Back to Journals » Cancer Management and Research » Volume 14
Feasibility and Tolerability of Lenvatinib, Plus PD-1 Blockades for Patients with Unresectable Hepatocellular Carcinoma: A Retrospective Exploratory Study
Authors Jia M, Jia JK, Xu J, Xue HZ
Received 24 April 2022
Accepted for publication 19 July 2022
Published 2 September 2022 Volume 2022:14 Pages 2625—2638
DOI https://doi.org/10.2147/CMAR.S372125
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Meng Jia, Jiang-Kun Jia, Jian Xu, Huan-Zhou Xue
Department of Hepatobiliary Surgery, Henan Provincial People’s Hospital, Zhengzhou, 450003, People’s Republic of China
Correspondence: Huan-Zhou Xue, Department of Hepatobiliary Surgery of Henan Provincial People’s Hospital, No. 7 Wei-Wu Road, Zhengzhou, 450003, People’s Republic of China, Email [email protected]
Objective: Lenvatinib was the standard first-line therapy for patients with unresectable HCC. PD-1 blockades demonstrated promising efficacy for patients with previously-treated HCC. Therefore, this study was to investigate the feasibility and tolerability of lenvatinib plus PD-1 blockades for patients with unresectable HCC retrospectively.
Methods: A total of 37 patients with unresectable HCC who received lenvatinib plus PD-1 blockades in first-line setting were included in this study retrospectively. Efficacy of the patients was evaluated with the change of target lesion using mRECIST criteria per investigator and all the subjects were followed up regularly. Adverse reactions were collected and documented. Exploratory analysis between prognosis and baseline characteristics was performed using log rank test and multivariate analysis were performed using Cox regression analysis.
Results: The best overall response of the 37 patients suggested that complete response was observed in one patient, partial response was noted in 11 patients, stable disease was noted in 16 patients and 9 patients had progressive disease, which yielded an objective response rate (ORR) of 32.4% (95%CI: 18.0– 49.8) and a disease control rate (DCR) of 75.7% (95%CI: 58.8– 88.2). Furthermore, the median progression-free survival (PFS) of the 37 patients with advanced HCC was 8.3 months (95%CI: 3.34– 13.26). And the median overall survival (OS) was 17.8 months (95%CI: 7.19– 28.41). In addition, the median duration of response (DoR) in 12 patients with response was 9.6 months (95%CI: 3.03– 16.17). Furthermore, adverse reactions that were attributed to the combination administration were detected in 36 patients (97.3%), among whom a total of 22 patients (59.5%) were observed of the grade ≥ 3 adverse reactions. And the most common adverse reactions were hypertension, fatigue, nausea and vomiting, and hepatotoxicity.
Conclusion: Lenvatinib plus PD-1 blockades demonstrated promising anticancer activity and acceptable toxicity for patients with unresectable HCC. And the conclusion should be validated in prospective clinical trials subsequently.
Keywords: hepatocellular carcinoma, lenvatinib, PD-1 blockades, efficacy, safety
Introduction
Primary liver cancer was estimated to be one of the most common solid tumors worldwide and the second leading cause of cancer-related death globally.1 And the incidence of liver cancer in China was higher, which was estimated that there were approximately 410,000 new cases and 391,000 deaths in China annually.2 Hepatocellular carcinoma (HCC) was the predominant category and accounted for almost 90% of liver cancer,3 which suggested that there were approximately 369,000 new cases and 352,000 deaths of HCC in China each year. Given that the etiology and the molecular characteristics in China was different from that in western countries, the treatment of HCC in China had its own styles and the therapeutic strategies for HCC should be developed separately to optimize the clinical outcomes of the patients.4 Although surgery or liver transplantation or ablation were the efficacious treatment for patients with early stage HCC, the majority of patients were diagnosed with unresectable disease owing to the inconspicuous symptoms and the prognosis was dismal with the five-year overall survival (OS) rate <15%.5
Given that most patients with HCC were insensitive to chemotherapy, sorafenib had become the only useful multi-kinase inhibitor for patients with unresectable advanced HCC over the past 10 years,6 which yielded an objective response rate (ORR) of 3.3%, a median progression-free survival (PFS) of 2.8 months and a median OS of 6.5 months in 2009 among Asia-Pacific population.7 Noteworthily, considerable trials were performed to improve the prognosis of patients with HCC until 2018, none of the trials achieved the predefined primary end points.6 Amazingly, another antiangiogenic targeted drugs lenvatinib that inhibited VEGFR1-3, FGFR1-4, PDGFRα, RET and KIT demonstrated promising efficacy and tolerable toxicity in first-line setting compared with sorafenib according to REFLECT Phase III clinical trial (median OS, 13.6 months with lenvatinib vs 12.3 months with sorafenib, HR=0.92, 95%CI: 0.79–1.06).8 As a result, lenvatinib had been approved as the standard of care in first-line setting for patients with unresectable advanced HCC. Unfortunately, to our knowledge, the ORR of antiangiogenic targeted drugs was disappointing in clinical practice, which indicated that sorafenib, donafenib and lenvatinib monotherapy as the first-line therapy for patients with advanced HCC was only 3.3%, 4.6%, and 24.1%, respectively,9 highlighting that the new combination strategies needed to be explored urgently.
