effectiveness of the Hybrid-PIPP compared to Standard Care. The feasibility study was designed to a) test the integrity of the study protocol including the eligibility criteria; recruitment and consent process; data collection methods; randomization process; and acceptability of the intervention, and b) inform sample size calculation and selection of primary outcomes for the randomized controlled trial. Exploratory pre-and post-intervention outcome measures are reported; however, as this was a feasibility study there was no hypothesis established for statistical significance of treatment efficacy.
Methods
Participants and Setting
Approval for the study was obtained from the Western Australian Child and Adolescent Health Service Human Research Ethics Committee (2014016EP). The study protocol was registered with the Australian and New Zealand Clinical Trial Registry (ANZCTR 12614000489695) http://www.anzctr.org.au. A non-blinded matched pair randomized controlled model with parallel assignment was used. A matched pair design was chosen to reduce allocation bias by minimizing the differences between the treatment streams that may occur with a non-matched randomization, particularly with small sample sizes and heterogeneous participants.16 At the point of entry of each participant to the study, the streams represent the best possible balance related to functional disability.16 As this was a feasibility study, it was not powered to show statistical differences. Following consultation between the clinicians (two of whom had experience in delivering group interdisciplinary pain programs to adults totalling over 30 years), it was determined that three cycles of Hybrid-PIPP groups (with 4 to 8 families in each group) would be used to test the protocol. Because Hybrid-PIPP participants were matched to Standard Care, the intended participant numbers would therefore be between 24 and 48, dependent upon how many participants were randomized to the Hybrid-PIPP groups in each cycle.
Families were referred from tertiary clinics at the state Child and Adolescent Health Service (CAHS), Western Australia if they were considered to meet criteria by the assessing pain consultant. They were then screened by the treating team that included a clinical psychologist, physiotherapist and occupational therapist. Inclusion criteria were: a) aged 11 to 17 years old, b) experiencing a primary pain disorder for more than 3 months, c) pain occurring at least 4 days a week, d) one adult family member/carer willing to participate, e) no major changes in the participant’s medical management anticipated in the next 4 months, and f) family access to phone, internet and e-mails at their home, community health center or other convenient location. Participants were not eligible if the adolescent had a) high dependency on analgesic medication, b) primary symptom of fatigue, c) current or previous treatment for cancer, d) pain explained by identified pathological process (eg, rheumatological disease or inflammatory bowel disease), e) documented developmental delay, f) co-existing psychiatric or psychosocial issues that were considered more relevant to the participant’s reduced function than their pain was, g) significant mental health disorder including bipolar disorder, psychosis, active suicidality or eating disorders, h) were currently participating in other rehabilitation programs or cognitive behavioral therapy, and i) barriers that would prevent the participant’s comprehension of principles taught in intervention.
Enrolment was performed by the treating clinicians. After obtaining consent from the carer and assent from the adolescent, participants underwent a matched allocation process using their Functional Disability Index (FDI)17 scores. Using the FDI they were classified as either No/Minimal Disability (0–12), Moderate Disability (13–29) or Severe Disability (30+).17 An independent person drew a marble from a concealed bag containing 24 white marbles (indicating Standard Care) and 24 colored marbles (indicating Hybrid-PIPP). Once selected, the marble was not placed back in the bag. This process did not occur if the participant’s FDI score matched a previously enrolled participant who had not yet been matched. When this occurred, they were assigned to the stream that did not include their match and the corresponding marble for their stream allocation was removed from the bag to maintain the probability. Participants were offered a place in the alternative stream after their involvement in the study was completed.
Feasibility Measures
Criteria for feasibility of success were established a priori and included: a) greater than 70% of participants who commence the intervention complete the intervention (Hybrid-PIPP or 7 weeks of Standard Care); b) greater than 60% of participants complete the Week 11 data collection; c) suitably qualified staff were available; and d) on average three participants per month were recruited of which 90% met criteria. In addition to these data, semi-structured interviews were conducted with the three treating clinicians to gain qualitative information on 1) eligibility criteria, 2) recruitment and consent process, 3) data collection forms, 4) randomization process, 5) acceptability of the interventions, and 6) selection of the primary outcome.
