Back to Journals » Cancer Management and Research » Volume 11
Feasibility and safety of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy in patients with advanced stage ovarian cancer: a single-center experience
Authors Gao T, Huang X, Wang W, Wu M, Lin Z, Li J
Received 29 April 2019
Accepted for publication 9 July 2019
Published 24 July 2019 Volume 2019:11 Pages 6931—6940
DOI https://doi.org/10.2147/CMAR.S213882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Tian Gao,1,* Xiao-xin Huang,1,* Wu-yun Wang,1,2 Miao-fang Wu,1 Zhong-qiu Lin,1 Jing Li1
1Department of Gynecologic Oncology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou 510120, People’s Republic of China; 2Department of Gynecology, Hangzhou Women’s Hospital, Hangzhou 310008, People’s Republic of China
*These authors contributed equally to this work
Objectives: For patients with advanced ovarian cancer, neoadjuvant chemotherapy (NACT) can significantly increase the rate of optimal cytoreduction. However, this does not translate into a survival benefit. The aim of this study was to investigate the feasibility and effect of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy (NLHIPEC).
Methods: Between March 2016 and February 2018, 14 patients with advanced ovarian cancer who were not candidates for optimal cytoreduction via primary debulking surgery (PDS) received NLHIPEC. Their clinical data were retrospectively analyzed.
Results: No patients experienced intraoperative complications during NLHIPEC. Grade 3 adverse events (AEs) were noted in two (14.3%) patients, and all patients received planned NACT without dose delay or dose reduction. Following NACT, CA125 levels <35 U/mL and <20 U/mL were observed in six (42.9%) patients and five (35.7%) patients, respectively. All patients underwent interval debulking surgery (IDS) after the last NACT cycle. After IDS, R0 resection was achieved in 10 (71.4%) patients without intraoperative injury, and one (7.1%) patient developed a grade 3 AE. During a median follow-up time of 16 months, no patients died of disease, and the median progression-free survival (PFS) was not achieved. Progression was noted in six (42.9%) patients (range, 9–21 months).
Conclusions: NLHIPEC appears to be a feasible option for ovarian cancer patients who have a low likelihood of achieving optimal cytoreduction during PDS.
Keywords: ovarian cancer, hyperthermic intraperitoneal chemotherapy, neoadjuvant, laparoscopy
Background
Among all invasive gynecologic cancers, ovarian cancer is the leading cause of death. Nearly 75% of women with ovarian cancer are diagnosed with advanced stage disease (International Federation of Gynecology and Obstetrics [FIGO] IIIC or IV) at presentation.1 Treatment with primary debulking surgery (PDS) followed by chemotherapy has been the standard of care for ovarian cancer patients. Because each 10% increase in maximal cytoreduction is associated with a 5.5% increase in median survival, the primary aim of debulking surgery is no gross residual disease.2 If it is difficult to achieve this aim via PDS, neoadjuvant chemotherapy (NACT) followed by interval debulking surgery (IDS) can be considered a reasonable alternative.1 Although NACT can significantly increase the optimal cytoreduction rate, this does not translate into a survival benefit.1,3,4 Therefore, novel approaches to enhance the therapeutic effects of NACT need to be explored.
The peritoneal cavity is the principal site of ovarian disease. Given that systemic chemotherapy has poor access to the peritoneum due to the plasma-peritoneal barrier, intraperitoneal chemotherapy has been proposed and validated as an effective therapy.5,6 Intraperitoneal chemotherapy can also be delivered under hyperthermic conditions, which is termed hyperthermic intraperitoneal chemotherapy (HIPEC). Because heat can propagate the cytotoxicity of selected chemotherapeutic drugs, HIPEC in the treatment of ovarian cancer has drawn increasing interest. Recent studies have shown that HIPEC can improve the survival outcomes of ovarian cancer patients.7,8 Considering the potential value of HIPEC, we hypothesized that it could be used in a neoadjuvant setting and might enhance the effect of NACT. The primary aim of this study was to evaluate the feasibility and primary effect of neoadjuvant laparoscopic HIPEC (NLHIPEC) in ovarian cancer patients who are not candidates for optimal cytoreduction via PDS.
