Back to Journals » International Journal of Nanomedicine » Volume 15
Fatty Acid Synthase (FASN) siRNA-Encapsulated-Her-2 Targeted Fab’-Immunoliposomes for Gene Silencing in Breast Cancer Cells
Authors Khan A , Aljarbou AN, Aldebasi YH , Allemailem KS , Alsahli MA , Khan S, Alruwetei AM , Khan MA
Received 4 April 2020
Accepted for publication 29 June 2020
Published 5 August 2020 Volume 2020:15 Pages 5575—5589
DOI https://doi.org/10.2147/IJN.S256022
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Arif Khan,1,* Ahmed N Aljarbou,2 Yousef H Aldebasi,3 Khaled S Allemailem,4 Mohammed A Alsahli,1 Shamshir Khan,5 Abdulmohsen M Alruwetei,4 Masood A Khan1,*
1Department of Basic Health Sciences, College of Applied Medical Sciences, Qassim University, Al-Qassim, Buraydah, Saudi Arabia; 2Department of Pharmaceutics, College of Pharmacy, Qassim University, Al-Qassim, Buraydah, Saudi Arabia; 3Department of Optometry, College of Applied Medical Sciences, Qassim University, Al-Qassim, Buraydah, Saudi Arabia; 4Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, Al-Qassim, Buraydah, Saudi Arabia; 5Dentistry and Pharmacy College, Buraydah Private Colleges, Al-Qassim, Buraydah, Saudi Arabia
*These authors contributed equally to this work
Correspondence: Arif Khan; Masood A Khan
Department of Basic Health Sciences, College of Applied Medical Sciences, Qassim University, Buraydah, Saudi Arabia
Email [email protected]; [email protected]
Purpose: The overexpression of Her-2 in 25– 30% breast cancer cases and the crosstalk between Her-2 and fatty acid synthase (FASN) establishes Her-2 as a promising target for site-directed delivery. The present study aimed to develop the novel lipid base formulations to target and inhibit the cellular proliferation of Her-2-expressing breast cancer cells through the silencing of FASN. In order to achieve this goal, we prepared DSPC/Chol and DOPE/CHEMS immunoliposomes, conjugated with the anti-Her-2 fab’ and encapsulated FASN siRNA against breast cancer cells.
Methods: We evaluated the size, stability, cellular uptake and internalization of various formulations of liposomes. The antiproliferative gene silencing potential was investigated by the cell cytotoxicity, crystal violet, wound healing and Western blot analyses in Her-2+ and Her-2® breast cancer cells.
Results: The data revealed that both nanosized FASN-siRNA-encapsulated liposomes showed significantly higher cellular uptake and internalization with enhanced stability. The cell viability of Her-2+ SK-BR3 cells treated with the targeted formulation of DSPC/Chol- and DOPE/CHEMS-encapsulating FASN-siRNA reduced to 30% and 20%, respectively, whereas it was found to be 45% and 36% in MCF-7 cells. The wounds were not only failed to close but they became broader in Her-2+ cells treated with targeted liposomes of siRNA. Consequently, the amount of FASN decreased by 80% in SK-BR3 cells treated with non-targeted liposomes and it was 30% and 60% in the MCF-7 cells treated with DSPC/Chol and DOPE/CHEMS liposomes, respectively.
Conclusion: In this study, we developed the formulation that targeted Her-2 for the suppression of FASN and, therefore, inhibited the proliferation of breast cancer cells.
Keywords: gene therapy, pH-sensitive liposomes, DRVs, stealth pegylated
Introduction
Recently, gene therapy has appeared as a critical approach that allows an efficient and specific alteration in the expression of a gene in tumor cells.1 The mainly activated proto-oncogenes are targeted as the overexpression of them can be restored to the normal level in cancer cells.2 However, the development of a secure and effective system for gene therapy is a challenging task. The targeted delivery of siRNAs using the competent system in the silencing of targeted genes has made revolutionary development as compared to other gene therapeutic approaches.3 However, siRNA therapy is still facing the challenge of site-directed delivery as of other organic compounds of therapeutic efficacy. These challenges include serum degradation, site-specific binding, internalization and distributions as well. Moreover, siRNA is required to be delivered into the cytosol of targeted cells in order to achieve the gene silencing effect.4 Several types of delivery systems have been employed in the clinical trials of gene therapy, that include viral and non-viral vectors. Noticeably, most of the trials were executed with various viral vectors due to the high transfection efficiency.5–8 Despite all of the advancements of viral vectors, there are certain limitations due to the immunogenicity, toxicity, complexity of vector design and carcinogenesis as well.9,10 Keeping these limits into consideration, the use of non-viral vectors, including liposomes, is a practical way and exhibits an excellent potential as it showed low immunogenicity, inexpensiveness, and suitable surface alteration.11 Specifically, coating of polyethylene glycol (PEG) to make it long circulating stealth liposomes has opened the new window of lipid-based drug delivery system.12 Liposomes comprising DOPE and CHEMS are the most recognized pH-sensitive liposomes due to the fusogenic property of DOPE. The fusogenicity and pH-sensitive properties of DOPE/CHEMS combination exploit higher cellular uptake and undergo endo/lysosomal escape upon acidification.13 Additionally, CHEMS is also the essential component to provide the sufficient stability to pH-sensitive liposomes.14,15 The incorporation of antibodies or their fragments could