Outcome Measures
The study was designed to capture data over 11 weeks, across all domains recommended by the Pediatric Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (PedIMMPACT)18 including pain intensity; physical functioning; emotional functioning; role functioning; adverse events; global satisfaction with treatment; sleep; and economic factors, as detailed in Table 1. Self-report measures were taken at standardized timepoints: Timepoint 1, baseline assessment; Time-point 2, Week 7 (to coincide with the end of Hybrid-PIPP intervention); and Time-point 3, Week 11 (end of study period), see Figure 1. Participants completed their questionnaires electronically either from home or at the hospital using a secure website (http://www.researchaustralia.net.au). This took approximately 30 mins for the adolescents, and 40 mins for the parents. The electronic data capture website had been custom-made to manage data from an adult pain service and was modified to administer the pediatric instruments required for this study.
 |
Table 1 Outcome Measures |
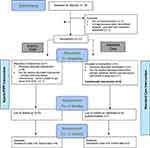 |
Figure 1 Participant flow. Notes: T1=Time point 1, T2= Time point 2, T3 = Time point 3. |
Interventions
Clinical Concepts Common to Both Streams
The treatment team for both intervention streams was the same and included a clinical psychologist, physiotherapist and occupational therapist who were all experienced in managing patients with complex pain. The intervention was informed by several sources5,19,20 and used an interdisciplinary cognitive behavioural pain management model, delivered within a neuroscience psycho-educational framework. The primary themes were the promotion of self-efficacy and increased engagement in functional activities despite pain; independence from medical and social care; and return to normal adolescent activity including school. The treatment model explored how pain can be amplified by thoughts and feelings; physiological responses (fight or flight); and maladaptive behavioural responses to pain. A contemporary pain neuroscience platform was used to describe the perpetuating factors of pain and provide the rationale for engaging in cognitive behavioural pain management strategies including “balanced thinking”, goal setting, activity management, physical conditioning, communication skills training, sleep health, relaxation and mindfulness, and graded exposure to challenging activities. Participants were encouraged to: identify their own activity/pain patterns; set goals; identify core daily exercises based on treatment goals; plan and prioritize activities; and schedule their days (including avoiding long periods of rest or activity). Parents received the same information as the adolescents in addition to skills training to promote reinforcement of pain management strategies at home. Parents and adolescents were also asked to identify avoidant behaviours and develop a plan to address them using a graded hierarchy. These concepts were presented in age-appropriate terms, using metaphors and analogies to support learning. A file with handouts and worksheets was provided for both adolescents and parents. Families also had support from a school liaison teacher. Clinicians met at least weekly to discuss the participant’s progress and provide interdisciplinary support to each other, with one clinician being allocated as a case manager for each participant.
Standard Care
The adolescent and their parent were assessed by the team during 3 one hour “face-to-face” individual sessions. Treatment was then provided in individual sessions by each therapist. The therapists endeavoured to co-ordinate their bookings with each other to reduce travel time for the participants. Sessions with each clinician were generally offered fortnightly to ensure similar treatment intensity in both streams. The number and length of treatment sessions was recorded. The treatment model and content was the same as Hybrid-PIPP but was less structured - the order of content delivery and time spent on each topic was determined on a case by case basis by each therapist for their particular session, which was standard practice in the hospital at the time.
Hybrid-PIPP
The Hybrid-PIPP program was conducted over 7 weeks (Table 2) with a structured schedule. The adolescent and their parent/carer attended a 2 hr face-to-face physical assessment (physiotherapy and occupational therapy combined) and a 1 hr face-to-face psychology assessment. They then commenced the next available Hybrid-PIPP program which consisted of an initial 7 hr face-to-face workshop (involving up to 8 families attending parallel group sessions for carers and adolescents); a five-week period involving four individual videoconference sessions in their home (detailed below); and a final 4 hr face-to-face workshop (total hours detailed in Table 2). The workshops were facilitated by the three treating clinicians plus a clinical assistant. The total contact time for the workshops and videoconference sessions was 14.5 hrs (plus 3 hr physical and psychological assessment).
