Back to Archived Journals » Journal of Vascular Diagnostics and Interventions » Volume 4
Favorable effects on arterial stiffness after renal sympathetic denervation for the treatment of resistant hypertension: a cardiovascular magnetic resonance study
Authors Hammer T, Langlo KA, Goa PE, Fadl Elmula FEM, Hoffmann P, Stensæth KH
Received 15 June 2016
Accepted for publication 5 August 2016
Published 22 September 2016 Volume 2016:4 Pages 45—51
DOI https://doi.org/10.2147/JVD.S115069
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rahmi Oklu
Tommy Arild Hammer,1 Knut Asbjørn Rise Langlo,2 Pål Erik Goa,1,3 Fadl Elmula M Fadl Elmula,4,5 Pavel Hoffmann,6 Knut Haakon Stensaeth1,7
1Department of Radiology and Nuclear Medicine, 2Department of Nephrology, St Olav’s University Hospital, Trondheim, Norway; 3Department of Physics, Norwegian University of Science and Technology, Trondheim, Norway; 4Institute of Cardiovascular and Renal Research, Department of Cardiology, Oslo University Hospital, Oslo, Norway; 5Faculty of Clinical Medicine, University of Oslo, Oslo, Norway; 6Department of Cardiology, Oslo University Hospital, Oslo, Norway; 7Institute of Circulation and Medical Imaging, Norwegian University of Science and Technology, Trondheim, Norway
Aims: Renal sympathetic denervation (RDN) has recently been suggested to be a novel treatment strategy for patients with treatment-resistant hypertension. However, the latest randomized studies have provided conflicting results and the influence of RDN on arterial stiffness remains unclear. Therefore, this study aimed to detect the effects of RDN on arterial stiffness as measured with aortic pulse wave velocity (PWV) and distensibility in addition to cardiac function and T1 mapping at baseline and at 6-month follow-up.
Methods: RDN was performed in a total of 16 patients with treatment-resistant hypertension, and the procedures were conducted at two university hospitals using two different RDN devices. All patients and age-matched controls underwent a comprehensive clinical examination and cardiac magnetic resonance protocols both at baseline and at a 6-month follow-up.
Results: In the treatment group, the systolic blood pressure (SBP) was found to be decreased at the follow-up visit (office SBP; 173±24 compared to 164±25 mmHg [P= 0.033]), the 24-hour ambulatory SBP had decreased (163±25 compared to 153±20 mmHg [P=0.057]), the aortic PWV had decreased from 8.24±3.34 to 6.54±1.31 m/s (P=0.053), and the aortic distensibility had increased from 2.33±1.34 to 3.96±3.05 10−3 mmHg−1 (P=0.013). The changes in aortic PWV and distensibility were independent of the observed reductions in SBP.
Conclusion: The arterial stiffness, as assessed with aortic PWV, and distensibility were improved at 6 months after RDN. This improvement was independent of the reduction in SBP.
Keywords: renal denervation, cardiovascular MR, arterial stiffness
Introduction
Arterial hypertension affects more than one-quarter of the adult population worldwide.1 Approximately 12% of patients who are treated for hypertension still have uncontrolled high blood pressure (BP) despite prescriptions for antihypertensive drugs.2 This fraction is reduced to ~7.5% with the use of 24-hour ambulatory BP, which comprises patients who are prescribed at least three antihypertensive drugs in full doses, including a diuretic. This condition is defined as treatment-resistant hypertension (TRH).
