Back to Journals » Cancer Management and Research » Volume 12
FDG PET Predicts the Effects of 131I and Prognosis for Patients with Bone Metastases from Differentiated Thyroid Carcinoma
Authors Wang D, Bai Y, Huo Y, Ma C
Received 30 September 2020
Accepted for publication 13 December 2020
Published 23 December 2020 Volume 2020:12 Pages 13223—13232
DOI https://doi.org/10.2147/CMAR.S284188
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Danyang Wang,1,* Yongli Bai,2,* Yanlei Huo,3,* Chao Ma1,3
1Department of Nuclear Medicine, Xinhua Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, People’s Republic of China; 2Department of Nuclear Medicine, Shaanxi Provincial People’s Hospital, Xian, People’s Republic of China; 3Department of Nuclear Medicine, Tenth People’s Hospital of Tongji University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Chao Ma
Department of Nuclear Medicine, Tenth People’s Hospital of Tongji University, Yanchangzhong Road 301, Shanghai 200072, People’s Republic of China
Tel +86-21-25078593
Email [email protected]
Background: 18F-FDG PET and 131I scans are important in the detection of metastases from differentiated thyroid carcinoma (DTC). The relationship of FDG and radioiodine (RAI) metabolism in bone metastases (BMs) from DTC and its prognostic value on RAI treatment is not clear.
Methods: The retrospective study included DTC patients with BMs from two medical centers, who underwent 18F-FDG PET/CT scans and RAI therapy. Therapeutic response was evaluated by serum thyroglobulin (Tg) levels and anatomical imaging changes.
Results: The analyses were performed on 30 patients with 72 BMs. Forty-two (42/72, 58%) lesions displayed simultaneous 131I and 18F-FDG uptake. BMs with positive 18F-FDG uptake had a less favorable response to RAI therapy in comparison to those with negative 18F-FDG uptake (p = 0.018), even in 131I-avid lesions (p = 0.033). Sixteen (53%) patients had disease progression with a median PFI of 26 months (range: 3 to 89 months). Compared to those with 131I-avid but non-FDG-avid BMs, patients presenting with 18F-FDG-avid BMs had shorter PFI, whether with 131I uptake (p = 0.002) or without (p = 0.002).
Conclusion: More than half of BMs (58%) from DTC show simultaneous 18F-FDG and 131I uptakes which are contrary to the flip-flop phenomenon (131I negative and 18F-FDG positive). Regardless of 131I uptake, 18F-FDG uptake of BMs portends a less favorable therapeutic response and poorer prognosis for patients with DTC.
Keywords: bone metastasis, differentiated thyroid carcinoma, FDG, PET, prognosis
Introduction
Differentiated thyroid carcinoma (DTC) has a favorable long-term prognosis in general. Most patients with DTC are well managed with a combined treatment of radical surgery, radioiodine (RAI) ablation and thyroid hormone replacement/suppression with a 20-year overall survival rate of approximately 90%.1,2 However, the presence of distant metastases, especially of bone metastases (BMs), can remarkably worsen the prognosis of DTC patients.3–7
For BMs, the preferred progression of treatment is surgical excision of loco-regional disease in potentially curable patients, followed by RAI therapy for RAI-responsive disease and external beam radiation therapy or other directed treatment modalities as dictated by disease response.2 Although RAI has been applied in the treatment of distant metastases from DTC for more than 60 years, the efficacy of RAI for BMs was reported variably.5,8–12 The question that who will benefit from RAI therapy remain unresolved. Recent investigations have indicated that 18F-fluorodeoxyglucose (18F-FDG) accumulation on a positron emission tomography (PET) scan is a strong predictor of poor prognosis for DTC patients.9,13–16 However, due to the lower frequency of BMs, these studies included only a small number of patients with BMs. In addition, the correlation of 18F-FDG uptake with the prognosis of BMs cannot be fully assessed, because it was analyzed in conjunction with metastases to other organs.16 Therefore, in our study, patients with BMs from DTC who underwent 18F-FDG PET/computed tomography (CT) before RAI treatment were collected and analyzed.