 |
Table 2 Service Delivery of Hybrid-PIPP |
Videoconference Support Sessions
Videoconference sessions used SCOPIA (https://www.avaya.com) that was the hospital’s recommended system at the time. SCOPIA provided an encrypted video link between the hospital and the participant’s home, community or school environment. The structure of the sessions was informed by an evidence-based internet delivered adult pain program described by Dear et al21 and aimed to:
- Summarize content from the face-to-face workshop, but not deliver new concepts or content.
- Answer questions.
- Reinforce progress and encourage practical application of pain management strategies.
- Normalize challenges of treatment.
- Gain feedback about the participant’s engagement in the program.
The adolescents were asked to complete daily charts related to activity management, cognitive strategies, and flare up and set back planning that were reviewed at the videoconference sessions. Parents were monitored by verbal questioning during the videoconference session with an emphasis on supporting parental skills acquisition utilizing a problem-solving-based approach.
The initial protocol determined that families participate in 30 min home videoconference sessions with their case manager in Weeks 2 to 5. However, following the assessment of the first group of participants in the Hybrid-PIPP group, the clinical team decided to increase the time allocated for these sessions to 60 mins in Weeks 2 to 5 so that they could do a 30-min session with the adolescent, and the remaining time with their carer. This was continued for all Hybrid-PIPP participants. Additionally, the original protocol described videoconference input from only the case manager whereas the adjusted protocol allowed for the case manager to request input from other clinicians in the team as required (eg, specialized input regarding mood was directed by the psychologist and advice on physical activity was directed by the physiotherapist).
Analysis
Data were analyzed using statistical software Stata 14 (College Station, TX: StataCorp LP). This was a feasibility study therefore the analysis used descriptive statistics with proportions, medians and ranges reported due to skewed distributions and small sample size.22
Results
Figure 1 shows the enrolment and progression of participants through the study. Recruitment commenced in August 2014 with the intention of recruiting for 3 Hybrid-PIPP groups with matched Standard Care participants. Recruitment ceased in March 2015 after 2 Hybrid-PIPP groups were delivered, when a higher than expected level of disability was identified in both streams (Standard Care FDI median 27, range 18–41, Hybrid PIIPP FDI median 28.5, range 18–45). It became clear that the 14.5 hr intervention was insufficient for the level of disability, and funding became available for a more intensive program. The clinical reasoning for this decision is described in the discussion.
Eighteen consecutive adolescent/parent dyads were assessed for eligibility and 2 were excluded as they did not meet criteria. The 3 families who were being triaged when the decision was made to stop the trial were provided with an alternative treatment in the same hospital. In total, 13 families were randomly allocated to the two treatment streams with 7 families allocated to the Hybrid-PIPP and 6 to Standard Care. One family chose not to commence Hybrid-PIPP because of problems arranging parental leave. This family requested a place on a future program, but the trial was ceased. Two families in the Standard Care stream chose to attend Hybrid-PIPP after their involvement in the study was complete. In total both Hybrid-PIPP groups had 4 participating families. All families that commenced Hybrid-PIPP completed the intervention and final data collection. Two families dropped out of the Standard Care stream – one actively withdrew before commencement to participate in a drug trial, and one passively withdrew during treatment by declining to attend (no reason given). Details can be seen in Figure 1. Table 3 presents demographic data for the two treatment streams, Table 4 presents the feasibility criteria results, Table 5 reports the attendance data, and Table 6 details the qualitative feedback from clinicians.
 |
Table 3 Adolescent Demographics |
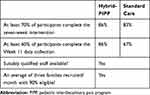 |
Table 4 Feasibility Criteria |
 |
Table 5 Attendance |
 |
Table 6 Qualitative Feedback |
The study met predetermined feasibility criteria for completion of intervention, data collection, recruitment rate and staffing (Table 4). Although treatment sessions in Standard Care were usually offered fortnightly (to have similar treatment intensity in both streams) the uptake of sessions varied. Only 63% of the recommended sessions were attended in the Standard Care stream, compared to 94% in the Hybrid-PIPP stream. The Hybrid-PIPP stream had superior attendance and used staff resources more efficiently than the Standard Care stream (Tables 5 and 6 Section 2). The Hybrid-PIPP stream had similar participant Global Satisfaction ratings to Standard Care (median 7, range 5–9), Hybrid-PIPP (median 8.5, range 5–10) on an 11-point scale. No adverse events were reported in either stream.