Renal sympathetic denervation (RDN) has been introduced as a new treatment for TRH, and several studies have indicated a profound antihypertensive effect.3–5
However, recent randomized studies have failed to demonstrate antihypertensive effects of RDN. In 2013, the Oslo RDN study reported that RDN had no effect on office or ambulatory 24-hour systolic BP (SBP) at 3 or 6 months in patients with true TRH after the witnessed intake of antihypertensive drugs,6 and in December 2014, the Symplicity HTN-3 reported no effect on ambulatory 24-hour SBP in RDN-treated patients compared with sham controls.7
Arterial stiffness and its hemodynamic consequences are established predictors of adverse cardiovascular outcomes. Arterial stiffness is positively associated with systolic hypertension, coronary artery disease, stroke, and heart failure, which all are among the leading causes of mortality in developed countries.8 Several measures of arterial stiffness have been employed; however, pulse wave velocity (PWV) and distensibility are the parameters that have gained the most attention, and their predictive accuracies have been demonstrated in a number of studies.9–11
To date, ultrasound and tonometry have been the most widely used methods for assessing PWV via measurements of the pulse wave on two levels (usually the carotid and femoral levels) and estimates of the distance between them (PWV = distance/time in m/s). However, body habitus and age-related changes affect the calculation when the length of the pulse wave is projected over the body surface.12 Cardiovascular magnetic resonance (CMR) is a noninvasive technique that allows for the direct imaging of the entire aorta without the use of geometric assumptions and is well suited for assessments of PWV.13,14 Additionally, CMR is precise and provides highly reproducible assessments of cardiac volumes and function.15
Long-standing hypertension is known to induce cardiac failure often by means of fibrotic cardiac remodeling.16 Pathological cardiac fibrosis is a result of the uncontrolled proliferation of fibroblasts and the excessive production of collagen fibers that accumulate in the interstitium. Several antihypertensive medications have been demonstrated to reduce or slow down the development of cardiac fibrosis; therefore, the detection of fibrosis at an early stage is important.17 CMR is sensitive in terms of the detection of focal fibrotic myocardial changes via late gadolinium enhancement, which provides large signal differences between normal and fibrotic myocardia. When the fibrotic changes are diffuse, a different approach is needed. Therefore, we included the novel method of myocardial T1 mapping in this study. This method directly measured the T1 relaxation time of the entire myocardium in a voxel-by-voxel manner. These measurements can then be used to build myocardial T1 maps that highlight tissue pathologies.18
In 2012, Mortensen et al published data from a pilot study19 that indicated that RDN improved the central hemodynamics and arterial stiffness, and these results were primarily based on PWV measurements performed with ultrasound and tonometry. However, the influence of RDN on arterial stiffness remains uncertain. Our study sought to investigate the effect of RDN on arterial stiffness through CMR parameters, such as aortic PWV and distensibility, in patients with true TRH. Additionally, we were interested in the possible influences of RDN on cardiac function, myocardial mass, and myocardial T1 values, both at baseline and at 6-month follow-up.
Methods
Population
Based on the Oslo RDN study (Clinicalgov.Trial Id NCT01673516) and the St. Jude EnligHTN (Clinicalgov.Trial Id NCT02006758) study, patients who were referred specifically for therapy from hospitals and specialist practices in eastern and central Norway were thoroughly examined by experienced physicians in the nephrology outpatient clinic of the Oslo University Hospital in Ullevaal, Norway, during the time period August 2012 through June 2013 and at the St. Olav’s Trondheim University Hospital during the period March 2013 through February 2014.
The inclusion criteria were the following: patients between 18 and 80 years of age with normal renal arteries on CT or MRI examination within 2 years prior to participation who had main renal arterial diameters >4 mm and renal arterial stenosis <30%. Patients with an estimated glomerular filtration rate <45 mL/min/1.73 m2 (Oslo RDN), and those with an estimated glomerular filtration rate <15 mL/min/1.73 m2 (EnligHTN) were excluded.
TRH was defined as an office SBP >140 mmHg despite taking the maximally tolerated doses of at least three antihypertensive drugs that included a diuretic. Additionally, an ambulatory 24-hour SBP >135 mmHg following the witnessed intake of antihypertensive drugs was required. Patients with documented intolerance of medications could be included in the study even if they had not used a diuretic and even if they had used fewer than three medications. Patients in this study used at the time of inclusion 5.5 (mean, standard deviation 1.9) different antihypertensive drugs, and no changes were made to the medications during follow-up.
Patients with true TRH underwent RDN performed with the SymplicityTM catheter system (Ardian, Mountain View, CA, USA) or the EnligHTNTM catheter system (St Jude Medical, St Paul, MN, USA).
A total of 88 patients were referred, 30 patients were accepted for therapy, and among these, 16 patients were found to be eligible for RDN and CMR. Nine healthy volunteers constituted the normotensive control group, and this group was age-matched with the RDN group (Table 1). The control group had only office BP measurements prior to the CMR examinations.