be exploited on the surface of targeted liposome for the site-specific targeting in cancer cells are known as immunoliposomes. The recent advancements in liposome research and the recombinant Mab technology are associated with the site-specific targeting to cancer cells.16,17 However, as evident from several studies, intact antibody engrafted on the liposomes was not competent to penetrate solid tumors due to its large molecular size.18–20 Moreover, it showed immunogenicity and rapidly removed through Fc-mediated phagocytosis by macrophages.21 Therefore, the use of fab’ fragments demonstrated superior pharmacokinetics for tissue penetration, exhibited more favorable than their full-length (185 kDa) counterparts, because of their small size (45 kDa). The Fab’ fragments retained the antigen-binding affinity of the parental antibody comprising both VL and VH domains.22–24
Her-2 has been shown to be overexpressed in 25–30% human breast cancer, making it an attractive target for site-specific siRNA delivery of target gene.25–27 Correspondingly, the overexpression of FASN appears frequently due to change in the mechanism of lipogenesis, as evident by the upregulation of associated enzymes of the same lipogenic pathway.28 Several studies suggested that the amplification in the activity of FASN plays a significant role in the development of cancer by regulating oncogenic proteins associated with malignant transformation.29–31 The role of FASN-mediated pathway, to regulate the expression, activity, and cellular localization of Her-2 in breast and ovarian cancer cells has already been discussed.32,33 Remarkably, there is a bidirectional crosstalk between Her-2 and FASN, as Her-2 directly phosphorylates and activates FASN, leading to its upregulation.25,34 The present study is focused to develop FASN siRNA-encapsulated Her-2 targeting fab’-immunoliposomes against breast cancer cells. We attempted to accomplish Her-2 mediated breast cancer-specific novel delivery system of pegylated stealth and pH-sensitive immunoliposomes.
Materials and Methods
Reagents
Distearoyl phosphatidylcholine (DSPC), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (PEG-DSPE-Mal), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-methoxy-polyethylene glycol (mPEG-DSPE), Cholesterol (Chol), Cholesteryl hemisuccinate (CHEMS), DiIC18(3)-DS [1,1-Dioctadecyl-3,3,3,3-tetramethylindocarbocyanine-5,5-disulfonic acid] were purchased from Sigma-Aldrich (St. Louis, MO, USA). The scrambled and FASN siRNAs, primary, and secondary antibodies, BCA protein assay kit were procured from the Santa Cruz Biotechnology (Heidelberg, Germany), Fab’ fragment of Her-2 was procured from the CD Biosciences, Shirley, NY, USA. The cell cytotoxicity assay kit was purchased from Abcam (Cambridge, USA). MCF-7 (ECAC 86012803) and MDA-MB-231 (ECACC 92020424) were commercially purchased from ECACC (European Collection of Cell Cultures), Salisbury, UK. SK-BR3 (ATCC HTB30) was procured from ATCC (American Type Culture Collection), VA, USA.
Liposome Preparation
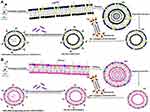
The pegylated stealth, DSPC/Chol and pH-sensitive, DOPE/CHEMS comprising mPEG-DSPE and/or PEG-DSPE-Mal liposomes were prepared as described in our earlier study with minor modifications.13,35 Briefly, DSPC: Chol (49 mmol-chol: 21 mmol), DOPE-CHEMS (54 m mol: 36 mmol) with mPEG–DSPE and/or PEG-DSPE-mal (3–6% of total phospholipids) were prepared by a lipid film method. In the preparation of fluorescent liposomes, 0.1% DiIC18(3)-DS of total lipid molar ratio were added in the liposomal formulation for tracing liposomes. Consequently, the unilamellar nano-scale liposomal vesicles (ULVs) were prepared by extruding the multilamellar liposome vesicles (MLVs) by using the automated extruder device or handheld extruder at ambient temperature. It was performed sequentially from 800, 400, 200, and/or 100 nm decreasing pore sized membranes with 5–10 cycles for each size of membrane. The liposomes were lyophilized for 16–18 h after mixing with 9% sucrose solution (Figure 1).
Conjugation of Anti-Her2 Fab’ to Liposomes
In the preparation of immunoliposomes, the Fab’ fragments of rhuMAb-HER2 were thiolated by Traut’s reagent in the molar ratio of 100:1 (Traut’s reagent: Fab’). Then, the thiolated Fab’ was mixed with the liposomes comprising DSPE-PEG-Mal at the molar ratio of 1:10 (Fab’: DSPE-PEG-Mal) in HEPES-buffered saline and incubated 12–16 hours at ambient temperature in N2 atmosphere. The unconjugated Fab’ fragments were separated by gel chromatography on Sepharose 4B following quenching of excessive maleimide groups with β-mercaptoethanol. Subsequently, the immunoliposomes were sterilized using 0.2 µm syringe filters following collection in the void volume fraction, and store at 4°C as illustrated in Figure 1. The conjugation of Fab’ in the liposomes was determined by BCA protein assay and converted into the number of Fab’ per liposome vesicle by Marsh 1990, considering the molecular weight of Fab’ fragment equal to 46 kDa.36
Entrapment of siRNA in Liposomes
siRNA was entrapped by the preparation of dried, reconstituted vesicles (DRVs) with minor modifications. The complex of targeted or non-targeted liposomes and siRNA solution were lyophilized following two to three freeze-thaw cycles as explained in Figure 1. The non-entrapped siRNAs were separated using 30k MW filter units after rehydrating the dried lyophilized powder with 120 mL distilled water followed by reconstitution with PBS.