Materials and methods
Patients
After Institutional Review Board (IRB) approval (#SYSEC-KY-KS-2019-018) was obtained from the Sun Yat-sen Memorial Hospital Institutional Review Board, we retrospectively identified patients who received NLHIPEC and underwent subsequent IDS for ovarian cancer at our institution between March 2016 and February 2018. Individualized treatment strategies were made by a multidisciplinary team (MDT), which consisted of three gynecologic oncologists, two pathologists and two radiologists. All patients deemed appropriate surgical candidates underwent an initial laparoscopic evaluation for pathological diagnosis and peritoneal disease assessment. The Fagotti scoring system was utilized to determine the possibility of optimal cytoreduction.9 Patients with a Fagotti score ≥8 were offered NACT and subsequent IDS, while patients with a Fagotti score < 8 were offered PDS. Eligibility criteria to receive NLHIPEC were as follows: Fagotti score ≥8, age 18–75 years, adequate bone marrow, normal hepatic and renal function and signed informed consent. Contraindications for NHIPEC were as follows: nonepithelial or borderline histology, American Society of Anesthesiologists (ASA) score IV and extensive abdominal adhesions, active inflammation or severe comorbidities.
Technique for NLHIPEC
The NLHIPEC was started with laparoscopic evaluation. The site of the first port placement was decided at the surgeon’s discretion based on imaging and clinical findings. The preferred technique for the creation of the pneumoperitoneum was via the optical access technique in the umbilical area. After induction of a CO2 pneumoperitoneum, an additional three trocars of 5 mm were placed under direction vision in order to enable complete dissection of adhesions and a thorough inspection of the abdominal cavity. The Fagotti score was generated according to Fagotti’s study, and biopsy was obtained and examined by frozen section analysis.9 All surgical evaluation procedures were performed by a team comprising two experienced gynecologic oncologists. Following surgical evaluation and histologic confirmation of the diagnosis, four tubes were placed via the laparoscopic ports (two in the bilateral subdiaphragmatic space for use as inlet tubes and two in the pelvic cavity for use as an outlet tubes) which were used to administrate HIPEC (Figure 1). Then, HIPEC was given within 24 hrs after primary laparoscopic evaluation. The NACT regimen was paclitaxel 175 mg/m2 administered by intravenous infusion over 3 hrs followed by cisplatin 70 mg/m2 administered by HIPEC. A high-precision hyperthermic intraperitoneal perfusion treatment system (approved by the State Food Drug Administration of China, approval No. 2009-3260924) was utilized, which has a precision of ±0.10 °C for temperature control and ±5% for flow control. Cisplatin was added to 3000 mL of saline solution, which was heated and circulated at a flow rate of 300–500 mL/min. The perfusion velocity was adjusted to ensure that the entire abdomen was exposed to the perfusate (an initial velocity was 300 mL/min, and then it was increased gradually until the patient felt floated or a flow rate of 500 mL/min was achieved). An intraabdominal temperature of 43 °C was maintained and measured by the treatment system using temperature monitoring probes in the infusion and outflow catheters. The HIPEC procedure took 90 mins in total, consisting of a 30 min preheating period and a 60 min perfusion period. After HIPEC treatment, the four tubes were removed immediately to retain as much cisplatin in the abdominal cavity as possible. NLHIPEC-related adverse events (AEs) which presented within three weeks of NLHIPEC were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Version 4.0. Following NLHIPEC, two additional cycles of NACT were planned. Both paclitaxel and platinum-based chemotherapy were given intravenously every 21 days.
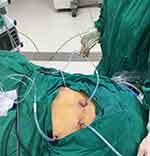 |
Figure 1 Placement of four tubes via the laparoscopic ports. |
Management and safety evaluation following NACT
During the NACT period, all patients were reviewed weekly, and the serum levels of CA125 were measured after each cycle of NACT. IDS was performed within four weeks after the last cycle of NACT. The result of IDS was classified using a cytoreductive completeness scoring (CCS) system, where CC-0 (R0 resection) is defined as no visible disease after cytoreduction and CC-1, 2 and 3 (CC-1+) scores (residual tumors less than 2.5 mm, between 2.5 mm and 2.5 cm, and greater than 2.5 cm, respectively) were grouped together.10 The extent and complexity of the surgical procedures were categorized according to the score.11 Complications that presented within two weeks of IDS were also graded according to the NCI CTCAE Version 4.0. Following IDS, all patients received at least three cycles of systemic platinum-based chemotherapy.