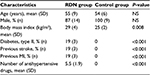  | Table 1 Baseline patient and control characteristics Abbreviations: MI, myocardial infarction; NS, not significant; RDN, renal sympathetic denervation; SD, standard deviation. |
This study was approved by the respective Regional Committees for Research Ethics in Norway and by the institutional research committees at Oslo University Hospital and St. Olav’s University Hospital. All patients provided written informed consent for participation in the study and the publication of the results.
CMR acquisition and analysis
CMR was performed at baseline and at 6 months in both groups, using a 1.5 T Achieva (Philips Healthcare, Best, the Netherlands) or 1.5-T Avanto (Siemens, Erlangen, Germany) MRI scanner under standardized patient conditions, which included a minimum of 10-minute supine rest in a quiet room with stable room temperature. No meals, caffeine, or smoking was allowed for at least 3 hours beforehand. The BP measurements were performed in conjunction with the MR acquisitions using semi-automatic sphygmomanometers (Champion, Riester, Ventura, CA, USA or Datex-Ohmeda S5, Instrumentarium Corp., Helsinki, Finland). All CMR examinations were performed by experienced radiologists (TAH and KHS). The images were analyzed quantitatively using dedicated software (cmr42 v 5.2, Circle Cardiovascular Imaging, Calgary, Canada).
Aortic PWV
A retrospective electrocardiography-gated gradient-echo pulse sequence with velocity encoding was applied to measure the through-plane flow at two predefined positions in the ascending and abdominal aorta. The imaging parameters on the 1.5 T scanners (Philips/Siemens) included the following: echo time =6.5–7.4/3.4 ms, repetition time =10–12/64 ms, flip angle =20/30 degrees, field of view =280/360 mm, Venc =160–190/200 cm/s, matrix size = 256×128, and slice thickness =10 mm.
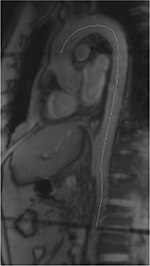
We calculated 50 phases to obtain a temporal resolution of approximately 25 ms depending on the heart rate. The aortic PWV was calculated as Δx/Δt (expressed in m/s), where Δx is the aortic path length between the two imaging levels (Figure 1), and Δt is the time delay between the arrivals of the feet of the pulse waves at these levels.13
Aortic distensibility
The distensibility of the aorta as derived from the flow measurements at the mid-ascending aorta was calculated using the following formula:
D = (Amax − Amin)/(Amin × pulse pressure)20
where D = distensibility (mmHg−1), Amax = maximal aortic area (mm2), Amin = minimal aortic area (mm2), and the pulse pressure = systolic BP – diastolic BP (mmHg).
Left ventricular function
The end-diastolic chamber volume (EDV) and ejection fraction (EF) were measured. The entire heart was imaged in the short axis orientation using electrocardiography-gated breath-hold multishot echo-planar imaging. The imaging parameters were as follows: (Philips/Siemens 1.5-T scanners); echo time =1.6/1.48 ms, repetition time =3.2/74.36 ms, flip angle =60/80 degrees, field of view =350/340 mm, matrix size =172×184/192×134, slice thickness =8 mm, and temporal resolution =35–40/2.48 ms per cardiac phase depending on the heart rate.
Myocardial T1 mapping
The acquisition of T1 data was successfully conducted in nine controls and eight patients in the RDN group. All examinations were performed using a 1.5 T Avanto scanner with the application of a modified look-locker inversion-recovery sequence21 with an acquisition in a 3-3-5 manner (field of view 380×380 mm, voxel size 2.5×2.8 mm, flip angle 35 degrees, trigger delay set for end-diastole). The data were collected from three planes of the left ventricule (LV) orthogonal to the two-chamber view, ie, the basal, mid, and apical levels.
Statistical analysis
Each categorical variable is expressed as the number and the percentage of patients. The demographic and clinical data are expressed as the mean values with standard deviations or proportions. The baseline characteristics were compared between groups using independent-sample t-tests. Shapiro–Wilk’s tests were used to assess the normalities of the distributions of the variables. Comparisons of continuous variables were conducted with two-sided Student’s t-tests. The correlations between variables were assessed with Pearson’s estimations of the correlation coefficients. In addition, we built a multiple linear regression model and assessed the influence of relevant variables upon PWV and distensibility. Significance was assumed when P< 0.05. The statistical analyses were performed using SPSS statistics version 23.0 (IBM, Armonk, NY, USA) for Windows.