Treatment Outcome Measures
Both streams had a similar trend for most variables with improvements seen at 7 weeks that were not maintained at 11 weeks. The median (range) for functional disability (FDI) in the Hybrid-PIPP stream at baseline, 7weeks, and 11 weeks, respectively, was 28.5 (18–45), 23 (10–42), and 26.5 (8–44). The median (range) for functional disability (FDI) in the Standard Care stream at baseline, 7weeks, and 11 weeks, respectively, was 27 (18–41), 26 (14–35), and 27 (8–36). Neither group showed improvements over the 30% threshold in any measure required for clinical significance.23
Feedback from Clinicians
Semi-structured interviews with the treating clinicians on completion of the study provided feedback detailed in Table 6.
Discussion
While the study met a priori feasibility criteria relating to the availability of suitably trained staff; the rate of recruitment; treatment completion; and data collection - qualitative data identified several factors which limit the protocol’s usefulness for a randomized controlled trial. These included the intervention intensity related to participant disability, and time inefficiencies related to attendance. Because of this, the protocol was considered impracticable without significant modification.
Attendance was identified as a significant issue in the Standard Care stream. Whilst intuitively it might appear that flexible appointment scheduling would lead to better attendance, in this study this was not the case. In the Standard Care stream, the families booked sessions at their convenience and were able to reschedule appointments as required. This required considerable administrative and clinician time to coordinate appointments between the 3 practitioners, made more complex by attempts to book sessions with all three clinicians in 1 day to reduce the travel burden on the participants. Despite this flexibility there was frequent poor attendance in this stream including both non-attendance of booked appointments, and poor uptake of offered sessions. Only 63% of the recommended sessions were attended by the families completing the Standard Care stream (that had flexible scheduling policy), compared to 94% in the Hybrid-PIPP stream (that had a rigid scheduling policy) see Table 5. Further inefficiencies in the Standard Care stream included the inability to leverage clinician time (such as by seeing more than one participant at once in a group), and time required to facilitate data collection. Taken as a whole, within this research protocol the Standard Care model was considered impractical by clinicians.
In the Hybrid-PIPP stream, the primary issue was lack of intensity in the initial workshop, limiting the capacity to teach pain management skills, shape behaviors, and develop therapeutic relationships. This lack of intensity in the face-to-face component resulted in the videoconference sessions being used to deliver basic pain management strategies rather than reinforcing progress and encouraging skills practice as was intended. In addition, the positive group effects seen in the workshop could not be developed further in the individual videoconference sessions. This significantly limited the potential benefits of peer problem solving and support for both the adolescents and their parents. For some participants, the home base treatment was believed to reinforce avoidance behavior (eg, adolescents staying in their bedroom) and diminish the value that participants placed on the intervention (eg, using mobile phones during sessions). Separate videoconference sessions with the adolescent and their parent were also difficult to arrange.
The decision to stop recruitment early was related to funding and clinical reasoning that was informed by a) evidence-based guidelines that recommend interventions of at least 60 hrs for individuals presenting with high levels of disability and distress,24 and b) comparison of participant disability levels to 5 published studies of children with chronic pain summarized by Gauntlett-Gilbert and Eccleston.25 The authors reported FDI values varying from 11.3 to 26.3. The functional disability in the study cohort was considered high in comparison (Standard Care median 27 (18 −41), Hybrid PIIPP median 28.5 (18–45)). The 14.5 hr Hybrid-PIPP intervention was determined to be too brief for the study cohort. At the time that the protocol was developed there was no funding allocated to interdisciplinary pediatric pain management in the health service where the study took place. During the study, funding for an intensive pain program became available. Recruitment was stopped at this time because it was considered unethical to continue if a more intensive option was available. This decision was affirmed when the outcome data were analyzed, and no clinically significant improvement was seen in either stream.