Characterization of Liposomes
Transmission Electron Microscopy (TEM)
A drop of liposome suspension was placed on parafilm. A formvar and carbon-coated grid were floated on the suspension for one to 2 minutes. The excess suspension was removed, and then 2% Potassium Phosphotungstic Acid (PTA) at pH 7.0 was placed on the grid for approximately 1 minute, followed by the removal of all the liquid. The grid was viewed in the transmission electron microscope using 120 kV.
The Size, Zeta (ζ) Potential and Poly Dispersity Index (PDI) of Liposomes
The mean particle sizes, zeta potentials and PDI of prepared liposomes were determined by dynamic light scattering (DLS) using the Zetasizer Nano system (Malvern Instruments, Malvern, Worcestershire, UK).
Entrapment Efficiency of siRNA in Liposomes
To determine the entrapment potential of siRNA using the Quant-iT RiboGreen RNA detection kit calibration line of various known concentrations of siRNAs were prepared following the manufacturer’s instructions. The concentration of siRNA in the liposomes was measured at 495Ext/525Em in the spectrofluorophotometer before and after the addition of 0.5% Triton X-100.
Serum Stability Assay
Free or liposome-encapsulated siRNA (0.25 µg/mL) were incubated in 50% FBS at 37°C followed by collection of aliquots at different lengths of time (0, 3, 6, 9, 12, 24h). The samples of siRNA were run on a nondenaturing 20% polyacrylamide TBE gel followed by the visualization of bands by ethidium bromide staining.
To confirm that nuclear protection of siRNA was conferred by lipid encapsulation, the lipid bilayer integrity was disrupted instantly using 0.1% before incubation with serum.
To determine the resistance to RNase, liposomes (siRNA-Lip and/or siRNA-Immunoliposomes) were mixed with 40 ng RNase at 37°C for 6 hours followed by the addition of 10% SDS to stop the reaction.
Binding and Internalization of Immunoliposomes to SK-BR3, MCF-7 and MDA-MB-231 Cells for Her-2
To determine the binding and internalization of immunoliposomes, 2.5 X 105 cells were incubated for 1 h, at 4°C with 25 nmol immunoliposomes (IL-DOPE/CHEMS) in the 100 µL cell growth medium with 10% FBS. Cell binding was analyzed indirectly by the incorporation of DiIC18(3)-DS in the liposomal membrane. After incubation, the cells were washed, and the images were taken by the confocal microscope.
In vitro Targeting of Fluorescent Liposomes to SK-BR3, MCF-7 and MDA-MB-231 Breast Cancer Cells
The in vitro targeting of fluorescent liposomes to the cells was measured by the flow cytometry. Briefly, the cells were treated with various formulations of liposomes incorporated with DiIC18(3)-DS in the liposomal membrane after achieving the density of 5 X 105 per well in 12-well plates. The cells were incubated at 37°C for 24 h and harvested at different time points viz. 1, 2, 4, 6, 12, 18 and 24 h following trypsinization. The mean fluorescence intensity (MFI) of samples was measured using MACS Quant Analyzer 10 (Miltenyi Biotec, Germany).
Cell Cytotoxicity Assay
Firstly, the primary screening of siRNA at various concentrations was conducted in SK-BR3 breast cancer cell lines to select the dose for further in vitro study. The cell cytotoxicity assay kit from Abcam was used to determine the percentage viability of SK-BR3 cells following exposure to various formulations. Briefly, the cells were grown into 96-well plates (1x104 cells per well) and incubated overnight, followed by the treatment of cells with various liposomal formulations containing 0, 10, 25, 50, 75, 100, 125, 150, 175 and 200 nM of siRNA for 48 h. Then, the reagent (20µL) from the kit was added in each well and incubated the plate at 37°C in a humidified 5% CO2 atmosphere for 4 h and the absorbance was measured at 590 nm in a microplate reader. The viability of the cells was measured using the following formula. 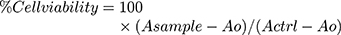
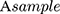 is an absorbance of liposomal formulation treated cells
is an absorbance of liposomal formulation treated cells
 is the absorbance of untreated cells
is the absorbance of untreated cells
 is the absorbance of the background of non-cell control (only media)
is the absorbance of the background of non-cell control (only media)
Following primary screening, the percentage viability of cells was determined in SK-BR3, MCF-7, MDA-MB-231 cells following exposure to various liposomal formulations entrapping 100 nM of siRNA using the same kit. The sham liposomes contained the scrambled siRNA instead of FASN.
Crystal Violet Staining Assay
In the crystal violet staining cell viability assay, the cells were treated with various liposomal formulations for 48 h. The cells were gently washed and stained with 0.2% crystal violet in 10% formalin solution. Then the staining solution was removed, and the cells were washed three times with phosphate-buffered saline (PBS) and were air-dried for taking macrographic images.
Wound Healing Assay
The potential of various liposomal formulations was assessed by directional cell migration and wound-healing assay by following standard method.37 Briefly, 1× 105 cells were grown in each well of a 12-well plate for 24 h, followed by making the straight scratch across the center with the help of pipette tip. The cells were then treated with various formulations followed by imaging of the cells at 0 h using an inverted microscope (Optika-TB-3W, Ponteranica, Italy) and incubated for 48 h. The width of the wound was measured and quantified. The cell migration ability was compared as the relative mobility index, to the gaps filled by untreated control in 48 h.