Results
The results are summarized in Table 2.
BP changes
In the RDN group, the office SBP decreased from 173±24 mmHg at baseline to 164±25 mmHg at the 6-month follow-up (P=0.033), while office diastolic BP(DBP) decreased from 106±17 mmHg to 100±15 mmHg (P=0.105). The ambulatory 24-hour SBP changed from 163±25 mmHg at baseline to 153±20 mmHg at the 6-month follow-up (P=0.057). The ambulatory 24-hour DBP decreased from 96±12 mmHg to 91±12 mmHg (P=0.071).
In the control group, there were no significant changes in either office SBP (P=0.719) or in office DBP (P=0.549).
Aortic PWV and distensibility
The arterial stiffness parameters revealed that PWV (m/s) in the RDN group decreased from 8.24±3.34 to 6.54±1.31 m/s (P=0.053), and the distensibility (10−3 mmHg−1) increased from 2.33±1.34 to 3.96±3.05 (P=0.013).
In the control group, there were no significant changes in either PWV (P=0.632) or in aortic distensibility (P=0.532).
Left ventricular measurements
At baseline, there were significant differences between the RDN and control groups regarding the LV EDV (165±39 vs 204±45 mL, P=0.031) and the LV ESV (54±28 vs 77±25 mL, P=0.048). There were no significant differences regarding the LV EF, LV myocardial mass, or LV T1 values.
From baseline to the 6-month follow-up, in the RDN group, there were no significant changes in the LV EF, LV EDV, LV ESV, LV myocardial mass, or LV T1 values.
The average T1 values in the RDN group changed from 944±32 to 961±36 ms, whereas the values among the controls changed from 959±45 to 952±38 ms. None of these changes were significant. There were also no significant differences between the control group and the RDN group.
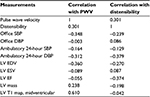
In the RDN group, there were no significant correlations between the changes in the PWV or aortic distensibility and the changes in the office SBP, office DBP, ambulatory 24-hour SBP, ambulatory 24-hour DBP, LV EDV, LV ESV, LV EF, LV mass, or LV T1 values (Table 3). All the above-mentioned variables were also used in a multiple linear regression model with both PWV and distensibility as dependent variables. The model showed that none of the independent variables significantly predict neither PWV nor distensibility.
Discussion
In the current study, the careful exclusion of secondary and spurious hypertension patients in combination with witnessed drug intake reduced the number of patients with apparent TRH to <1/3rd based on qualifying ambulatory 24-hour BP measurements. Sixteen true TRH patients underwent RDN using two different catheter systems. Additionally, there was an age-matched control group. The main findings were that RDN improved hemodynamics as demonstrated by significantly increased distensibility and a borderline significantly decreased PWV at 6 months after RDN.
Several studies have illustrated the antihypertensive effects of RDN; however, studies have failed to demonstrate RDN’s superiority over placebo when using sham-treated patients as controls.7 Our findings are indeed debatable; however, we present reduced arterial stiffness, which significantly influences distensibility, in 16 patients who were treated with RDN. These changes occurred independently of the BP-lowering effect.
RDN uses a radiofrequency pulse to disrupt the sympathetic nerve fibers going both to and from the kidneys and is known to have several effects. Among these effects are concomitant reduced stimulation of β1 receptors in the juxtaglomerular apparatus that leads to reduced release of renin and a weakened sympathetic tone that may lead to both reduced sodium reabsorption and vasodilation of the renal vasculature, which hastens the increased excretion of both water and sodium.22
Research has demonstrated that the sympathetic drive plays an important role in the regulation of BP, the strength of cardiac muscle contraction, and vessel wall tonus.22 RDN disrupts the sympathetic nerves going both to and from the kidneys, which are well known to regulate a wide array of hemodynamics and not only regulate the BP. Our work has potentially demonstrated the beneficial and independent effect of RDNs on the cardiovascular hemodynamics.