In view of the physiological role of liver in processing extraneous drugs and the first-pass metabolism, HCC might naturally exhibit a relatively immunosuppressed state that was heavily regulated by the immune system, which suggested that immunotherapy (PD-1 blockades) might play a pivotal role to reverse the process in the tumor and help T cells to kill the tumor cells.10 Consequently, the potential significance of PD-1 blockades in HCC had been proved by pembrolizumab, nivolumab, and camrelizumab as second-line treatment in China based on the results of Phase II clinical trials.11 Unfortunately, the ORR of the PD-1 blockades monotherapy among patients with HCC ranged from 15–20% and all the PD-1 blockades failed to improve OS ultimately, which highlighted that immune checkpoint inhibitor-based regimens might be of potential significance for patients with unresectable HCC clinically.12
Interestingly, recent years had witnessed that the combination of PD-1 blockades with antiangiogenic targeted drugs demonstrating promising efficacy for patients with HCC, which suggested that the antiangiogenic drugs reprogrammed the tumor milieu from an immunosuppressive to an immune permissive microenvironment and the activated immunity by PD-1 blockades also potentiated antiangiogenesis by downregulating the expression of vascular endothelial growth factor and alleviating hypoxia condition.13 As a result, we observed that atezolizumab plus bevacizumab showed encouraging feasibility and acceptable toxicity for patients with unresectable HCC, thus becoming the standard of care as first-line therapy for HCC according to the IMbrave150 clinical trial.14 In addition, lenvatinib plus pembrolizumab also achieved amazing efficacy (ORR: 46%, median PFS: 9.3 months, median OS: 22 months) and tolerable safety as first-line therapy for patients with advanced HCC according to the Keynote 524 trial.15 Notably, the lack of validated biomarkers of response represented an important issue since only a proportion of HCC might benefit from PD-1/PD-L1 administration. A previous study indicated that some potential biomarkers including PD-L1 expression, tumor mutational burden (TMB), microsatellite instability (MSI) status and gut microbiota might be promising biomarkers to predict the efficacy of PD-1 blockades.16 However, the feasibility and safety of lenvatinib plus PD-1 blockades in first-line setting for Chinese patients with advanced HCC remained scanty currently.
Consequently, this study was to investigate the feasibility and tolerance of lenvatinib combined with PD-1 blockades for Chinese patients with unresectable advanced HCC retrospectively.
Patients and Methods
Procedures and Eligibility Criteria
Given that lenvatinib was approved as the first-line treatment for previously-untreated patients with unresectable HCC since September 2018 and PD-1 blockades were licensed as second-line therapy for patients with advanced HCC, those who received lenvatinib plus PD-1 blockades were ethical in clinical practice. And this study was implemented as a real-world study. Patients with unresectable advanced HCC who received lenvatinib plus PD-1 blockades as first-line treatment in the Department of Hepatobiliary Surgery of Henan Provincial People’s Hospital from November 2018 to November 2021 were as included in this study consecutively. Specifically, the main inclusion criteria included: (1) histologically or cytologically diagnosed of unresectable HCC, those who were radiologically confirmed HCC based on the American association for the study of liver disease criteria were also eligible; (2) aged ≥18 years old; (3) the Eastern Cooperative Oncology Group (ECOG) performance status ranged from 0 to 2 score; (4) stage B or C according to Barcelona Clinic Liver Cancer (BCLC) staging system;17 (5) measurable tumor lesion based on the response criteria (RECIST 1.1);18 (6) patients were treated with lenvatinib plus PD-1 blockades as first-line therapy in clinical practice. The exclusion criteria were: (1) histologically diagnosed of fibrolamellar, sarcomatoid and mixed hepatocholangiocellular carcinoma; (2) patients were presence of a history of autoimmune diseases or patients were treating with steroids or other immunosuppressive drugs; (3) patients who were suitable for locoregional therapies. However, those who had progressed the previous locoregional therapies were permitted to be included; (4) concomitant with another cancer or serious diseases that might compromise survival of the patients; (5) data of efficacy assessment was not available. The study profile was illustrated in Figure 1. A total of 37 patients with unresectable advanced HCC were included in this retrospective study ultimately. And the primary endpoint of this study was PFS, secondary endpoints were ORR, disease control rate (DCR), duration of response (DoR), OS and safety profile of the 37 patients with advanced HCC who received lenvatinib plus PD-1 blockades.
 |
Figure 1 Study profile of this retrospective study regarding the feasibility and tolerance of lenvatinib plus PD-1 blockades for patients with unresectable advanced hepatocellular carcinoma. |
The protocol of this study was approved by the ethics committees of Henan Provincial People’s Hospital. Written informed consent was obtained from all the patients included in this study according to the recommendations of the Declaration of Helsinki.