In vitro Silencing Efficiency by the Western Blotting
The potential of various formulations on in vitro silencing of FASN was evaluated by the Western blotting. The cells were seeded in 6-well plates with a density of 0.3 × 106 cells per well for overnight and treated under the same conditions as described above for cell viability. The immunoblotting analyses were done by probing the protein on the PVDF membrane with α-FAS Ab, after harvesting of the cells, 48 of the treatment.
Statistical Analysis
The mean values and standard deviation for all samples were calculated for different treated groups. The significant difference between the groups of various formulations was measured by the Student’s t-test for the paired samples with the help of Sigma Stat 3.5. P-value <0.05 was considered statistically significant.
Results
Characterization of Liposomes
Size, Zeta Potential, Polydispersity Index and Encapsulation Efficiency
The TEM micrographs demonstrated the size of the IL-DSPC/Chol and IL-DOPE/CHEMS within the range of 150 nm (Figure 2A). The DLS data revealed the mean particle size of the prepared liposomes between 125 and 155 nm with <0.2 PDI homogeneity among all formulations. The targeted liposomes exhibited a size increase to approximately 155 nm from 125 nm and 112 nm to 140 nm in DSPC/Chol and DOPE/CHEMS liposomes, respectively. The increase in the overall negative charge was also measured in the zeta potential of immunoliposomes (Figure 2B). Considering the mean particle size (140–155 nm) of the immunoliposomes, the conjugation of Fab’ was estimated to be 112–124 Fab’/liposome vesicle (data not shown). The entrapment efficiency of siRNA was found to be 90–93% in targeted and non-targeted DSPC/Chol, as well as DOPE/CHEMS liposomes (Figure 2B).
siRNA Degradation in Serum
The degradation of siRNA in various liposomal formulations was assessed to characterize their stability in 50% FBS. The data demonstrated that free form of siRNA was degraded completely as no band was seen after 9 h, but 25% of total siRNA remained in non-targeted liposome. The siRNA in IL-DSPE/Chol and IL-DOPE/CHEMS showed the lowest degrading rate, the remaining 35% of total siRNA provides better protection for siRNA than non-targeted liposomes (Figure 2C). As depicted in Figure 2C, 15–20% of total siRNA still remained in targeted liposomes even after 24 h of incubation in the serum.
In vitro Cellular Uptake of Liposomes
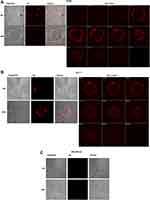
The specific binding of immunoliposomes to the Her-2 target was examined indirectly by the incorporation of DiIC18(3)-DS in the liposomal membrane using the confocal microscopy. As shown in Figure 3, the cellular uptake and internalization were seen in all Her-2 expressing SK-BR3 (Figure 3A), and MCF-7 cells (Figure 3B) as fluorescence were diffused on the cells, but no fluorescence was visibly observed in Her-2¯ MDA-MB-231 breast cancer cells (Figure 3C). These results demonstrated that Her-2 targeting immunoliposomes specifically bind to Her-2 and get the entry inside the cells through receptor-mediated endocytosis.
The in vitro Targeting of Liposomes to Breast Cancer Cells
The mean fluorescence intensity of DiIC18(3)-DS, incorporated in the liposomal membrane was measured among all formulations in the cells at various time points by the flow cytometry. The data showed the uptake of targeted IL-PEG-DSPC/Chol and IL-PEG-DOPE/CHEMS were prominently higher in Her2+ SK-BR3 (Figure 4A) and MCF-7 cells (Figure 4B) as compared to non-targeted liposomes at each time point. However, similar uptake was detected in Her-2¯ MDA-MB-231 cells (Figure 4C), among all formulations at corresponding time points, but significantly lower cellular uptake of immunoliposomes in comparison to Her2+ cells.
FASN siRNA Encapsulated Immunoliposomes Enhanced the Inhibition of Proliferation in Her-2+ Cells
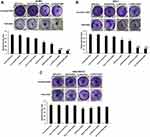
The data from the primary screening of FASN siRNA in SKBR-3 cells by cell cytotoxicity assay assisted us to select the dose for further analyses (Supplementary Figure 1). Following the selection of 100 nM FASN siRNA, we tested the cytotoxic potential of various liposomal formulations after treating the cells for 48 hours. Sham liposomes of DSPE/Cho, and DOPE/CHEMS were found to be nontoxic to all types of cells. As shown in Figure 5, a slight decrease (insignificant) was observed in the viability of cells treated with sham immunoliposomes. However, the exposure to non-targeted liposomes resulted in significant inhibition in Her2+ SKBR3 and MCF-7 cells relative to the Her2¯ MDA-MB-231 cells were recorded against sham liposomes treated cells (P<0.05). As expected, there was 70% and 80% inhibition in SK-BR3 (Figure 5A) treated with IL-DSPE/Chol and IL-DOPE/CHEMS, whereas MCF-7 showed 55% and 64% inhibition in cell growth (Figure 5B), respectively. Interestingly, no significant change was documented in Her2¯ MDA-MB-231 cells (Figure 5C) treated with any liposomal formulation.