The Symplicity HTN-2 study3 included 106 patients who were randomized to RDN or control groups, and the office SBP was reduced by 32±12 mmHg in the intervention group at 6 months but remained unchanged in the control group. The Symplicity HTN-3 study,7 which included a sham operation, reported that HTN-3 does not reduce the SBP more than the sham operation at 6 months. The Oslo RDN and HTN-3 studies seem to agree that RDN is ineffective as an antihypertensive intervention. The same conclusion was also reached in a large study in ten European centers.23
In our patient population, which included subgroups from the Oslo RDN study and the St Jude EnligHTN study, we observed a significant reduction in office SBP and a nonsignificant reduction in ambulatory 24-hour systolic BP from baseline to 6 months in patients who were treated with RDN. The insignificant reduction in ambulatory 24-hour SBP accords with the results of recent studies.7,24
This study demonstrated that aortic stiffening (as characterized by PWV and distensibility) was improved in the treatment group. We also demonstrated that this improvement was independent of reductions in BP. The mechanism of the observed BP independent improvement of PWV and distensibility remains unclear, but it seems reasonable to suggest that the changes done to the sympathetic drive are of importance. It is possible that central hemodynamics is more affected by changes in sympathetic drive than peripheral hemodynamics. If this is the case, it could explain why the improved aortic distensibility is independent of changes in BP.
An important advantage of our study is that CMR provided precise and direct measurements of the pulse wavelengths of the aorta even in very sinuous conditions and thereby provided accurate PWV values. Furthermore, CMR is capable of locally assessing the pulse waves in the aorta and thus minimizes the influences of peripheral arteries on the aortic PWV. For noninvasive assessments of PWV, estimations of the pulse wave travel distance are critical.25
The European Society of Hypertension guidelines propose an aortic stiffness cutoff value of 12 m/s based on PWV data from direct tape measure distances; however, the use of the more accurate 80% cutoff value of 9.6 m/s should be adapted.26 When using the subtracted distance, as in the Framingham study, the cutoff value should be 8.4 m/s. The Framingham study demonstrated that with the use of this cutoff value, the probability of a first major cardiovascular event within the next 8 years was ~3%.11 No reference values exist for more accurate CMR measurements. The PWV serves as an excellent and well-accepted predictor of cardiovascular mortality,27 and it has been stated that arterial stiffness serves as a better predictor of cardiovascular risk and end-organ damage than peripheral brachial pressures.28
The baseline measurements of the PWV in the RDN group had a mean of 8.24 m/s, which is close to the suggested cutoff value mentioned earlier. This finding indicates that this group of patients had a greatly increased risk of a major cardiovascular event.
We did not observe any significant changes in the left ventricular measurements in the treatment group. There were no changes in the T1 values in the RDN-treated patients between baseline and follow-up. Because hypertensive disease is known to induce cardiac fibrosis,16 our study of hypertensive patients who were treated with RDN aimed to identify differences in the T1 values between hypertensive patients and controls. We also hypothesized that RDN would lead to a reduction in cardiac fibrosis and a subsequent reduction in native T1 values. We were unable to observe any changes, most likely because the hypertensive patients did not in fact have any cardiac fibrosis. The recorded EF at baseline of 69% substantiates this assumption.
Limitations
The data in our study were generated from a modest number of patients, and all associated limitations apply. Moreover, there was no blinding regarding the RDN treatment, and it is possible that this influenced the behaviors of the patients. Nevertheless, the size of our study is comparable to those of other publications that have investigated RDN, and the results were consistent even though we applied more thorough methods regarding patient selection than in the Symplicity HTN-1 and -2 studies.3,4 Moreover, the patients who were included in this study were thoroughly examined to identify those who had real TRH, which makes it unlikely that patients without true TRH were included in the study.
The modest number of patients in this study yields a somewhat low statistical power. Due to this, we have deliberately chosen not to perform a Bonferroni (or similar) correction of multiple comparisons, as we considered this correction to be very conservative and likely to produce false negatives.
Only a few studies have examined the possible relationship between aortic and cardiac function using CMR as a noninvasive tool, and our observations are similar to those of previous less-precise studies that required larger sample sizes29 due to the high reproducibility of CMR measurements.
The T1 values were measured using a nonmotion-corrected modified look-locker inversion-recovery sequence, which resulted in values with a somewhat large standard deviation. The global T1 standard deviation values in our material ranged from 32 to 45 ms, which reduced the probability of detecting subtle changes in the T1 values.
The cohort of our study did not allow for analyses of clinical outcomes. Future results of trials with longer follow-ups and larger cohorts of treated patients will therefore be of interest.