Statistical analysis
All statistical analyses were conducted using IBM SPSS (version 13.0, SPSS, Chicago, IL, USA). The initial data analysis was conducted by employing a descriptive statistical approach and analysis of variance. The Kolmogorov-Smirnov test was used to verify standard normal distribution assumptions of continuous variables. Survival times were calculated from the date of laparoscopic evaluation to the date of relapse, death from any reason, and last follow-up.
Results
Patient demographics
During the study period, 14 patients with a Fagotti score ≥8 received NLHIPEC. Their baseline characteristics are outlined in Tables 1 and S1. Relevant comorbidities, including cardiovascular disease, hypertension, type 2 diabetes and a history of chronic obstructive pulmonary disease and stroke, were present in five (35.7%) patients.
 |
Table 1 Patient and disease characteristics |
Safety of NLHIPEC
We did not observe any intraoperative complications during laparoscopic evaluation and HIPEC tube placement. Following NLHIPEC, no patient was admitted to the intensive care unit, and the median length of hospital stay was 2 days (range, 1–3 days). Table 2 presents AEs that manifested within three weeks of NLHIPEC. AEs of grade 4 were not observed, and AEs of grade 3 were noted in two (14.3%) patients. The most common events were neutropenia and abdominal pain. No febrile neutropenia, thromboembolic events, infection, gastrointestinal perforation or renal toxicity events were observed.
 |
Table 2 Neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy related adverse events |
Effects of NLHIPEC
Following NLHIPEC, 9 patients with ascites at diagnosis showed ascite regression; all patients received two subsequent cycles of NACT without dose delay or dose reduction. Following the third NACT, normalization of CA125 levels was observed in six (42.9%) patients, and a CA125 level <20 U/mL was observed in five (35.7%) patients. All patients in our study received IDS within 4 weeks of the last NACT cycle. Table 3 summarizes the surgical characteristics and outcomes. Aggressive complex surgical cytoreduction (surgical complexity score ≥8) was performed in two (14.3%) patients. CC-0 was achieved in 10 (71.4%) patients. In the IDS procedure, no intraoperative injuries were recorded, and blood transfusion was required in one patient (7.1%). Following IDS, one (7.1%) patient developed grade 3 thrombosis, and no patients developed AEs of grade 4 (Table S1). During a median follow-up time of 16 months (range, 9–26 months), no patient died of disease, and the median progression-free survival (PFS) was not achieved. Progression was noted in six (42.9%) patients, with PFS ranging from 9 months to 21 months (mean, 14 months). No patient developed platinum-resistant recurrence.
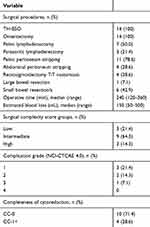 |
Table 3 Features of surgical complexity and outcomes |
Discussion
The current study describes our experience of delivering NACT with a laparoscopic HIPEC technique. NLHIPEC could combine the pharmacokinetic advantages of HIPEC with the advantages of minimally invasive surgery. Hyperthermia has a direct antitumor effect, increases the penetration of chemotherapy at the peritoneal surface and augments the cytotoxic effects of platinum-based compounds.12 Intraperitoneal chemotherapy has also been proven to improve the survival outcomes of ovarian cancer patients.13,14 Adding hyperthermia to intraperitoneal cisplatin results in synergistic effects, which could further enhance the effect of intraperitoneal chemotherapy.15,16 In addition, laparoscopy allows for adhesiolysis with adequate catheter placement, which results in optimal chemotherapy exposure to the peritoneal surfaces. Compared to an open approach, laparoscopy is associated with an increased abdominal pressure, which may improve drug penetration into the tumor tissue.17 Additionally, obviating the need for nontherapeutic laparotomies could reduce postoperative discomfort, thereby decreasing the risk of delay in starting subsequent NACT.
In the present study, we administered NLHIPEC to 14 patients. No NLHIPEC-related serious AEs were noted, and NLHIPEC did not prevent the administration of subsequent NACT and IDS. In the literature, few studies on NLHIPEC in the treatment of ovarian cancer are available. However, the safety of this technique has been demonstrated in gastric cancer patients with inoperable disease. Yonemura et al reported 105 gastric cancer patients who received NLHIPEC for peritoneal metastasis.18 Following NLHIPEC, serious AEs (grade 3 and grade 4) were noted in 4 (7.7%) patients. Although it is difficult to make a cross-study comparison, we believe that Yonemura’s result is in line with ours suggesting that NLHIPEC is safe and tolerable.