Therapeutic Regimens and Protocol of Assessment
Given that this study was conducted as a retrospective study, the 37 eligible patients were administered with lenvatinib plus PD-1 blockades in clinical practice. Lenvatinib was administered orally and once daily with the initial dosage of 8 mg or 12 mg (8 mg when body weight <60 kg, 12 mg when body weight ≥60 kg). PD-1 blockades (PD-1 inhibitors that were approved in China) were consisted of camrelizumab (200 mg), sintilimab (200 mg) and pembrolizumab (200 mg), which were intravenously administered on day 1, every 21 days were deemed as one therapeutic cycle. Dosage adjustment of lenvatinib was permitted based on the adverse reactions during the treatment. Noteworthily, those who transiently or permanently terminated either lenvatinib or PD-1 blockades owing to the potential adverse reactions were permitted to continue single-agent administration in the subsequent treatment.
Efficacy assessment was performed based on mRECIST criteria.19 Target lesions of the tumors were assessed using computed tomography (CT) or magnetic resonance imaging (MRI) scans at baseline and every two cycles or depended on the actual situation when it was necessary for the patients. ORR and DCR in this study were analyzed according to the results of the best overall response evaluated during the treatment of lenvatinib plus PD-1 blockades. Furthermore, safety profile was analyzed using Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 criteria. All adverse reactions of the patients who were treated with lenvatinib plus PD-1 blockades were recorded specifically to present the safety profile of the combination regimen.
Procedure of Follow-up
Baseline demographic characteristics, adverse reactions and status of disease progression of each patient were collected through the electronic medical record system when the patients underwent hospitalization. Subsequently, the follow-up was mainly performed by telephone. Patients were followed up monthly after the progression on lenvatinib combined with PD-1 blockades and the death status were mainly inquired. Ultimately, the date of data cut-off was March 5, 2022.
Statistical Analysis
In detail, ORR was defined as the proportion of complete response (CR) and partial response (PR) among all the patients included. DCR was defined as the proportion of CR and PR and stable disease (SD) among all the patients included. All the data were carried out and analyzed using SPSS version 25.0. Quantitative variables and qualitative variables were presented as median (range) and number of patients (percentage), respectively. PFS and OS was defined according to the previous study.20 DoR was defined as the duration from the date of first assessment of the tumor as CR or PR to the date of first assessment of PD or death from any cause. PFS and OS survival curves were generated using Stata 14.0 software. Association between PFS and baseline characteristic subgroups was analyzed using log rank test. Multivariate Cox regression analysis was adopted for PFS including the variables that were significant in univariate analysis. P<0.05 was considered significant.
Results
Baseline and Demographic Characteristics
Baseline characteristics of the 37 patients with unresectable advanced HCC were shown in Table 1. The median age of the patients was 62 years with the ranges from 28 to 78 years. Patients with age ≥62 and <62 were observed in 20 and 17 cases, respectively. ECOG performance status of 0–1 score and 2 score was reported in 29 and 8 patients, respectively. Male and female was noted in 25 and 12 patients, respectively. With regard to the BCLC staging, majority patients were stage of C (75.7%). And the Child–Pugh classification indicated that most patients were of grade A (83.8%). In addition, hepatitis B virus (HBV) was the predominant etiology for HCC (73.0%) and hepatitis C virus (HCV) was another etiology for HCC (16.2%). A total of seven patients had received local therapy previously. Furthermore, 25 patients were present of extrahepatic metastasis. Noteworthily, patients with alpha fetoprotein (AFP) of <400 and ≥400 (ng/mL) were observed in 24 and 13 patients, respectively. Patients with number of metastatic lesions ≤3 and >3 were found in 19 and 18 cases, respectively. Besides, initial dosage of lenvatinib with 12 mg and 8 mg was observed in 20 and 17 patients, respectively. Interestingly, three PD-1 inhibitors were used in this study. And camrelizumab, sintilimab, and pembrolizumab were administered in 17, 12, and 8 patients, respectively.
 |
Table 1 Baseline Characteristics of the 37 Patients with Unresectable Advanced HCC |
Efficacy of the 37 Patients with Unresectable Advanced HCC Who Received Lenvatinib, Plus PD-1 Blockades
From November 2018 to November 2021, a total of 37 patients with unresectable advanced HCC were recruited in this study. As illustrated in Figure 1, a total of 30 patients terminated the treatment and 7 patients were still in the treatment at the date of data cut-off. In addition, radiological evidence regarding the target lesions of the 37 patients included in this study was collected and analyzed retrospectively. The best overall response of each patient during the treatment of lenvatinib plus PD-1 blockades suggested that CR was observed in one patient, PR was noted in 11 patients, SD was found in 16 patients and progressive disease (PD) was reported in 9 patients, which yielded an ORR of 32.4% (95% confidence interval, CI: 18.0–49.8% and a DCR of 75.7%, 95%CI: 58.8–88.2%). Specifically, the waterfall plot for the change in target lesions of the 37 patients with advanced HCC who received lenvatinib plus PD-1 blockades was illustrated in Figure 2. Obviously, the treatment of lenvatinib plus PD-1 blockades demonstrated promising antitumor activity among patients with advanced HCC. And majority of the target lesions in the 37 patients with advanced HCC shrank dramatically.