Patients and Methods
Patients
From January 2009 to August 2019, patients with BMs from DTC who received RAI therapy and 18F-FDG PET/CT for pre- or post-treatment evaluation in the Department of Nuclear Medicine at Xinhua Hospital and Tenth People’s Hospital of Tongji University were retrospectively selected. A total of 37 patients were diagnosed with BMs from DTC based on either of the following criteria: (1) histological confirmation of BMs from primary DTC; (2) no histological records, but either 131I-whole body scintigraphy (WBS) or 18F-FDG PET/CT revealing suspicious BMs with the confirmation of at least one of the other imaging findings (X-ray, computed tomography [CT] or magnetic resonance imaging [MRI]) and elevated level of serum thyroglobulin (Tg). Patients were diagnosed either at the time of their initial evaluation or during subsequent follow-up. Six patients who showed a limited number of BMs and who underwent surgical resection before or during the RAI treatment were excluded, because of concerns that the therapeutic effects of RAI might overlap the effects of surgery. Another patient who received only one RAI therapy was also excluded for insufficient follow-up time and data. Finally, 30 patients were included in this study.
This study was conducted in accordance with the Declaration of Helsinki. Written ethics approval from the Hospital Research Ethics Committee of Shanghai Jiaotong University, School of Medicine affiliated Xinhua Hospital and The Tenth People’s Hospital was obtained, and participants provided their written informed consent to participate in this study.
RAI Therapy and Follow-Up
All the patients withdrew from taking levothyroxine and began a low-iodine diet 3–4 weeks before RAI therapy, with achieved serum thyroid-stimulating hormone (TSH) levels above 30 mIU/L. Patients with BMs were admitted to our department after thyroid hormone withdrawal (THW). On the first day after admission, 18F-FDG PET/CT scans together with conventional measurements, including physical examination, serum TSH, serum Tg, and serum anti-thyroglobulin antibodies (TgAb) were performed. An 18F-FDG PET/CT scan was performed at the diagnosis of BM for all patients, and subsequent PET/CT scans were performed selectively. RAI was administered to these patients on the second day after admission. A 131I post-therapy scan was performed three days after oral administration of RAI. A RAI activity of 1.85–7.4 gigabecquerel (GBq) was used to ablate the thyroid remnants according to patients’ risk stratification and pathology features or for the treatment of known metastases at the same time. For the subsequent treatment of BMs, the activities of oral administration of RAI were empirically divided into three tiers (5.55, 7.4 and 9.25 GBq) for each treatment according to the tumor burden. If the number of BMs from DTC patient were less than 5, 5.55–7.4 GBq 131I would be administered, and 7.4–9.25 GBq 131I was administered in patients with the number of BMS equal/more than 5 without other organ (lymph node or lung) metastases based on post-therapy scans and CT or PET/CT. The doses of 131I were 7.4–9.25 GBq in BMs from DTC with other organ metastases. For the RAI positive BMs on the first post RAI therapy scan, the second treatment will be started four to six months later. Later on, treatment intervals between the two subsequent RAI treatments of BMs varied from six to 12 months according to the therapeutic response. The follow-up period was one to 11 years (yrs) with a median follow-up of 3.21 yrs.
18F-FDG PET/CT Imaging
18F-FDG PET/CT examinations were performed using a Biograph mCT-64 scanner (Siemens/CTI, Knoxville, TN) in a state of thyroid hormone withdrawal (above 30 mIU/L) before RAI treatment. Before the examination, patients fasted for at least six hours to ensure a serum glucose concentration of less than 150 mg/dL. Patients were injected intravenously with 5.55 MBq/kg of 18F-FDG, and no intravenous or oral contrast agents were administered. Sixty minutes later, patients were scanned with a whole-body, three-dimensional PET/CT scan from the base of the skull to the mid-thigh. The acquisition consisted of a CT scan (120 kV; 300 mAs; slice thickness, 3 mm; pitch, 1.5) for the purpose of attenuation correction for anatomical co-registration of PET data, followed by three-dimensional emission imaging for two minutes per bed position. The standard uptake value (SUV) of BMs was obtained.