Following NACT, both patients with a CA125 level <35 U/mL and those with a CA125 level <20 U/mL were reported to have improved survival outcomes.19–23 Therefore, we used these measurements as cutoff values. Of our patients, 42.9% had a CA125 level <35 U/mL, and 35.7% had a CA125 level <20 U/mL following NACT. The results are consistent with previous studies, where 17.8–57% of patients were reported to have a pre-IDS CA125 level <35 U/mL,19,22,24–26 and 31.3–53.3% of patients had a pre-IDS CA125 level <20 U/mL.21,23 In addition, the median percent reduction in CA125 of our cohort was 94.7% (range, 86.3–99.4), which is consistent with Mahdi’s study, where the reported median percentage was 94.5% (range, 33–97.7).21 Of note, most patients in the abovementioned studies underwent more than three cycles of NACT,19,21,24,25 while all patients in our study received exactly three cycles of NACT. Considering that each additional cycle of NACT between 3 and 6 cycles is associated with a 4-month decrease in OS,27 we believe that using HIPEC in a neoadjuvant setting could allow NACT to more efficiently decrease the level of CA125 and thus enhance the therapeutic effect of NACT.
Any remaining disease following IDS contains stem cells that may induce chemo-resistance, and converging evidence has highlighted the incremental survival benefit with cytoreduction to no gross residual disease.28 Therefore, for ovarian cancer patients with advanced disease, the ultimate goal of IDS should be R0 resection. Previous randomized controlled trials (RCTs) have validated that R0 resection can be more easily achieved among NACT patients than PDS patients.3,4,29 In these trials, NACT was administered intravenously, and the reported rate of R0 resection following NACT ranged from 39% to 55%.3,4,29 The rate of R0 resection was 71.4% in the current study, which is much better than that in previous studies. The increased rate may be attributed to the effect of NLHIPEC.
Although NACT decreases treatment-related morbidity and mortality, many types of complicated surgical procedures are still involved in IDS. Researchers from the Mayo Clinic showed that 14.9% of ovarian cancer patients still needed to undergo high-complexity debulking surgery (surgical complexity scores ≥8) following NACT.30 That result is in line with ours. However, 11% of their patients developed grade 3/4 complications after IDS, which is higher than the rate (7.1%) noted in our cohort. Three RCTs, EORTC/NCIC, CHORUS and JGOG0602, compared PDS with NACT followed by IDS.3,4,29 In these trials, post-IDS death and IDS-related grade 3/4 AEs were recorded in 0.4–0.7% and 4.6–14% of NACT patients, respectively; the most common grade 3/4 AE after IDS was hemorrhage with an incidence of 4.1–6%.3,4,29 In the present study, no IDS-related death, grade 4 AE or postoperative hemorrhage was noted; a grade 3 AE was only observed in only one (7.1%) patient. Moreover, compared with JGOG0602,29 where blood transfusion was recorded in 52.7% of patients in the NACT group, our study indicated fewer patients (7.1%) who required blood transfusion. Given the published data and our results, we believe that NLHIPEC would not adversely affect the subsequent IDS and may even make the complicated surgical procedures much easier to perform.
NACT may increase the risk of developing platinum resistance by exposing large tumor volumes to chemotherapy, which presents a major concern in clinical settings. Previous studies have shown that NACT patients are more likely to develop platinum-resistant recurrence than patients undergoing PDS.31–33 In the literature, the reported incidence of platinum-resistant recurrence among NACT patients was 27.8–50%.31,32,34 In our cohort, surprisingly, no patient experienced platinum-resistant recurrence. A possible explanation for this finding is that hyperthermia can target and eliminate cancer stem cells that drive therapy resistance and tumor recurrence.35
Several limitations of the current study should be acknowledged, including its small sample size, retrospective nature and limited follow-up time. In addition, peritoneal cancer index (PCI), which is a useful tool to assess tumor burden and extend of metastases, was not recorded in every patient. An efficacy analysis using PCI could not be conducted. Therefore, our results should be interpreted with caution. In addition, because of the absence of a control group, it is difficult to explore the difference between intraperitoneal NACT and NLHIPEC.