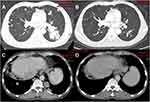
Noteworthy was the chest CT scan of the target lesions in liver and lung sites of one male patient before and after the administration of lenvatinib plus pembrolizumab shown in Figure 3. The target lesions disappeared completely after the treatment of lenvatinib plus sintilimab, which was deemed as CR and suggested that this patient benefited dramatically from the combination administration.
PFS and OS of the 37 Patients with Unresectable Advanced HCC Who Received Lenvatinib, Plus PD-1 Blockades
The data cut-off date of this study was March 5, 2022, resulting in a median follow-up duration among the 37 patients with advanced HCC of 15.8 months (follow-up range: 0.9–36.5 months). Regarding the PFS data, a total of 24 progression or death events were observed at the date of data cut-off, which produced a maturity of PFS data with 64.9%. Specifically, the PFS survival curve was shown in Figure 4, indicating that the median PFS of the 37 patients with advanced HCC who were treated with lenvatinib plus PD-1 blockades was 8.3 months (95%CI: 3.34–13.26). Interestingly, the 6-month PFS and 12-month PFS rate was 58.9% (95%CI: 41.2–72.8%) and 39.8% (95%CI: 23.4–55.7%), respectively.
 |
Figure 4 Progression-free survival curve of the 37 patients with unresectable advanced hepatocellular carcinoma who received lenvatinib plus PD-1 blockades administration. |
Simultaneously, OS data was also mature and available to be analyzed. A total of 21 death events were observed at the date of data cut-off, which yielded a maturity of OS data with 56.8%. As illustrated in Figure 5, the median OS of the 37 patients with advanced HCC who received lenvatinib plus PD-1 inhibitors was 17.8 months (95%CI: 7.19–28.41). In addition, the 12-month OS and 24-month OS rate was 57.8% (95%CI: 39.9–72.2%) and 34.3% (95%CI: 16.6–52.9%), respectively.
 |
Figure 5 Overall survival curve of the 37 patients with unresectable advanced hepatocellular carcinoma who received lenvatinib plus PD-1 blockades administration. |
The DoR of the 12 patients who achieved CR or PR in this study was also analyzed to present the response duration. As exhibited in Figure 6, the median DoR of the 12 patients who achieved CR or PR response was 9.6 months (95%CI: 3.03–16.17). Furthermore, the 12-month DoR and 20-month DoR rate was 47.6% (95%CI: 18.2–72.4%) and 35.7% (95%CI: 9.8–63.3%), respectively.
Furthermore, association analysis between PFS and baseline characteristic subgroups was performed separately to identify the potential significance of baseline characteristic subgroups. And the median PFS and 95%CI according to baseline characteristic subgroups were shown in Table 2. Obviously, almost all the patients benefited from lenvatinib plus PD-1 blockades treatment uniformly regardless of the baseline characteristic subgroups. Notably, ECOG performance status, alpha fetoprotein (AFP) status and number of metastatic lesions were dramatically correlated with PFS in the univariate analysis as illustrated in Table 2, suggesting that patients with ECOG performance status of 0–1 score conferred a significantly longer PFS than those with 2 score (median PFS: 9.2 vs 6.7 months, P=0.038), patients with AFP of <400 ng/mL were associated with superior PFS compared with those with ≥400 ng/mL (median PFS: 9.2 vs 6.7 months, P=0.021) and patients with number of metastatic lesions of ≤3 were relevant to better PFS than those with number of metastatic lesions of >3 (median PFS: 8.3 vs 5.9 months, P=0.019). Subsequently, those variables that were significantly associated with PFS were included in multivariate Cox regression analysis, which was presented in Table 2 and the Cox multivariate analysis indicated that both AFP status (hazard ratio, HR=0.73, P=0.033) and number of metastatic lesions (HR=0.69, P=0.026) were potential predictors for PFS independently. In addition, it should be noted that ECOG performance status conferred a significant association with PFS in univariate analysis. However, the difference was not statistically significant after Cox regression adjustment (HR=0.75, P=0.061).