Criteria of Remission
Per Lesion Evaluation
The local efficacy of RAI was evaluated in view of the structural and size changes of a well-defined lesion on CT. A lesion was defined as responsive if it met one of the following criteria during the follow-up and showed no more signs of disease progression until the end of the follow-up: (1) complete or partial sclerotic rim or sclerotic fill-in was seen in a lytic lesion; (2) the lesion showed over 50% decrease in size, compared with pretreatment. The size of a well-defined lesion was deemed as the sum of perpendicular bidimensional measurements of its greatest diameters. Otherwise, the lesion was regarded as unresponsive. To ensure adequate follow-up times, all patients included in the study underwent at least two cycles of RAI treatment.
Per Patient Evaluation
The response assessment on a per patient level was based on CT and serum Tg changes after the last cycle of RAI therapy, compared with the time point when BMs were found. With regard to CT changes, the therapeutic response of each patient was assigned to one of four categories based on MD Anderson criteria,17 namely complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD). Similar to the evaluation of local response, both structural and size changes were taken into consideration when assessing the therapeutic response. The percentage change of serum Tg was also calculated, and according to that, the outcomes of assessment were classified into three categories: (1) effective, Tg decrease ≥ 25%; (2) stable, Tg increase or decrease <25%; and (3) ineffective, Tg increase ≥ 25%.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics 19.0. Factors that might relate to 18F-FDG uptake of BMs and the correlation between 18F-FDG uptake and serum Tg variation or anatomical imaging changes were analyzed by univariate analysis and confirmed by χ2 test or Fisher’s exact test. Odds ratios (ORs) and 95% confidence intervals (CIs) for therapeutic response per lesion were calculated. Differences of the distribution of SUVmax between groups were compared using the Mann–Whitney U-test. P <0.05 was considered statistically significant. The progression-free interval (PFI), as measured from the date of BMs diagnosis to the date of death or disease progression according to MD Anderson criteria, was described as the primary end point of this study. The effect of different variables involving in PFI was estimated by Kaplan–Meier survival analysis and the differences between groups were compared using the Log rank test. Multivariate analysis based on Cox proportional hazards model was applied in order to analyze the relationship between PFI and several variables simultaneously.
Results
Patient Characteristics
Thirty patients (19 females, 11 males; mean age: 59; age range: 28–81) were included. Pathology was papillary thyroid carcinoma (PTC) in 17 cases and follicular thyroid carcinoma (FTC) in 13 cases. According to the American Joint Committee on Cancer (AJCC) 7th TNM classification, a primary tumor was postoperatively staged as pT1 in six patients, pT3 in one patient and pT4 in five patients. In 18 patients, T feature was not reported (Tx). Lymph node metastases (pN1) were found in nine subjects. Characteristics of the patients are listed in Table 1. The diagnosis of BM was established at the time of the initial evaluation in 25 patients on the post RAI treatment imaging, SPECT/CT, CT or MRI, FDG PET/CT and elevated Tg, and three to four months later during subsequent follow-up in five patients with elevated Tg and positive lesions on FDG PET/CT. Among the cases, 17 patients had only BMs, and 13 patients had other metastatic sites (12 lung and one lung + adrenal). By the end of the study, all of the patients went through two to 15 cycles of RAI therapy. Among them, 13 (43%) patients had fewer than five cycles, 14 (47%) had five to 10 cycles, and 3 (10%) had more than 10 cycles. The RAI treatment interval between the first and second administration ranged from four to six months, and the subsequent RAI was administered every six to 12 months according to the therapeutic response. The median cumulated dose of 131I was 36.3 GBq, ranging from 9.3 GBq to 122.1 GBq. The median pre-therapeutic serum Tg was 217.6 ng/mL, ranging from 4.7 ng/mL to 2363.8 ng/mL. Twelve patients (22/30, 73%) complained bone pain, and one of them had pathological fracture. BMs were distributed on spine (32/72), pelvis (17/72), the limbs including humerus and femur (9/72), ribs (10/72), sternum (3/72) and jaw (1/72).