Conclusion
RDN has been introduced as a novel treatment for TRH, but recent studies have demonstrated controversies regarding the effect of RDN in TRH. Additionally, the influence of RDN on arterial stiffness remains unclear. With a comprehensive CMR protocol, our study demonstrated that RDN may improve the known predictors of adverse cardiovascular outcomes as illustrated by a significantly improved aortic distensibility. Furthermore, the improvements in the stiffness parameters were independent of decreased SBP.
Disclosure
The authors report no conflicts of interest in this work.
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. | ||
de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57(5):898–902. | ||
Symplicity HTN-I, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–1909. | ||
Symplicity HTN-I. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57(5):911–917. | ||
Tsioufis CP, Papademetriou V, Dimitriadis KS, et al. Catheter-based renal denervation for resistant hypertension: twenty-four month results of the EnligHTN I first-in-human study using a multi-electrode ablation system. Int J Cardiol. 2015;201:345–350. | ||
Fadl Elmula FE, Hoffmann P, Fossum E, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62(3):526–532. | ||
Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–1401. | ||
Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. | ||
Laurent S, Boutouyrie P, Asmar R, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. | ||
Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113(5):664–670. | ||
Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121(4):505–511. | ||
Wenn CM, Newman DL. Arterial tortuosity. Australas Phys Eng Sci Med. 1990;13(2):67–70. | ||
Mohiaddin RH, Firmin DN, Longmore DB. Age-related changes of human aortic flow wave velocity measured noninvasively by magnetic resonance imaging. J Appl Physiol (1985). 1993;74(1):492–497. | ||
van der Meer RW, Diamant M, Westenberg JJ, Doornbos J, Bax JJ, de Roos A, Lamb HJ. Magnetic resonance assessment of aortic pulse wave velocity, aortic distensibility, and cardiac function in uncomplicated type 2 diabetes mellitus. J Cardiovasc Magn Reson. 2007;9(4):645–651. | ||
Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48(7):1475–1497. | ||
Piek A, de Boer RA, Silljé HH. The fibrosis-cell death axis in heart failure. Heart Fail Rev. 2016;21(2):199–211. | ||
Hale TM. Persistent phenotypic shift in cardiac fibroblasts: impact of transient renin angiotensin system inhibition. J Mol Cell Cardiol. 2015;93:125–132. | ||
Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 mapping: basic techniques and clinical applications. JACC Cardiovasc Imaging. 2016;9(1):67–81. | ||
Mortensen K, Franzen K, Himmel F, Bode F, Schunkert H, Weil J, Reppel M. Catheter-based renal sympathetic denervation improves central hemodynamics and arterial stiffness: a pilot study. J Clin Hypertens (Greenwich). 2012;14(12):861–870. | ||
Resnick LM, Militianu D, Cunnings AJ, Pipe JG, Evelhoch JL, Soulen RL. Direct magnetic resonance determination of aortic distensibility in essential hypertension: relation to age, abdominal visceral fat, and in situ intracellular free magnesium. Hypertension. 1997;30(3 Pt 2):654–659. | ||
Messroghli DR, Greiser A, Fröhlich M, Dietz R, Schulz-Menger J. Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007;26(4):1081–1086. | ||
Barrett CJ. Renal sympathetic nerves - what have they got to do with cardiovascular disease? Exp Physiol. 2015;100(4):359–365. | ||
Persu A, Jin Y, Azizi M, et al. Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens. 2014;28(3):150–156. | ||
Fadl Elmula FE, Hoffmann P, Larstorp AC, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension. 2014;63(5):991–999. | ||
Weber T, Ammer M, Rammer M, et al. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens. 2009;27(8):1624–1630. | ||
Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: executive summary: Fourth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (Constituted by representatives of nine societies and by invited experts). Eur Heart J. 2007;28(19):2375–2414. | ||
Mitchell GF, Wang N, Palmisano JN, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122(14):1379–1386. | ||
Lartaud-Idjouadiene I, Lompré AM, Kieffer P, Colas T, Atkinson J. Cardiac consequences of prolonged exposure to an isolated increase in aortic stiffness. Hypertension. 1999;34(1):63–69. | ||
Eren M, Gorgulu S, Uslu N, Celik S, Dagdeviren B, Tezel T. Relation between aortic stiffness and left ventricular diastolic function in patients with hypertension, diabetes, or both. Heart. 2004;90(1):37–43. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.