In crystal violet staining assay, when Her-2+ SKBR-3 and MCF-7 cells transfected with siRNA encapsulated immunoliposomes, there was a consistent trend showing more efficient inhibition of cell proliferation against non-targeted liposomes. As depicted in Figure 6, the quantitative analysis of cell growth inhibition was estimated to be 40% and 50% in SK-BR3 cells (Figure 6A), whereas 30% and 35% in MCF-7 cells (Figure 6B) treated with siRNA encapsulated in DSPE/Chol and DOPE/CHEMS non-targeted liposomes, respectively. Correspondingly, it was shown to be 80% and 85% in SK-BR3 cells, and 70% and 75% in MCF-7 cells transfected with siRNA-encapsulated IL-DSPE-Chol and IL-DOPE/CHEMS formulations. However, there was no significant difference in the staining of Her-2¯ MDA-MB-231 cells (Figure 6C) exposed to various formulations.
Targeted Immunoliposomes Augment the Inhibition of Cell Migration of Her+ Breast Cancer Cells
We evaluated the effect of various formulations of liposomes with or without siRNA on cell migration by wound healing assay in Her-2+ and Her-2¯ cells. The results showed that the wound gaps were failed to be closed in siRNA-encapsulated targeted and non-targeted formulations. However, it was more prominent in Her-2+ SK-BR3 (Figure 7A), and MCF-7 cells (Figure 7B) treated with siRNA-encapsulated targeted liposome in comparison to the treatment with non-targeted system. Interestingly, the gaps did not close in the Her-2¯ MDA-MB-231 cells (Figure 7C), but they became broader in non-targeted liposomes in comparison to targeted liposomes.
Immunoliposomes Silenced the Expression of FASN in Her+ Breast Cancer Cells
Finally, we evaluated the potential of various liposomal formulations in the silencing of FASN by the Western blotting for which the siRNA was entrapped to develop a novel system. As depicted in Figure 8, the expression of FASN was negligible and/or faintly detected in the HER-2+ cells treated with targeted liposomes. Interestingly, the significant change in the reduction of FASN was also observed in the cells treated with sham IL-DOPE/CHEMS liposomes. The amount of FASN decreased by 80% in SKBR-3 cells (Figure 8A) treated with non-targeted liposomes, whereas it was 30% and 60% in the MCF-7 cells (Figure 8B) treated with DSPC/Chol and DOPE/CHEMS liposomes, respectively. However, it was found that any of the formulations failed to make significant changes in the expression of FASN Her-2¯ MDA-MB-231 cells (Figure 8C).
Discussions
The continuous efforts are being made to develop the new strategies that allow an efficient and specific alteration in the expression of the gene in tumor cells.38 Gene silencing using siRNA reduces the expression of the gene at the transcriptional level, has been acknowledged progressively as a preferred approach in the treatment of various types of cancers.39–41 Therefore, the site-directed delivery of siRNA to downregulate the expression of chosen gene targets is likely to lead to a promising therapeutic strategy. The major bottleneck in site-specific delivery of siRNA is the development of an effective system.42–44 A molecular association between Her-2 and FASN has made Her-2 fab’ as targeting ligands and FASN siRNA as gene target.45 In the present study, we prepared and demonstrated the potential of Her-2 targeted two different phospholipids-based pegylated liposomal carrier systems for the delivery of FASN siRNA against breast cancer cells.
The conjugation of fab’ fragments with PEG-DSPE-Mal on the surface of targeted liposomes had shown greater targeting ability than the non-pegylated immunoliposomes.46–48 Noticeably the addition of maleimide-terminated linkers for the conjugation of ligands in the pegylated stealth liposomes provides spacer arm for improved binding and internalization. The attachment of targeting moieties at distal end of PEG also support to minimize the steric hindrances by neighboring polymer chain.49,50 Such type of targeted liposomes reduces the association with plasma proteins due to the availability of PEG chains that are not conjugated with the fab’ or scFv fragments. Therefore, it escapes the uptake of immunoliposomes by the cells of the reticuloendothelial system (RES), leading to increased blood concentration and enhanced site-specific delivery of the payloads.51
The pore sizes are varied from 100 to 800 nm in tumor tissues due to discontinuous vasculature, while it appears 2 nm in most of the healthy vascular endothelium. In order to make liposomes to extravasate (to pass through the walls of a vessel into the surrounding tumor tissues), they should be around 200 nm or less to pass through the pores.52 To prepare nano-scale unilamellar vesicles, liposomal formulations were extruded through an extruder by using progressively decreasing pore-sized membranes. Our results demonstrated the size of prepared siRNA encapsulating immunoliposomes within the range of 140–155 nm (Figure 2).