To our knowledge, this is the first study to evaluate the feasibility of NLHIPEC among ovarian cancer patients with advanced disease. Although the present study has no statistical power, the results are encouraging and suggest excellent tolerance of NLHIPEC. Moreover, we observed good disease control among our patients. The findings of the current study should be confirmed prospectively. If validated, NLHIPEC could be considered a more efficient way to deliver NACT, which may confer survival benefits for ovarian cancer patients.
Ethics
Patient data were kept to a minimum and stored in a secure manner on a database under the control of the Sun Yat-sen Memorial Hospital to which only the corresponding author has access, and patient consent to review their medical records is not required by the Sun Yat-sen Memorial Hospital Institutional Review Board. The present study was performed in accordance with the 1975 Declaration of Helsinki.
Data availability
The datasets used in the present current study are available from the corresponding author on reasonable request.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant 81702549). The authors declare that neither the article nor any part of its essential substance, tables, or figures has been or will be published or submitted elsewhere.
Disclosure
Dr Jing Li reports grants from the National Natural Science Foundation of China, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(28):3460–3473. doi:10.1200/JCO.2016.68.6907z
2. Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi:10.1200/JCO.2002.20.5.1248
3. Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–953. doi:10.1056/NEJMoa0908806
4. Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–257. doi:10.1016/S0140-6736(14)62223-6
5. Sehouli J, Senyuva F, Fotopoulou C, et al. Intra-abdominal tumor dissemination pattern and surgical outcome in 214 patients with primary ovarian cancer. J Surg Oncol. 2009;99(7):424–427. doi:10.1002/jso.21288
6. Berek JS, Kehoe ST, Kumar L, Friedlander M. Cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2018;143(Suppl 2:):59–78. doi:10.1002/ijgo.12614
7. van Driel WJ, Koole SN, Sikorska K, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230–240. doi:10.1056/NEJMoa1708618
8. Spiliotis J, Halkia E, Lianos E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22(5):1570–1575. doi:10.1245/s10434-014-4157-9
9. Fagotti A, Fanfani F, Ludovisi M, et al. Role of laparoscopy to assess the chance of optimal cytoreductive surgery in advanced ovarian cancer: a pilot study. Gynecol Oncol. 2005;96(3):729–735. doi:10.1016/j.ygyno.2004.11.031
10. Hoskins WJ, McGuire WP, Brady MF, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170(4):
11. Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197(6):676 e1–7. doi:10.1016/j.ajog.2007.10.495
12. Zivanovic O, Chi DS, Filippova O, Randall LM, Bristow RE, O’Cearbhaill RE. It’s time to warm up to hyperthermic intraperitoneal chemotherapy for patients with ovarian cancer. Gynecol Oncol. 2018;151(3):555–561. doi:10.1016/j.ygyno.2018.09.007
13. Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. doi:10.1056/NEJMoa052985
14. Tewari D, Java JJ, Salani R, et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2015;33(13):1460–1466. doi:10.1200/JCO.2014.55.9898
15. Crile G
16. Van der Speeten K, Stuart OA, Sugarbaker PH. Pharmacokinetics and pharmacodynamics of perioperative cancer chemotherapy in peritoneal surface malignancy. Cancer J. 2009;15(3):216–224. doi:10.1097/PPO.0b013e3181a58d95
17. Gesson-Paute A, Ferron G, Thomas F, de Lara EC, Chatelut E, Querleu D. Pharmacokinetics of oxaliplatin during open versus laparoscopically assisted heated intraoperative intraperitoneal chemotherapy (HIPEC): an experimental study. Ann Surg Oncol. 2008;15(1):339–344. doi:10.1245/s10434-007-9571-9
18. Yonemura Y, Ishibashi H, Hirano M, et al. Effects of neoadjuvant laparoscopic hyperthermic intraperitoneal chemotherapy and neoadjuvant intraperitoneal/systemic chemotherapy on peritoneal metastases from gastric cancer. Ann Surg Oncol. 2017;24(2):478–485. doi:10.1245/s10434-016-5487-6