Geographical barriers will remain for this high-intensity group until effective remote delivery methods are developed. More work needs to be done in area of e-Health delivery of pain interventions to determine which components of treatment, for which families, can be delivered in this way.7 To facilitate this, studies should describe the intervention in detail so that possible active components can be determined.7,11 This knowledge, along with a clearer understanding of factors that predict success, will provide a structured model for triage into various treatment delivery streams11 and intensities.
Limitations of the study include the inability to blind the participants and clinicians to the stream allocation, and fewer participants than anticipated. There was a variation in treatment intensity between streams because of poor uptake of the Standard Care sessions offered. Although time spent on scheduled clinical interactions and non-attendance of appointments was recorded, non-acceptance of offered treatment, and administrative time spent coordinating appointments was not. Fidelity checking of the intervention curriculum was not completed. There was a significant burden on clinical staff to recruit participants and ensure data were collected and processed accurately which reduced their capacity to provide clinical services and was a potential source of experimental bias. The electronic database we used made it difficult for clinicians to identify and rectify missing data points and did not have the functionality required as raw data could not be exported for the cohort.
Recommendations
Considering the inefficiencies demonstrated in the Standard Care stream, the original protocol is not considered practicable within our hospital funding environment without a significant increase in staff funding. We recommend that further research should focus on either a single-case experimental design or a single stream study to investigate a modified Hybrid-PIPP program (with a waitlist control if practicable). Modifications should include: eligibility requirements changed from pain disorder >3 months to >6 months, and the addition of “all investigations completed and parents accepting of a biopsychosocial model”; capacity to provide at least two levels of treatment intensity (according to levels of adolescent pain-related disability, and adolescent and parent distress) with the intensive intervention having at least 60/30 hrs for the adolescent/parent; group size limited to 6 families at one time; group videoconference sessions rather than individual to facilitate peer support and engagement for adolescent and parents (run separately); documentation of type and duration of administrative tasks related to intervention delivery; development of digital resources to support learning and to leverage clinician time; research support and an intuitive electronic database; technical support for clinicians; selection of the FDI17, parent or school reported school attendance last term, and the Parent Behavior scale of the Bath Adolescent Pain Parent Impact Questionnaire26 as the primary outcome measures; and adverse events data to be recorded with a separate question rather than limited to inclusion in global satisfaction.
In summary, although the a priori feasibility criteria were met, the limitations identified in the qualitative analysis meant that the protocol requires significant modification for a future study to be feasible.
Conclusion
There are significant challenges evaluating technology-based service delivery methods within real word health environments. This study describes the development of a protocol to evaluate a pediatric pain intervention delivered in part using videoconferencing. The study determined that the protocol met a priori feasibility criteria, but to be practicable in our hospital environment requires significant modifications related to issues identified regarding 1) attendance, time efficiency and data collection in the Standard Care stream, 2) the intensity of both interventions related to the disability of the participants, and 3) the study design.
Recommendations are made for either a single-case experimental design or a single stream study using a modified version of Hybrid-PIPP with an increased number of face-to-face contact hours and/or online resources. The significant challenges identified in this study highlight the importance of feasibility studies in the development of evaluation protocols for complex interventions such as PIPPs, particularly when evaluating new service delivery methods such as videoconferencing.
Generalizability
These results could be cautiously generalized to other pediatric pain populations if consideration is taken of comparative levels of disability (measured by the FDI), tertiary referrals and eligibility criteria. This paper was written and accepted for publication before COVID-19. Some of the recommendations may not be feasible during a pandemic where face to face clinical contact is limited.
Ethics Statement
The participants, and parents or legal guardians involved in this study provided written informed consent, and the trial was conducted in accordance with the Declaration of Helsinki.