The stability of liposomes depends on the types and the ratio of constituents used in their preparation.53,54 The usage of suitable stabilizer can protect the liposomal damage occurred by the process of lyophilization. The addition of disaccharides can provide the cushion to the liposomes and prevent the membrane fusion and leakage during lyophilization. Several studies suggest that sucrose maintains the integrity of liposomes in the dried state. Therefore, the physical properties of the DRVs, ie, size, fluidity showed the resemblance to the fully hydrated liposomes by which the integrity is maintained following the interaction between sugar and lipid.55–59
However, the immunoliposomes of these characteristics maybe failed to release the entrapped content into the cytoplasm of the cells if degraded by the lysosomes following endocytosis.14,60–62 Keeping this view into consideration, we prepared pH-sensitive liposomes that could release the entrapped siRNA into the cytoplasm following internalization and endosomal escape due to the mild acidic pH of early endosomes. The binding and internalization of siRNA encapsulated targeted pH-sensitive liposomes to Her-2+ and Her-2¯ cell lines were investigated indirectly by the incorporation of DiIC18(3)-DS in the liposomal membrane (Figure 3). The mean fluorescence intensity (MFI) of both types of targeted liposomes was significantly higher in Her-2+ cells on nontargeted liposomes at each time point recorded from 1 to 24 h. Noticeably, significantly lower uptake was detected in Her2− MDA-MB-231 cells, among the targeted liposomes at corresponding time points in comparison to non-targeted liposomes. However, the MFI was considerably very low at each time point among all the formulations in MDA-MB-231 cells (Figure 4).
The data revealed that the inhibition of cellular proliferation, as well as silencing FASN expression, were significantly higher in targeted liposomes in comparison to non-targeted liposomes. Besides, pH-sensitive IL-DOPE/CHEMS immunoliposomes showed greater efficacy as compared to stealth IL-DSPC/Chol formulation.
Obviously, the idea of any type, the preparation of pegylated sterically stabilized long circulatory liposomes, pH-sensitive liposomes, and ligand-mediated targeting have already been explored, as evident by several studies. As a proof-of-concept, the present study demonstrated the development of effective novel formulations for the delivery of siRNA against the breast cancer. We inducted the benefit of each of these properties while combining selectively, ensuing improved therapeutic efficacy in vivo.
Conclusions
This study is the first report to demonstrate the potential use of lipid-based nanoformulations in the suppression of FASN using ligand-mediated site-specific delivery in Her-2 expressing breast cancer system. The cell specificity of Her-2 targeted FASN-encapsulated immunoliposomes, drastically enhanced, and reduced FASN that led to inhibit the proliferation of breast cancer cells.
Acknowledgments
We acknowledge all the staff members of the College of Applied Medical Sciences for their co-operation to conduct this research.
Disclosure
The authors have no conflict of interest to declare.
References
1. Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 – an update. J Gene Med. 2013;15(2):65–77. doi:10.1002/jgm.2698
2. Dang CV, Reddy EP, Shokat KM, Soucek L. Drugging the ‘undruggable’ cancer targets. Nat Rev Cancer. 2017;17(8):502–508. doi:10.1038/nrc.2017.36
3. Jain S, Pathak K, Vaidya A. Molecular therapy using siRNA: recent trends and advances of multi target inhibition of cancer growth. Int J Biol Macromol. 2018;116:880–892. doi:10.1016/j.ijbiomac.2018.05.077
4. Lorenzer C, Dirin M, Winkler AM, Baumann V, Winkler J. Going beyond the liver: progress and challenges of targeted delivery of siRNA therapeutics. J Control Release. 2015;203:1–15. doi:10.1016/j.jconrel.2015.02.003
5. Touzot F, Moshous D, Creidy R, et al. Faster T-cell development following gene therapy compared to haploidentical hematopoietic stem cell transplantation in the treatment of SCID-X1. Blood. 2015;125(23):1–18. doi:10.1182/blood-2014-12-616003
6. Hacein-Bey Abina S, Gaspar HB, Blondeau J, et al. Editorial outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313(15):550–1563. doi:10.1001/jama.2015.3253
7. Nathwani AC, Reiss UM, Tuddenham EGD, et al. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371(21):1994–2004. doi:10.1056/NEJMoa1407309
8. Schooley RT, Spritzler J, Wang H, et al. AIDS clinical trials group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. 2010;92093(5):705–776. doi:10.1086/655468
9. Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev. 2011;12(5):341–355. doi:10.1038/nrg2988
10. Yin H, Kanasty RL, Eltoukhy AA, et al. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi:10.1038/nrg3763
11. Moreira JN, Santos A, Moura V, et al. Non-viral lipid-based nanoparticles for targeted cancer systemic gene silencing. J Nanosci Nanotechnol. 2008;8(5):2184–2204. doi:10.1166/jnn.2008.18270
12. Mastrotto F, Brazzale C, Bellato F, et al. In vitro and in vivo behavior of liposomes decorated with pegs with different chemical features. Mol Pharm. 2020;17(4):472–487. doi:10.1021/acs.molpharmaceut.0c00149