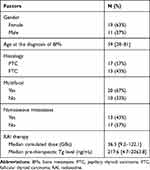 |
Table 1 Characteristics of the Patients |
Per Lesion Analysis
With regard to the distribution of bone lesions, 10 patients presented with solitary BMs and 20 patients presented with multifocal lesions. In total, 72 BMs in 30 patients were analyzed. BMs were distributed on skull, spine (32/72), pelvis (17/72), the limbs including humerus and femur (9/72), ribs (10/72), sternum (3/72) and jaw (1/72). The size of the osteolytic disease was 2.77±1.64cm, ranging from 0.4cm to 7.6cm. Fifty-four BMs showed positive accumulation on 18F-FDG PET/CT at the diagnosis of BMs, which included four (7%, 4/54) lesions that were regarded as responsive, and the other 50 (93%, 50/54) were unresponsive. Eighteen BMs showed no accumulation of FDG on 18F-FDG PET/CT, of which six (33%, 6/18) lesions responded to RAI, whereas the other 12 (67%, 12/18) were unresponsive. According to univariate analysis, BMs with negative 18F-FDG uptake were more inclined to respond to RAI (p = 0.018; Table 2) than were 18F-FDG-avid BMs. However, in the quantitative analysis of 18F-FDG avidity, the distribution of SUVmax between responsive (25th, 50th and 75th percentile SUVmax: 1.25, 1.88 and 9.72) and unresponsive (25th, 50th and 75th percentile SUVmax: 2.33, 5.17 and 11.17) lesions showed no significant difference according to the Mann–Whitney U-test (p = 0.130).
 |
Table 2 18F-FDG/131I Uptake and Local Therapeutic Response |
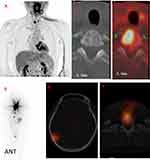
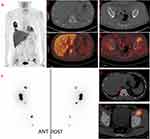
In terms of 131I avidity, 58 lesions showed positive accumulation on 131I WBS at the diagnosis of BMs, of which 10 lesions (17%, 10/58) responded to RAI therapy. Although there was no statistically significant association between 131I uptake and the local therapeutic response (p = 0.214; Table 2), none of the 131I-negative lesions responded to RAI therapy. Further, we divided the 131I-avid BMs into two subgroups according to 18F-FDG PET/CT results: (1) simultaneously positive accumulation on 18F-FDG PET/CT (F+/I+, n = 42), see Figure 1 and Figure 2; (2) no accumulation of FDG on 18F-FDG PET/CT (F-/I+, n = 16). Ten percent (4/42) of the F+/I+ and 38% (6/16) of the F-/I+ BMs were regarded as responsive after RAI therapies. Statistically, F-/I+ BMs were more likely to respond to RAI therapy than were F+/I+ BMs (OR = 0.175; 95% CI = 0.041–0.744; p = 0.033, Table 3).
 |
Table 3 18F-FDG Uptake and Local Therapeutic Response for 131I Positive Lesions |
Per Patient Analysis
Twenty-three (77%) patients showed positive accumulation on 18F-FDG PET/CT and 25 (83%) patients had positive accumulation on 131I WBS at the diagnosis of BMs. A simultaneous uptake of 18F-FDG and 131I was observed in 18 (60%)
Response Assessment Based on Anatomical Imaging
According to MD Anderson criteria, the numbers of patients who obtained PR, SD, and PD were six (6/30, 20%), 11 (11/30, 37%), and 13 (13/30, 43%), respectively. No patients showed CR based on CT results. Univariate analyses results demonstrated that the 18F-FDG uptake had significant impact on the therapeutic response of RAI therapy based on anatomical imaging changes (p = 0.026; Table 4). Fifty-two percent (12/23) of 18F-FDG positive patients had PD, whereas the ratio was 14% (1/7) in patients with negative 18F-FDG uptake. Although the 131I uptake was not statistically related to anatomical imaging evaluation outcomes, none of the 131I negative patients obtained CR or PR (Table 4).