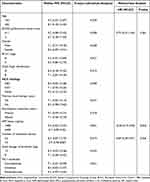 |
Table 2 Progression-free Survival of the 37 Patients with Advanced HCC According to Baseline Characteristic Subgroups in Univariate Analysis and Multivariate Cox Analysis |
Safety Profile of the 37 Patients with Unresectable Advanced HCC Who Received Lenvatinib Plus PD-1 Blockades Administration
All the adverse reactions occurred during the administration of lenvatinib plus PD-1 blockades were recorded retrospectively. During the combination administration, no new or unexpected adverse reactions were detected. And the adverse reactions regardless of attribution were found in 36 patients among the 37 patients included (97.3%). Furthermore, adverse reactions of grade 3–5 were noted in 22 patients among the 37 patients with advanced HCC (59.5%), including only one patient (2.7%) with grade 5 adverse reactions (hepatic failure) during the treatment of lenvatinib plus PD-1 blockades.
As shown in Table 3, the common adverse reactions detected in this study were hypertension (51.4%), fatigue (45.9), nausea and vomiting (40.5%), hepatotoxicity (35.1%), diarrhea (35.1%), rash (29.7%), proteinuria (24.3%), pneumonia (21.6%), reactive cutaneous capillary endothelial proliferation (RCCEP, 13.5%),and constipation (10.8%). Notably, the adverse reactions of grade 3–5 were hypertension (13.5%), fatigue (8.1%), nausea and vomiting (8.1%), hepatotoxicity (8.1%), diarrhea (5.4%), rash (5.4%), proteinuria (2.7%), and pneumonia (2.7%), respectively. One patient was dead from hepatic failure among the 13 patients with hepatotoxicity.
 |
Table 3 Safety Profile of the 37 Patients with Advanced HCC Who Received Lenvatinib Plus PD-1 Blockades |
Discussion
This study provided real-world evidence regarding the feasibility and tolerability of lenvatinib combined with PD-1 blockades as first-line therapy for patients with unresectable advanced HCC retrospectively. Collectively, the treatment of lenvatinib plus PD-1 inhibitors might be an efficacious and promising regimen for patients with unresectable advanced HCC clinically.
Although sorafenib became the only available multi-kinase inhibitor for patients with unresectable advanced HCC over the past 10 years, new antiangiogenic targeted drugs and PD-1/PD-L1 inhibitors dramatically changed the therapeutic landscape for patients with unresectable HCC both in first-line and second-line setting recently.21 As a result, we noticed that sorafenib, lenvatinib, donafenib, and atezolizumab plus bevacizumab regimens were all available therapeutic regimens as first-line therapy for patients with advanced HCC in China.9 Among these regimens, lenvatinib seemed to be the most commonly used regimen for the patients in clinical practice. Unfortunately, similar to the clinical activity of PD-1 inhibitors monotherapy clinically (ORR <20%),10 the ORR of lenvatinib monotherapy was also disappointing clinically (ORR <30%),22 which highlighted the necessity to investigate new combination regimens to expand the potential patients who might benefit from lenvatinib-based administration.
Patients included in present study were the unresectable advanced HCC who had not been treated with systemic therapy. Notably, seven patients received previous local treatment including TACE or RFA or radiotherapy, which was similar as the baseline characteristics in REFLECT clinical trial,8 indicating that the combination administration of lenvatinib plus PD-1 blockades in first-line setting was reasonable. In addition, lenvatinib and PD-1 blockades had indications as the first-line and second-line treatment for patients with advanced HCC in China, respectively. From the objective view, ORR of the 37 patients with unresectable advanced HCC who received lenvatinib plus PD-1 blockades was 32.4%, DCR was 75.7%, the median PFS was 8.3 months and median OS was 17.8 months, which was higher than the ORR, DCR and median PFS and OS among those who received lenvatinib monotherapy in REFLECT clinical trial (ORR=24.1%, DCR=75.5%, median PFS=7.3 months and median OS=13.6 months) numerically.8 These results preliminarily indicated that combination of lenvatinib plus PD-1 blockades might result in synergistic action.
However, it should be noted that 168 Japanese patients were assigned to the lenvatinib arm (N=81) or the sorafenib arm (N=87) among the 954 patients in the overall population according the REFLECT clinical trial, which produced a better OS in Japanese population than that in overall population (17.6 months and 17.8 months vs 13.6 months and 12.3 months, respectively). It seemed that the efficacy of lenvatinib in Japanese population was similar to that of lenvatinib plus PD-1 blockades in our study (ORR: 29.6% vs 32.4%, median PFS: 7.2 months vs 8.3 months, median OS: 17.6 months vs 17.8 months).23 Moreover, this finding should be interpreted with caution, we noticed that Japanese patients had a higher proportion of intermediate stage of BCLC-B (38% vs 24.3%) and ECOG performance status of 0 score (94% vs 56.8%) in REFLECT study than those in our study. In addition, the nationwide HCC surveillance program had been conducted in Japan for decades. All these factors might contribute to the superior prognosis of patients with HCC in Japan irrespective of the therapeutic regimens (similar median OS in lenvatinib and sorafenib). Consequently, lenvatinib plus PD-1 blockades might be a promising regimen for patients in China and the conclusion should be confirmed subsequently.