13. Nasti TH, Khan MA, Owais M. Enhanced efficacy of pH-sensitive nystatin liposomes against Cryptococcus neoformans in murine model. J Antimicrob Chemother. 2006;57(2):349–352. doi:10.1093/jac/dki454
14. Simard P, Leroux JC. pH-sensitive immunoliposomes specific to the CD33 cell surface antigen of leukemic cells. Int J Pharm. 2009;381(2):86–96. doi:10.1016/j.ijpharm.2009.05.013
15. Kim IY, Kang YS, Lee DS, et al. Antitumor activity of EGFR targeted pH-sensitive immunoliposomes encapsulating gemcitabine in A549 xenograft nude mice. J Control Release. 2009;140(1):55–60. doi:10.1016/j.jconrel.2009.07.005
16. Merino M, Zalba S, Garrido MJ. Immunoliposomes in clinical oncology: state of the art and future perspectives. J Control Release. 2018;275:162–176. doi:10.1016/j.jconrel.2018.02.015
17. Gao J, Xia Y, Chen H, et al. Polymer-lipid hybrid nanoparticles conjugated with anti-EGF receptor antibody for targeted drug delivery to hepatocellular carcinoma. Nanomedicine. 2014;9(2):279–293. doi:10.2217/nnm.13.20
18. Harding JA, Engbers CM, Newman MS, Goldstein NI, Zalipsky S. Immunogenicity and pharmacokinetic attributes of poly(ethylene glycol)-grafted immunoliposomes. Biochim Biophys Acta. 1997;1327(2):181–192. doi:10.1016/S0005-2736(97)00056-4
19. Bendas G, Rothe U, Scherphof GL, Kamps JA. The influence of repeated injections on pharmacokinetics and biodistribution of different types of sterically stabilized immunoliposomes. Biochim Biophys Acta. 2003;1609(1):63–70. doi:10.1016/S0005-2736(02)00655-7
20. Heath TD, Montgomery JA, Piper JR, Papahadjopoulos D. Antibody-targeted liposomes: increase in specific toxicity of methotrexate-gamma-aspartate. Proc Natl Acad Sci USA. 1983;80(5):1377–1381. doi:10.1073/pnas.80.5.1377
21. Koning GA, Morselt HW, Gorter A, et al. Interaction of differently designed immunoliposomes with colon cancer cells and Kupffer cells. An in vitro comparison. Pharm Res. 2003;20(8):1249–1257. doi:10.1023/A:1025009300562
22. Lee CH, Hsiao M, Tseng YL, Chang FH. Enhanced gene delivery to HER-2-overexpressing breast cancer cells by modified immunolipoplexes conjugated with anti-HER-2 antibody. J Biomed Sci. 2003;10(3):337–344. doi:10.1007/BF02256453
23. Maruyama K, Takahashi N, Tagawa T, Nagaike K, Iwatsuru M. Immunoliposomes bearing polyethyleneglycol-coupled Fab’ fragment show prolonged circulation time and high extravasation into targeted solid tumors in vivo. FEBS Lett. 1997;413(1):177–180. doi:10.1016/S0014-5793(97)00905-8
24. Shahinian, Silvius JR, Silvius JR. A novel strategy affords high-yield coupling of antibody Fab’ fragments to liposomes. Biochim Biophys Acta. 1995;1239(2):157–167. doi:10.1016/0005-2736(95)00145-S
25. Sinha CK, Ignatoski KW, Lippman ME, Ethier SP, Chinnaiyan AM. Transcriptome analysis of HER2 reveals a molecular connection to fatty acid synthesis. Cancer Res. 2003;63(1):132–139.
26. Slamon D, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244(4905):707–712. doi:10.1126/science.2470152
27. Gao J, Sun J, Li A, et al. Lyophilized HER2-specific PEGylated immunoliposomes for active siRNA gene silencing. Biomaterials. 2010;9(9):2655–2664. doi:10.1016/j.biomaterials.2009.11.112
28. Currie E, Schulze A, Zechner R, Walther TC, Farese RJ. Cellular fatty acid metabolism and cancer. Cell Metab. 2013;18(2):153–161. doi:10.1016/j.cmet.2013.05.017
29. Santos CR, Schulze A. Lipid metabolism in cancer. FEBS J. 2012;279(15):2610–2623. doi:10.1111/j.1742-4658.2012.08644.x
30. Hong Y, Qiao JL, Cui LF, et al. Increased expression of FAS is a prognostic marker for patients with breast cancer. Int J Clin Exp Med. 2016;9:185–189.
31. Zhou L, Zhao YH, Wang XD, Jiang SF, Li H. Expression of fatty acid synthase and adipocyte fatty acid-binding protein and the relationship with the clinicopathological characteristics in human infiltrating ductal breast cancer. Sichuan Da Xue Xue Bao Yi Xue Ban. 2015;46(1):22–33.
32. Cai Y, Wang J, Zhang L, et al. Expressions of fatty acid synthase and HER2 are correlated with poor prognosis of ovarian cancer. Med Oncol. 2015;32(1):391. doi:10.1007/s12032-014-0391-z
33. Khan A, Aljarbou AN, Aldebasi YH, Faisal SM, Khan MA. Resveratrol suppresses the proliferation of breast cancer cells by inhibiting fatty acid synthase signaling pathway. Cancer Epidemiol. 2014;386(6):765–767. doi:10.1016/j.canep.2014.09.006
34. Jin Q, Yuan LX, Boulbes D, et al. Fatty acid synthase phosphorylation: a novel therapeutic target in HER2-overexpressing breast cancer cells. Breast Cancer Res. 2010;1(6):R96. doi:10.1186/bcr2777
35. Khan A, Shukla Y, Kalra N, et al. Potential of diallyl sulfide bearing pH-sensitive liposomes in chemoprevention against DMBA-induced skin papilloma. Mol Med. 2007;13(7–8):443–451. doi:10.2119/2006-00111.Khan
36. Marsh DA. CRC Handbook of Lipid Bilayers. Boca Raton, FL: CRC Press; 1990:163–168.
37. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protocol. 2007;2(2):329–333. doi:10.1038/nprot.2007.30
38. Das SK, Menezes ME, Bhatia S, et al. Gene therapies for cancer: strategies, challenges and successes. J Cell Physiol. 2015;230(2):259–271. doi:10.1002/jcp.24791
39. Chalbatani GM, Dana H, Gharagouzloo E, et al. Small interfering RNAs (siRNAs) in cancer therapy: a nano-based approach. Int J Nanomedicine. 2019;14:3111–3128. doi:10.2147/IJN.S200253
40. Jia LT, Chen SY, Yang AG. Cancer gene therapy targeting cellular apoptosis machinery. Cancer Treat Res. 2012;38:868–876.