 |
Table 4 18F-FDG/131I Uptake and Therapeutic Response Based on Anatomical Imaging and Serum Tg Changes |
Response Assessment Based on Serum Tg
The median pre-therapeutic serum Tg was 303.97 ng/mL and 171.31 ng/mL in 18F-FDG-positive patients and 18F-FDG-negative patients, respectively, and no significant difference was found in the distribution of serum Tg between the two groups (p = 0.446, Mann–Whitney U-test). After the last cycle of RAI therapy, the median serum Tg values in 18F-FDG-positive and 18F-FDG-negative patients were 241.07 ng/mL and 109.02 ng/mL, respectively. The serum TgAb value of each individual was also recorded, because TgAb can interfere with the detected values of serum Tg. In this study, three patients showed increased serum TgAb levels after 3, 4 and 5 courses of 131I treatment with simultaneous high serum Tg. And later on, the three patients’ TgAb returned to negative again. Serum Tg decrease 25%, representing effectiveness, was seen in nine (9/30, 30%) patients. Twenty-two percent (5/23) of patients with positive accumulation on 18F-FDG PET/CT and 57% (4/7) with negative 18F-FDG uptake achieved a Tg decrease ≥25%. However, as shown in Table 4, univariate analyses indicated no significant correlation between serum Tg level variation and 18F-FDG uptake (p = 0.212). 131I uptake proved to be correlated with serum Tg variation according to the analysis (p = 0.013). Stable disease or ineffective therapy was defined when patients with negative 131I accumulation after two cycles of RAI therapies.
Progression-Free Survival
Of all the patients, 16 (53%) had disease progression. The median PFI of these patients was 26 months (range: three to 89 months). According to univariate analysis, only the 18F-FDG uptake and 131I uptake were correlated with PFI (p < 0.001; Table 5). Subsequent pairwise comparisons were performed. Compared with F-/I+ patients, a less favorable PFI was seen in both the F+/I+ group and the F+/I- group (p = 0.002 and 0.002, respectively). In this study, patients with both positive 18F-FDG PET/CT and 131I WBS outcomes were categorized as F+/I+ group. However, three patients in this group showed mixed 18F-FDG and 131I uptake in the BMs; namely, both F+/I+ and F-/I+ lesions were seen in the same patient. We then conducted a subgroup analysis to examine the difference of PFI between the patients with mixed 18F-FDG and 131I uptake and the other F+/I+ patients, but no significant difference was found (p = 0.376).
 |
Table 5 Univariate and Multivariate Analyses of Factors Predicting Progression-Free Survival |
Multivariate analysis based on a Cox proportional hazard model also confirmed that disease progression was correlated with 131I and 18F-FDG uptake (p = 0.004; Table 5). When the 18F-FDG uptake was substituted for the number of 18F-FDG-avid lesions in the multivariate analysis, no significant correlation was found between the number of 18F-FDG-avid lesions and PFI (p = 0.192).
Discussion
The alternating 131I and 18F-FDG uptake pattern, the flip-flop phenomenon (ie, 131I negative and 18F-FDG positive) has been regarded as a common feature of metastases from DTC and observed in approximately 90% of the patients.16,18 Our previous study also found that out of the 83 patients, 25 showed F-FDG uptake in the pulmonary metastases of DTC.19 Metastases of DTC including lymph node, lung and bone in 37 patients with 44 lesions were evaluated and found that sixteen lesions (70%) were increased and 7 (30%) showed no change or reduction when there was positive accumulation on FDG PET/CT and no accumulation of FDG on I-131 group.21 In the study, BMs from DTC were included and in this series, only 39% (28/72) of the BMs displayed alternating 131I and 18F-FDG uptake, and over half of the metastases (58%, 42/72) displayed simultaneous uptake of both tracers. These discordant findings may result from differences in the study population, because our study focused on BMs, which could represent a more progressive state of disease. 18F-FDG and 131I avidity of a metastasis from DTC may reflect the grade of tumor differentiation and malignancy. 18F-FDG-avid tumors tend to be less differentiated and more aggressive in behavior, whereas the presence of 131I uptake could imply a more differentiated and benign phenotype.16 Therefore, the simultaneous uptake of 131I and 18F-FDG might indicate that a mixture of variously differentiated tumor cells exist in the same lesion, including dedifferentiated cells with elevated 18F-FDG uptake and differentiated cells with retained 131I uptake. We assume that differentiated BMs cells from DTC with retained 131I uptake tend to dedifferentiate and become hypermetabolic; based on that, the simultaneous uptake of 131I and 18F-FDG may indeed be a depiction of such a dedifferentiation process.