Data Sharing Statement
The dataset supporting the conclusions of this article is included within the article.
Acknowledgment
The authors would like to thank Channel 7 Telethon for partially funding the project. The valuable input from the following people is also acknowledged: Dr Kevin Murray, Dr Pavla Walsh, Ms Kate Holtzman, and Dr Vance Locke.
Funding
The study was funded by Channel 7 Telethon Trust.
Disclosure
Anna Hilyard, Susan Taylor, Natasha Bear and Julia Kingsley report grant funding from Channel 7 Telethon Trust during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. King S, Ct C, Huguet A, et al. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738.
2. Noel M, Groenewald CB, Beals-erickson SE, Gebert JT, Palermo TM. Chronic pain in adolescence and internalizing mental health disorders: a nationally representative study. Pain. 2016;157(6):1333. doi:10.1097/j.pain.0000000000000522
3. Shelby GD, Shirkey KC, Sherman AL, et al. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics. 2013;132(3):475–482. doi:10.1542/peds.2012-2191
4. Hestbaek L, Leboeuf-yde C, Kyvik KO, Manniche C. The course of low back pain from adolescence to adulthood: eight-year follow-up of 9600 twins. Spine. 2006;31(4):468–472. doi:10.1097/01.brs.0000199958.04073.d9
5. Palermo T, Eccleston C, Goldschneider K, et al. Assessment and Management of Children with Chronic Pain. American Pain Society; 2012.
6. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol. 2014;69(2):119. doi:10.1037/a0035514
7. Caes L, Fisher E, Clinch J, Eccleston C. Current evidence-based interdisciplinary treatment options for pediatric musculoskeletal pain. Curr Treatm Opt Rheumatol. 2018;4(3):223–234. doi:10.1007/s40674-018-0101-7
8. Hogg MN, Gibson S, Helou A, DeGabriele J, Farrell M. Waiting in pain: a systematic investigation into the provision of persistent pain services in Australia. Med J Aust. 2012;196(6):386–390. doi:10.5694/mja2.2012.196.issue-6
9. Slattery BW, Haugh S, O’connor L, et al. An evaluation of the effectiveness of the modalities used to deliver electronic health interventions for chronic pain: systematic review with network meta-analysis. J Med Internet Res. 2019;21(7):e11086. doi:10.2196/11086
10. Fisher E, Law E, Palermo TM, Eccleston C. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2015;(3). doi:10.1002/14651858.CD011118.pub2.
11. Canter KS, Christofferson J, Scialla MA, Kazak AE. Technology-focused family interventions in pediatric chronic illness: a systematic review. J Clin Psychol Med Settings. 2019;26(1):68–87. doi:10.1007/s10880-018-9565-8
12. Annual Report Business Highlights 2017 - 2018 Health Direct Australia. Available from: http://media.healthdirect.org.au/publications/healthdirect-australia-annual-report_17-18_business-highlights.pdf. Accessed Feburary 26, 2020.
13. Safe, seamless and secure: Evolving healthcare and care to meet the needs of modern Australia. Australia’s National Digital Health Strategy. Australian Government. Available from: https://conversation.digitalhealth.gov.au/sites/default/files/adha-strategy-doc-2ndaug_0_1.pdf. Accessed February 26, 2020.
14. McGeary DD, McGeary CA, Gatchel RJ, Allison S, Hersh A. Assessment of research quality of telehealth trials in pain management: a meta‐analysis. Pain Pract. 2013;13(5):422–431. doi:10.1111/papr.2013.13.issue-5
15. Adamse C, Dekker-van Weering MG, van Etten-jamaludin FS, Stuiver MM. The effectiveness of exercise-based telemedicine on pain, physical activity and quality of life in the treatment of chronic pain: A systematic review. J Telemed Telecare. 2018;24(8):511–526. doi:10.1177/1357633X17716576
16. Blair E. Discussion: Gold is not always good enough: the shortcomings of randomization when evaluating interventions in small heterogeneous samples. J Clin Epidemiol. 2004;57(12):1219–1222.