An attractive trial of Keynote 524 initiated by RS Finn et al investigated the efficacy and safety of lenvatinib plus pembrolizumab among patients with unresectable HCC.15 A total of 100 patients with no previously systemic treatment unresectable HCC who were administered with lenvatinib plus pembrolizumab in first-line setting were included this phase Ib single-arm study, yielding an ORR of 46% (mRECIST), a DCR of 86% and a median PFS of 9.3 months, a median OS of 22.0 months. These results were basically consistent with those in our study even though the efficacy and prognosis in our study seemed to be slightly worse numerically. We speculated the discrepancy of the clinical outcomes between Keynote 524 and our study might be attributed to the following two aspects: firstly, patients with ECOG performance status of 2 score were also included in our study, whereas patients included in the Keynote 524 were only those with ECOG 0–1 score. Furthermore, the association analysis in our study suggested that patients with ECOG of 2 score conferred a worse prognosis, which was consistent with the previous study.24 Secondly, it should be noted that our study was designed as a retrospective analysis. Management of the patients in retrospective study was not sufficient and normative compared with a well-designed clinical trial, thus compromising the efficacy and prognosis in our study to some extent, which was also proved by the previous retrospective study among patients with HCC.25 Interestingly, we noticed that a recently reported retrospective study initiated by Sun et al explored the real-world effectiveness of lenvatinib plus PD-1 inhibitors in advanced HCC.26 A total of 84 patients with HCC receiving lenvatinib plus PD-1 blockades were recruited for retrospective analysis, including 31 patients with tumor occupation ≥50% and 30 patients with Vp4 invasion, which resulted in an ORR of 20.2%, a DCR of 40.5%, a median PFS of 6.6 months and a median OS of 11.4 months. Obviously, the clinical outcomes in this study seemed to be worse compared with those in our study. The possible explanation might be that considerable patients with tumor occupation ≥50% and Vp4 invasion were included in Sun et al's study, which might have compromised the efficacy of the regimen.27
We also observed that another retrospective study initiated by Huang et al investigated the efficacy of lenvatinib plus pembrolizumab or nivolumab among patients with previously treated HCC.28 The regimen produced an ORR of 24.1%, a DCR of 65.5%, a median PFS of 5.5 months and a median OS over 12 months. Furthermore, among the 12 patients with CR or PR response, the median DoR was 9.6 months (95%CI: 3.03–16.17), which was consistent with the DoR of the Keynote 524 trial (median DoR was 12.6 month).15 All these above studies had confirmed the synergistic action of lenvatinib plus PD-1 blockades preliminarily. Although the possible explanation of the superior efficacy was still not substantially interpreted, the previous study had indicated that hyperangiogenic nature of the tumor microenvironment might deteriorate and attenuate the efficacy of PD-1 blockades.29 Therefore, the immunomodulatory effect of lenvatinib might contribute to the therapeutic activity of PD-1 blockades, thus improving the sensitivity of HCC to this combination therapy.30 Consequently, we also observed that atezolizumab plus bevacizumab exhibited convincing clinical outcomes for patients with unresectable HCC, becoming the standard of care as first-line therapy.14 As a result, all these studies suggested that the combination of lenvatinib plus PD-1 blockades might confer a synergistic antitumor activity theoretically and clinically.
Meanwhile, the association between PFS and baseline characteristic subgroups was also analyzed in our study. It seemed that patients with advanced HCC might benefited from lenvatinib plus PD-1 blockades administration uniformly regardless of the majority baseline characteristic subgroups, which was basically consistent with a previous study initiated by Zhu et al that investigated the efficacy of lenvatinib monotherapy and lenvatinib-based combination therapy for patients with unresectable HCC.31 The exploratory analysis demonstrated that almost all the baseline characteristic subgroups might benefit from lenvatinib and lenvatinib-based combination administration. These conclusions highlighted that the effectiveness of lenvatinib plus PD-1 blockades was stable and balanced. Nevertheless, it seemed that ECOG performance status, AFP status and number of metastatic lesions were significantly associated with PFS in univariate analysis. And AFP status and number of metastatic lesions were independent factors to predict PFS in multivariate Cox analysis. These results exhibited that patients with AFP ≥400 ng/mL and metastatic lesions of >3 might not benefit from the administration of lenvatinib plus PD-1 blockades. However, this conclusion should be interpreted with caution. As we know, a previous relevant study had found that patients with metastatic lesions of >3 trended to confer a worse prognosis regardless of the therapeutic regimens.32 In addition, another exploratory study suggested that patients with AFP ≥400 ng/mL possessed worse prognosis among patients with HCC who received hepatectomy.33 Collectively, whether number of metastatic lesions and AFP status might be used as prognostic biomarkers for the administration of lenvatinib plus PD-1 blockades should be validated in subsequent prospective clinical trials. Another promising result of our study was that patients with HBV positive who accounted for 73% of the 37 patients with HCC might benefit from the administration of lenvatinib plus PD-1 blockades uniformly, which was an encouraging finding in our opinion. To our knowledge, different from the etiology of HCC in western countries, considerable HCC in China could result from the HBV infection and the etiology of HCC from HBV infection accounted for approximately 80% among all the HCC diagnosed in China.34 Therefore, patients enrolled in this study were representative of the patients with HCC in China and the finding that patients with HBV infection might benefit from lenvatinib plus PD-1 blockades were in concert with the study reported previously.26 Collectively, clinical outcomes of lenvatinib plus PD-1 blockades among patients with unresectable advanced HCC should be elucidated in prospective clinical trials in the future.