Hitherto, RAI therapy was the only systemic treatment modality that demonstrated therapeutic efficacy against widely metastatic disease from thyroid carcinoma.20 The efficacy of RAI is primarily based on the 131I avidity of local lesions, whereas metastases with no accumulation on 131I WBS may not be able to concentrate or retain enough RAI to achieve a therapeutic benefit. As shown by the aforementioned results, none of the BMs with negative 131I uptake responded to RAI therapy. However, the existence of 131I uptake did not necessarily relate to a better therapeutic response, and a surprisingly low response rate was observed even in 131I-avid BMs. Instead of the presence of 131I uptake, the absence of 18F-FDG uptake was more likely to predict a higher rate of local therapeutic response, because our study revealed that the coexisting 18F-FDG avidity would lower the response rate of 131I-avid BMs. These findings are consistent with the results of a previous investigation that concluded that 18F-FDG-avid metastases whether with or without 131I uptake are resistant to RAI therapy.21
We also tried to evaluate the impact of quantitative 18F-FDG uptake (ie, SUV) on the therapeutic response to RAI in BMs. However, no significant difference was found between the distribution of SUVmax of responsive lesions and that of unresponsive lesions. Therefore, we assumed that although 18F-FDG-avid BMs are more likely to be resistant to RAI therapy, the additional value of quantitative SUV in predicting therapeutic response seemed to be limited. Nevertheless, further studies are still needed with a larger sample size to confirm our findings.
Serum Tg was considered a prognostic indicator that could reflect disease progression and tumor burden in the evaluation of recurrent and metastatic DTC.22 A major problem that hampers accurate Tg measurement is the interference by TgAb. Therefore, TgAb should be measured in the same serum sample taken for Tg assay. Although for clinical purposes the measurement of Tg and TgAb before thyroidectomy for a suspected or proven DTC is not recommended, a pre-thyroidectomy Tg and TgAb measurement might be used as an “in-vivo” recovery test in order to assess the reliability of Tg for use as a postoperative tumour marker. In this study, three patients (3/30, 10%) showed increased serum TgAb levels with simultaneous high serum Tg. Our study indicates that 18F-FDG uptake was not significantly related to serum Tg variations, which may discredit the ability of 18F-FDG PET/CT to predict therapeutic response. However, in five patients with positive 18F-FDG uptake who exhibited a decrease of more than 25% of serum Tg, only two achieved partial response according to the post-treatment anatomical imaging, and the remaining three were regarded as having stable or progressive disease. Therefore, we assume that the decrease of serum Tg in patients with BMs from DTC, especially in those with 18F-FDG-avid lesions, might not only reflect a decrease in tumor burden but also signal dedifferentiation of the tumor cells.15,23 The variety in tumor burden, as indicated by serum Tg, among the patients included in this study may interfere with the analysis of the impact of 18F-FDG avidity on patient outcome. Thus, we performed a Kruskal–Wallis H-test to compare the distribution of pretreatment serum Tg among different groups of patients who showed PR, SD or PD according to follow-up CT results after RAI therapies, and no significant difference was found (p = 0.278).