17. Kashikar-Zuck S, Flowers SR, Claar RL, et al. Clinical utility and validity of the functional disability inventory among a multicenter sample of youth with chronic pain. Pain. 2011;152(7):1600–1607. doi:10.1016/j.pain.2011.02.050
18. McGrath PJ, Walco GA, Turk DC, et al. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: pedIMMPACT recommendations. J Pain. 2008;9(9):771–783. doi:10.1016/j.jpain.2008.04.007
19. Eccleston C, Malleson P, Clinch J, Connell H, Sourbut C. Chronic pain in adolescents: evaluation of a programme of interdisciplinary cognitive behaviour therapy. Arch Dis Child. 2003;88(10):881–885. doi:10.1136/adc.88.10.881
20. Palermo T. Cognitive-Behavioral Therapy for Chronic Pain in Children and Adolescents. Part III Interventions. New York, NY: Oxford University Press Inc; 2012.
21. Dear BF, Titov N, Perry KN, et al. The pain course: a randomised controlled trial of a clinician-guided internet-delivered cognitive behaviour therapy program for managing chronic pain and emotional well-being. Pain. 2013;154(6):942–950. doi:10.1016/j.pain.2013.03.005
22. Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. Eval Clin Pract. 2004;10(2):307–312. doi:10.1111/j.2002.384.doc.x
23. Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. 2008;33(1):90–94. doi:10.1097/BRS.0b013e31815e3a10
24. Pain management programs - Which patient for which program? A guide for NSW tier 3 and tier 2 public health facilities providing pain programs. New South Wales Agency for Clinical Innovation. Available from: https://www.apsoc.org.au/PDF/Publications/20131216_ACI13-015-pain-programs.pdf. Accessed February 26, 2020.
25. Gauntlett-gilbert J, Eccleston C. Disability in adolescents with chronic pain: patterns and predictors across different domains of functioning. Pain. 2007;131(1–2):132–141. doi:10.1016/j.pain.2006.12.021
26. Jordan A, Eccleston C, McCracken LM, Connell H, Clinch J. The Bath Adolescent Pain–Parental Impact Questionnaire (BAP-PIQ): development and preliminary psychometric evaluation of an instrument to assess the impact of parenting an adolescent with chronic pain. Pain. 2008;137(3):478–487. doi:10.1016/j.pain.2007.10.007
27. Eccleston C, Jordan A, McCracken LM, Sleed M, Connell H, Clinch J. The Bath Adolescent Pain Questionnaire (BAPQ): development and preliminary psychometric evaluation of an instrument to assess the impact of chronic pain on adolescents. Pain. 2005;118(1–2):263–270. doi:10.1016/j.pain.2005.08.025
28. Chorpita BF, Yim L, Moffitt C, Umemoto LA, Francis SE. Assessment of symptoms of DSM-IV anxiety and depression in children: A revised child anxiety and depression scale. Behav Res Ther. 2000;38(8):835–855
29. Bursch B, Tsao JC, Meldrum M, Zeltzer LK. Preliminary validation of a self-efficacy scale for child functioning despite chronic pain (child and parent versions). Pain. 2006;125(1–2):35–42. doi:10.1016/j.pain.2006.04.026
30. Simons LE, Sieberg CB, Carpino E, Logan D, Berde C. The Fear of Pain Questionnaire (FOPQ): assessment of pain-related fear among children and adolescents with chronic pain. J Pain. 2011;12(6):677–686. doi:10.1016/j.jpain.2010.12.008
31. Varni JW, Stucky BD, Thissen D, et al. PROMIS pediatric pain interference scale: an item response theory analysis of the pediatric pain item bank. J Pain. 2010;11(11):1109–1119. doi:10.1016/j.jpain.2010.02.005
32. Ho IK, Goldschneider KR, Kashikar-Zuck S, Kotagal U, Tessman C, Jones B. Healthcare utilization and indirect burden among families of pediatric patients with chronic pain. J Musculoskelet Pain. 2008;16(3):155–164. doi:10.1080/10582450802161853
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