19. Matsuhashi T, Takeshita T, Yamamoto A, et al. Serum CA 125 level after neoadjuvant chemotherapy is predictive of prognosis and debulking surgery outcomes in advanced epithelial ovarian cancer. J Nippon Med Sch. 2017;84(4):170–176. doi:10.1272/jnms.84.170
20. Pelissier A, Bonneau C, Chereau E, et al. CA125 kinetic parameters predict optimal cytoreduction in patients with advanced epithelial ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2014;135(3):542–546. doi:10.1016/j.ygyno.2014.09.005
21. Mahdi H, Maurer KA, Nutter B, Rose PG. The impact of percent reduction in CA-125 levels on prediction of the extent of interval cytoreduction and outcome in patients with advanced-stage cancer of mullerian origin treated with neoadjuvant chemotherapy. Int J Gynecol Cancer. 2015;25(5):823–829. doi:10.1097/IGC.0000000000000434
22. Le T, Faught W, Hopkins L, Fung-Kee-Fung M. Importance of CA125 normalization during neoadjuvant chemotherapy followed by planned delayed surgical debulking in patients with epithelial ovarian cancer. J Obstet Gynaecol Can. 2008;30(8):665–670. doi:10.1016/S1701-2163(16)32914-0
23. Furukawa N, Sasaki Y, Shigemitsu A, et al. CA-125 cut-off value as a predictor for complete interval debulking surgery after neoadjuvant chemotherapy in patients with advanced ovarian cancer. J Gynecol Oncol. 2013;24(2):141–145. doi:10.3802/jgo.2013.24.2.141
24. Tate S, Hirai Y, Takeshima N, Hasumi K. CA125 regression during neoadjuvant chemotherapy as an independent prognostic factor for survival in patients with advanced ovarian serous adenocarcinoma. Gynecol Oncol. 2005;96(1):143–149. doi:10.1016/j.ygyno.2004.09.020
25. Rodriguez N, Rauh-Hain JA, Shoni M, et al. Changes in serum CA-125 can predict optimal cytoreduction to no gross residual disease in patients with advanced stage ovarian cancer treated with neoadjuvant chemotherapy. Gynecol Oncol. 2012;125(2):362–366. doi:10.1016/j.ygyno.2012.02.006
26. Lee YJ, Chung YS, Lee JY, et al. Prognostic significance of CA-125 re-elevation after interval debulking surgery in patients with advanced-stage ovarian cancer undergoing neoadjuvant chemotherapy. Eur J Surg Oncol. 2018;45:644–649.
27. Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol. 2006;103(3):1070–1076. doi:10.1016/j.ygyno.2006.06.025
28. Lim MC, Song YJ, Seo SS, Yoo CW, Kang S, Park SY. Residual cancer stem cells after interval cytoreductive surgery following neoadjuvant chemotherapy could result in poor treatment outcomes for ovarian cancer. Onkologie. 2010;33(6):324–330. doi:10.1159/000313823
29. Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer. 2016;64:22–31. doi:10.1016/j.ejca.2016.05.017
30. Gill SE, McGree ME, Weaver AL, Cliby WA, Langstraat CL. Optimizing the treatment of ovarian cancer: neoadjuvant chemotherapy and interval debulking versus primary debulking surgery for epithelial ovarian cancers likely to have suboptimal resection. Gynecol Oncol. 2017;144(2):266–273. doi:10.1016/j.ygyno.2016.11.021
31. Luo Y, Lee M, Kim HS, Chung HH, Song YS. Effect of neoadjuvant chemotherapy on platinum resistance in stage IIIC and IV epithelial ovarian cancer. Medicine (Baltimore). 2016;95(36):e4797. doi:10.1097/MD.0000000000004864
32. Rauh-Hain JA, Nitschmann CC, Worley MJ
33. Da Costa AA, Valadares CV, Baiocchi G, et al. Neoadjuvant chemotherapy followed by interval debulking surgery and the risk of platinum resistance in epithelial ovarian cancer. Ann Surg Oncol. 2015;22(Suppl 3):S971–S978. doi:10.1245/s10434-015-4623-z
34. Kessous R, Laskov I, Abitbol J, et al. Clinical outcome of neoadjuvant chemotherapy for advanced ovarian cancer. Gynecol Oncol. 2017;144(3):474–479. doi:10.1016/j.ygyno.2016.12.017
35. Oei AL, Vriend LE, Krawczyk PM, Horsman MR, Franken NA, Crezee J. Targeting therapy-resistant cancer stem cells by hyperthermia. Int J Hyperthermia. 2017;33:1–12.
Supplementary material
 |
Table S1 Characterization of patients |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