With regard to prognostic factors for patients with BMs from DTC, inconsistent findings were reported in the previous literature. Overall, factors including the absence of non-osseous metastases, the cumulative dose of RAI therapy, BM surgery, solitary BMs, and 131I uptake by BMs were listed as the prognostic factors that were associated with improved survival.8,12,24 However, neither age at the diagnosis of BMs nor the involvement of non-osseous organs had significant impact on the PFI in our study. This difference may be caused by the small sample size of the study population with only two patients less than 45 years of age. Patients who underwent surgical resection of BMs were excluded from this study to eliminate the overlapping effect of local surgery on RAI therapy while analyzing the efficacy of RAI.
18F-FDG PET/CT is primarily considered in high-risk DTC patients with elevated serum Tg and negative 131I WBS. A couple of studies proposed that 18F-FDG PET/CT can also act as a prognostic tool that can identify patients at high risk of rapid disease progression and cancer-associated mortality.13–15 The aforementioned studies dealt with the prognostic performance of 18F-FDG uptake in all types of distant metastases from DTC, whereas our study mainly focused on BMs, which is considered a more dire type of metastatic disease. Based on our data, 18F-FDG uptake in BMs was correlated with a worse PFI, regardless of 131I uptake. Thus, both high frequency of positive 18F-FDG uptake in BMs and the predictive value of 18F-FDG uptake for disease progression emphasizes the importance of applying 18F-FDG PET/CT scans in the management of patients with BMs from DTC.
The main limitation that may restrain the validity of our conclusions is the limited number for subgroup analysis in this study. Larger-scaled studies are needed to further confirm our findings, especially those that systematically analyze the impact of variable 18F-FDG and 131I avidities among different metastatic sites of thyroid carcinoma.
In conclusion, the relationship between 18F-FDG and 131I uptake in BMs does not conform to the “flip-flop” phenomenon. The higher frequency of simultaneous 18F-FDG and 131I in BMs may indicate the tendency toward dedifferentiation and aggressiveness of BMs from DTC, compared to other common sites of metastases. 18F-FDG-avid BMs are less likely to respond to RAI therapy, even in those that can concentrate 131I. Our findings also suggest that the presence of 18F-FDG uptake in BMs may predict disease progression and be associated with a worse prognosis in patients with BMs from DTC. For patients with 18F-FDG-avid BMs, treatments other than RAI therapy, such as surgery, external beam radiation or targeted ablation (eg, cryotherapy) in combination with supportive therapies (bisphosphonates, denosumab) should be considered.
Funding
This work was supported by the National Natural Science Fund (grant number 81771859), Shanghai Science and Technology Commission Project (grant number 18401901300), Shanghai Health Bureau Fund (grant 202040085) and the Clinical Research Plan of SHDC (grant number 16CR3114B).
Disclosure
The authors declare that they have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported, and no financial or other potential conflict of interest.
References
1. Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet (London, England). 2002;360(9340):1131–1135. doi:10.1016/S0140-6736(02)11199-8
2. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi:10.1089/thy.2015.0020
3. Dinneen SF, Valimaki MJ, Bergstralh EJ, Goellner JR, Gorman CA, Hay ID. Distant metastases in papillary thyroid carcinoma: 100 cases observed at one institution during 5 decades. J Clin Endocrinol Metab. 1995;80(7):2041–2045. doi:10.1210/jcem.80.7.7608252
4. Ruegemer JJ, Hay ID, Bergstralh EJ, Ryan JJ, Offord KP, Gorman CA. Distant metastases in differentiated thyroid carcinoma: a multivariate analysis of prognostic variables. J Clin Endocrinol Metab. 1988;67(3):501–508. doi:10.1210/jcem-67-3-501
5. Schlumberger M, Challeton C, De Vathaire F, et al. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996;37(4):598–605.
6. Schlumberger M, Tubiana M, De Vathaire F, et al. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63(4):960–967. doi:10.1210/jcem-63-4-960
7. Haq M, Harmer C. Differentiated thyroid carcinoma with distant metastases at presentation: prognostic factors and outcome. Clin Endocrinol (Oxf). 2005;63(1):87–93. doi:10.1111/j.1365-2265.2005.02304.x
8. Qiu ZL, Song HJ, Xu YH, Luo QY. Efficacy and survival analysis of 131I therapy for bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab. 2011;96(10):3078–3086. doi:10.1210/jc.2011-0093
9. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91(8):2892–2899. doi:10.1210/jc.2005-2838
10. Robbins RJ, Schlumberger MJ. The evolving role of (131)I for the treatment of differentiated thyroid carcinoma. J Nucl Med. 2005;46(Suppl 1):28s–37s.