With regard to the safety profile of the combination regimen, since no new or unexpected adverse reactions were detected during the combination administration, it seemed that the safety profile of lenvatinib plus PD-1 blockades were acceptable and manageable, which was in accordance with the overall safety profile in the previous study regarding the combination therapy of lenvatinib plus PD-1 blockades among patients with advanced HCC.35 However, it should be noted that we observed one male patient had experienced severe hepatic failure that resulted in death after two months administration of lenvatinib plus camrelizumab. In addition, the incidence of grade ≥3 adverse reactions was 59.5%, which was slightly higher than that observed in the study regarding lenvatinib monotherapy or PD-1 monotherapy among patients with advanced HCC (grade 3–4 adverse reaction was approximately 55% and 19%, respectively).22,36 Consequently, it seemed that patients with worse hepatic function should be cautioned when they were treated with lenvatinib plus PD-1 blockades in real-world clinical practice. Moreover, the common adverse reactions such as hypertension and proteinuria might be attributed to the administration of lenvatinib. Other adverse reactions such as rash, pneumonia and reactive cutaneous capillary endothelial proliferation (RCCEP) might have resulted from the administration of PD-1 blockades.37 Of note, RCCEP was deemed as the specific adverse reaction of camrelizumab that was administered among 17 patients in our study. As a result, the actual incidence for RCCEP could be 29.4%, which might be slightly lower than that observed for camrelizumab monotherapy in the other cancer (approximately 60%).38 This discrepancy regarding the incidence of RCCEP might be attributed to the administration of Lenvatinib that might play a key role to attenuate the incidence of RCCEP during camrelizumab therapy. Therefore, the overall adverse reactions of lenvatinib plus PD-1 blockades for patients with unresectable advanced HCC were acceptable and manageable. Collectively, lenvatinib plus PD-1 blockades exhibited promising effectiveness (ORR of 32.4%, DCR of 75.7%, median PFS of 8.3 months and median OS of 17.8 months) and acceptable toxicity in first-line setting for patients with unresectable HCC, which might be a meaningful therapeutic option for the patients clinically in our opinion. However, it should be noted that patients with ECOG performance 2 score, AFP ≥400 (gm/mL), number of metastatic legions of >3 and those with worse hepatic function should be caution for the combination regimen in clinical practice own to the inferior efficacy or worse adverse reaction. We thought that some potential biomarkers such as PD-L1 expression, TMB and MSI status should be explored to identify the potential significance to predict the efficacy of lenvatinib plus PD-1 blockades in the subsequent exploratory study.
From an objective view, the major limitation of our study was that the sample size was relatively small as a retrospective study, only 37 patients were recruited for analysis. Feasibility and tolerability of lenvatinib plus PD-1 blockades was still needed to be confirmed in more patients with HCC. Furthermore, multi-PD-1 blockades were administered in our study, which might yield heterogeneous and diverse efficacy. Besides, as a retrospective study, unintentional biases could not be avoided and the PFS and OS data maturity was limited compared with clinical trials. Still and all, our study was of promising clinical implication to provide the real-world evidence for lenvatinib plus PD-1 blockades for patients with unresectable advanced HCC in first-line setting.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–791. doi:10.1097/cm9.0000000000001474
3. Grandhi MS, Kim AK, Ronnekleiv-Kelly SM, et al. Hepatocellular carcinoma: from diagnosis to treatment. Surg Oncol. 2016;25(2):74–85. doi:10.1016/j.suronc.2016.03.002
4. Xu XF, Xing H, Han J, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: a multicenter study from China. JAMA Surg. 2019;154(3):209–217. doi:10.1001/jamasurg.2018.4334
5. Wang H, Lu Z, Zhao X. Tumorigenesis, diagnosis, and therapeutic potential of exosomes in liver cancer. J Hematol Oncol. 2019;12(1):133. doi:10.1186/s13045-019-0806-6
6. Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5(1):146. doi:10.1038/s41392-020-00264-x
7. Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/s1470-2045(08)70285-7
8. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/s0140-6736(18)30207-1
9. Qin S, Bi F, Gu S, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled Phase II-III trial. J Clin Oncol. 2021;39(27):3002–3011. doi:10.1200/jco.21.00163