41. Devi G. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006;13(9):819–829. doi:10.1038/sj.cgt.7700931
42. Biswas S, Deshpande PP, Navarro G, Dodwadkar NS, Torchilin VP. Lipid modified triblock PAMAM-based nanocarriers for siRNA drug co-delivery. Biomaterials. 2013;34(4):1289–1301. doi:10.1016/j.biomaterials.2012.10.024
43. Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8(2):129–138. doi:10.1038/nrd2742
44. Rettig GR, Behlke MA. Progress toward in vivo use of siRNAs-II. Mol Ther. 2012;20(3):483–512. doi:10.1038/mt.2011.263
45. Blancafort A, Giró-Perafita A, Oliveras G. Dual fatty acid synthase and HER2 signaling blockade shows marked antitumor activity against breast cancer models resistant to anti-HER2 drugs. PLoS One. 2015;10(6):e0131241. doi:10.1371/journal.pone.0131241
46. Gao J, Feng SS, Guo Y. Antibody engineering promotes nanomedicine for cancer treatment. Nanomedicine. 2010;5(8):1141–1145. doi:10.2217/nnm.10.94
47. Nosova AS, Koloskova OO, Nikonova AA, et al. Diversity of PEGylation methods of liposomes and their influence on RNA delivery. Med Chem Comm. 2019;10(3):369–377. doi:10.1039/C8MD00515J
48. Maruyama K. PEG-immunoliposome. Biosci Rep. 2002;22(2):25–266. doi:10.1023/A:1020138622686
49. Kirpotin D, Park JW, Hong K, et al. Sterically stabilized anti-HER2 immunoliposomes: design and targeting to human breast cancer cells in vitro †. Biochemistry. 1997;36(1):66–75. doi:10.1021/bi962148u
50. Maruyama K, Takizawa T, Yuda T, Kennel SJ, Huang L, Iwatsuru M. Targetability of novel immunoliposomes modified with amphipathic poly(ethylene glycol)s conjugated at their distal terminals to monoclonal antibodies. Biochim Biophys Acta. 1995;1234(1):74–80. doi:10.1016/0005-2736(94)00263-O
51. Sawant RR, Torchilin VP. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012;14(2):303–315. doi:10.1208/s12248-012-9330-0
52. Sapra P, Allen TM. Ligand-targeted liposomal anticancer drugs. Prog Lipid Res. 2003;42(5):39–462. doi:10.1016/S0163-7827(03)00032-8
53. Hua S, Wu SY. The use of lipid-based nanocarriers for targeted pain therapies. Front Pharmacol. 2013;4:143. doi:10.3389/fphar.2013.00143
54. Monteiro N, Martins A, Reis RL, Neves NM. Liposomes in tissue engineering and regenerative medicine. J R Soc Interface. 2014;11(101):20140459. doi:10.1098/rsif.2014.0459
55. Franze S, Selmin F, Samaritani E, Minghetti P, Cilurzo F. Lyophilization of liposomal formulations: still necessary, still challenging. Pharmaceutics. 2018;10(3):139. doi:10.3390/pharmaceutics10030139
56. Cacela C, Hincha DK. Low amounts of sucrose are sufficient to depress the phase transition temperature of dry phosphatidylcholine, but not for lyoprotection of liposomes. Biophys J. 2006;90(8):2831–2842. doi:10.1529/biophysj.105.074427
57. Kannan V, Balabathula P, Thoma LA, Wood GC. Effect of sucrose as a lyoprotectant on the integrity of paclitaxel-loaded liposomes during lyophilization. J Liposome Res. 2015;25(4):270–278. doi:10.3109/08982104.2014.992023
58. Wolkers WF, Oldenhof H, Tablin F, Crowe JH. Preservation of dried liposomes in the presence of sugar and phosphate. Biochim. Biophys. Acta. 2004;1661(2):125–134. doi:10.1016/j.bbamem.2003.12.006
59. Miyajima K. Role of saccharides for the freeze-thawing and freeze drying of liposome. Adv Drug Deliv Rev. 1997;24(2–3):151–159. doi:10.1016/S0169-409X(96)00454-1
60. Torchilin V. Fluorescence microscopy to follow the targeting of liposomes and micelles to cells and their intracellular fate. Adv Drug Deliv Rev. 2005;57(1):95–109. doi:10.1016/j.addr.2004.06.002
61. Varga C, Wickham T, Lauffenburger D. Receptor-mediated targeting of gene delivery vectors: insights from molecular mechanisms for improved vehicle design. Biotechnol Bioeng. 2000;70(6):593–605. doi:10.1002/1097-0290(20001220)70:6<593::AID-BIT1>3.0.CO;2-N
62. Paliwal SR, Paliwal R, Vyas SP. A review of mechanistic insight and application of pH-sensitive liposomes in drug delivery. Drug Deliv. 2015;22(3):231–242. doi:10.3109/10717544.2014.882469
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.