11. Proye CA, Dromer DH, Carnaille BM, et al. Is it still worthwhile to treat bone metastases from differentiated thyroid carcinoma with radioactive iodine? World J Surg. 1992;16(4):640–646. doi:10.1007/BF02067343
12. Pittas AG, Adler M, Fazzari M, et al. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty-six patients. Thyroid. 2000;10(3):261–268. doi:10.1089/thy.2000.10.261
13. Deandreis D, Al Ghuzlan A, Leboulleux S, et al. Do histological, immunohistochemical, and metabolic (radioiodine and fluorodeoxyglucose uptakes) patterns of metastatic thyroid cancer correlate with patient outcome? Endocr Relat Cancer. 2011;18(1):159–169. doi:10.1677/ERC-10-0233
14. Robbins RJ, Wan Q, Grewal RK, et al. Real-time prognosis for metastatic thyroid carcinoma based on 2-[18F]fluoro-2-deoxy-D-glucose-positron emission tomography scanning. J Clin Endocrinol Metab. 2006;91(2):498–505. doi:10.1210/jc.2005-1534
15. Wang W, Larson SM, Fazzari M, et al. Prognostic value of [18F]fluorodeoxyglucose positron emission tomographic scanning in patients with thyroid cancer. J Clin Endocrinol Metab. 2000;85(3):1107–1113. doi:10.1210/jcem.85.3.6458
16. Feine U, Lietzenmayer R, Hanke JP, Held J, Wohrle H, Muller-Schauenburg W. Fluorine-18-FDG and iodine-131-iodide uptake in thyroid cancer. J Nucl Med. 1996;37(9):1468–1472.
17. Costelloe CM, Chuang HH, Madewell JE, Ueno NT. Cancer response criteria and bone metastases: RECIST 1.1, MDA and PERCIST. J Cancer. 2010;1:80–92. doi:10.7150/jca.1.80
18. Hong CM, Ahn BC, Jeong SY, Lee SW, Lee J. Distant metastatic lesions in patients with differentiated thyroid carcinoma. Clinical implications of radioiodine and FDG uptake. Nuklearmedizin Nucl Med. 2013;52(4):121–129. doi:10.3413/Nukmed-0541-12-11
19. Zhu X, Wu S, Yuan X, Wang H, Ma C. Progression free survival related to (18)F-FDG PET/CT uptake and (131)I uptake in lung metastases of differentiated thyroid cancer. Hell J Nucl Med. 2019;22(2):123–130. doi:10.1967/s002449911005
20. Sciuto R, Romano L, Rea S, Marandino F, Sperduti I, Maini CL. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann Oncol. 2009;20(10):1728–1735. doi:10.1093/annonc/mdp050
21. Yoshio K, Sato S, Okumura Y, et al. The local efficacy of I-131 for F-18 FDG PET positive lesions in patients with recurrent or metastatic thyroid carcinomas. Clin Nucl Med. 2011;36(2):113–117. doi:10.1097/RLU.0b013e318203bb6c
22. Baskin HJ. Effect of postoperative 131I treatment on thyroglobulin measurements in the follow-up of patients with thyroid cancer. Thyroid. 1994;4(3):239–242. doi:10.1089/thy.1994.4.239
23. Spencer CA, LoPresti JS, Fatemi S, Nicoloff JT. Detection of residual and recurrent differentiated thyroid carcinoma by serum thyroglobulin measurement. Thyroid. 1999;9(5):435–441. doi:10.1089/thy.1999.9.435
24. Bernier MO, Leenhardt L, Hoang C, et al. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001;86(4):1568–1573. doi:10.1210/jcem.86.4.7390
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.