10. Liu JKH, Irvine AF, Jones RL, Samson A. Immunotherapies for hepatocellular carcinoma. Cancer Med. 2022;11(3):571–591. doi:10.1002/cam4.4468
11. Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, Phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi:10.1016/s1470-2045(20)30011-5
12. Rizzo A, Ricci AD, Gadaleta-Caldarola G, Brandi G. First-line immune checkpoint inhibitor-based combinations in unresectable hepatocellular carcinoma: current management and future challenges. Expert Rev Gastroenterol Hepatol. 2021;15(11):1245–1251. doi:10.1080/17474124.2021.1973431
13. Song Y, Fu Y, Xie Q, et al. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020;11:1956. doi:10.3389/fimmu.2020.01956
14. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
15. Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi:10.1200/jco.20.00808
16. Rizzo A, Ricci AD. PD-L1, TMB, and other potential predictors of response to immunotherapy for hepatocellular carcinoma: how can they assist drug clinical trials? Expert Opin Investig Drugs. 2022;31(4):415–423. doi:10.1080/13543784.2021.1972969
17. Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21(36):10327–10335. doi:10.3748/wjg.v21.i36.10327
18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline. Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
19. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
20. Song PF, Xu N, Li Q. Efficacy and Safety of anlotinib for elderly patients with previously treated extensive-stage SCLC and the prognostic significance of common adverse reactions. Cancer Manag Res. 2020;12:11133–11143. doi:10.2147/cmar.s275624
21. Galle PR, Dufour JF, Peck-Radosavljevic M, Trojan J, Vogel A. Systemic therapy of advanced hepatocellular carcinoma. Future Oncol. 2021;17(10):1237–1251. doi:10.2217/fon-2020-0758
22. Ikeda K, Kudo M, Kawazoe S, et al. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52(4):512–519. doi:10.1007/s00535-016-1263-4
23. Yamashita T, Kudo M, Ikeda K, et al. REFLECT-A phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55(1):113–122. doi:10.1007/s00535-019-01642-1
24. Hao YY, Qiao YP, Cheng JD. Clinical activity and safety of anlotinib combined with PD-1 blockades for patients with previously treated small cell lung cancer. Int J Gen Med. 2021;14:10483–10493. doi:10.2147/ijgm.s337316
25. Wang DX, Yang X, Lin JZ, et al. Efficacy and safety of lenvatinib for patients with advanced hepatocellular carcinoma: a retrospective, real-world study conducted in China. World J Gastroenterol. 2020;26(30):4465–4478. doi:10.3748/wjg.v26.i30.4465
26. Sun X, Zhang Q, Mei J, et al. Real-world efficiency of lenvatinib plus PD-1 blockades in advanced hepatocellular carcinoma: an exploration for expanded indications. BMC Cancer. 2022;22(1):293. doi:10.1186/s12885-022-09405-7
27. Chuma M, Uojima H, Hiraoka A, et al. Analysis of efficacy of lenvatinib treatment in highly advanced hepatocellular carcinoma with tumor thrombus in the main trunk of the portal vein or tumor with more than 50% liver occupation: a multicenter analysis. Hepatol Res. 2021;51(2):201–215. doi:10.1111/hepr.13592
28. Huang X, Xu L, Ma T, et al. Lenvatinib plus immune checkpoint inhibitors improve survival in advanced hepatocellular carcinoma: a retrospective study. Front Oncol. 2021;11(751159). doi:10.3389/fonc.2021.751159
29. Lotfinejad P, Kazemi T, Mokhtarzadeh A, et al. PD-1/PD-L1 axis importance and tumor microenvironment immune cells. Life Sci. 2020;259:118297. doi:10.1016/j.lfs.2020.118297
30. Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109(12):3993–4002. doi:10.1111/cas.13806
31. Zhu Y, Sun P, Wang K, et al. Efficacy and safety of lenvatinib monotreatment and lenvatinib-based combination therapy for patients with unresectable hepatocellular carcinoma: a retrospective, real-world study in China. Cancer Cell Int. 2021;21(1):503. doi:10.1186/s12935-021-02200-7
32. Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117(19):4475–4483. doi:10.1002/cncr.25960
33. Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013;11(212). doi:10.1186/1477-7819-11-212
34. Jia L, Gao Y, He Y, Hooper JD, Yang P. HBV induced hepatocellular carcinoma and related potential immunotherapy. Pharmacol Res. 2020;159:104992. doi:10.1016/j.phrs.2020.104992
35. Wu JY, Yin ZY, Bai YN, et al. Lenvatinib combined with anti-PD-1 antibodies plus transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2021;8:1233–1240. doi:10.2147/jhc.s332420
36. Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi:10.1200/jco.19.01307
37. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–2471. doi:10.1016/s0140-6736(17)31827-5
38. Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13(1):47. doi:10.1186/s13045-020-00886-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.